Abstract
Brain-Machine Interfaces (BMI) provide a framework to study cortical dynamics and the neural correlates of learning. Neuroprosthetic control has been associated with tuning changes in specific neurons directly projecting to the BMI (hereafter ‘direct neurons’). However, little is known about the larger network dynamics. By monitoring ensembles of neurons that were either causally linked to BMI control or indirectly involved, here we show that proficient neuroprosthetic control is associated with large-scale modifications to the cortical network in macaque monkeys. Specifically, there were changes in the preferred direction of both direct and indirect neurons. Interestingly, with learning, there was a relative decrease in the net modulation of indirect neural activity in comparison to the direct activity. These widespread differential changes in the direct and indirect population activity were remarkably stable from one day to the next and readily coexisted with the long-standing cortical network for upper limb control. Thus, the process of learning BMI control is associated with differential modification of neural populations based on their specific relation to movement control.
Multiple studies have shown that during natural motor control, neurons in motor areas experience a change in their firing properties after visuomotor adaptation1–3 or adaptation to a new dynamical environment4–8. While the observed changes in neural activity are closely linked to improvements in performance, it remains difficult to place such modifications in the context of the large cortical network for motor control. For instance, the specific neural correlates of learning and memory formation may depend upon a neuron’s causal role in movement control.
Brain-Machine Interfaces (BMI)9–23 offer the possibility of understanding the cortical network dynamics associated with learning to move a novel actuator in awake-behaving primates. During ‘brain control’, actuator movements are causally linked to an ensemble of neurons (i.e. ‘direct’ neurons), typically from the primary motor cortex (M1). Several studies have shown that such direct neurons experience a change in their tuning properties during the process of learning neuroprosthetic control12–13,21,23.
In contrast, the vast majority of neurons embedded in the larger M1 cortical network do not have a direct ‘projection’ to the BMI. While little is known about the function of such ‘indirect’ neurons during ensemble control, they are hypothesized to play a supportive role during the process of learning and recalling proficient brain control24. To characterize the large-scale cortical dynamics associated with learning neuroprosthetic control, we recorded ensembles of M1 neurons while only a subset were assigned to have a causal role during control. Here we characterize the differential plasticity of neural properties depending on the specific link to movement control.
METHODS
Surgery
Two adult male rhesus monkeys (Macaca mulatta) were chronically implanted bilaterally in primary motor and premotor cortex with 3–4 arrays of 64 teflon-coated tungsten microelectrodes (8×8 array separated by ~500µm; Supplementary Fig. 4) (Innovative Neurophysiology, Durham, NC). Our recent publication describes the specific location of implants in these two monkeys (Monkey P & R)23. All procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of California at Berkeley Institutional Animal Care and Use Committee.
Extracellular unit recordings
Unit activity was recorded using the MAP system (Plexon Inc, Dallas, TX). Activity was sorted on-line prior to recording sessions (Sort-client, Plexon Inc, Dallas, TX). The new experiments described in this manuscript exclusively used recordings from contralateral M1. Near, far and direct units were from the contralateral M1. Two datasets from our recent publication20 were also included in the analysis. While one dataset was from the ipsilateral M1, the other was from the contralateral M1.
Consistent with reports in the literature 12,23,28–30, several months post-surgery, we found a subset of units whose waveform shape, amplitude and relationship to other units on a channel varied little from day to day (i.e. sorting template in Sort-client required no modifications, see Fig. 5 a, b). The stationarity of such properties was the first criterion for a putative stable unit. Offline, we confirmed stationarity using principal component analysis (Wavetracker, Plexon Inc, Dallas, TX). We also examined the interspike-interval distribution (ISI). A Kolmogorov-Smirnov test was used to compare ISI distributions. After sorting, units with a clear refractory period (1.5 – 2 ms) were designated as putative single units. We also estimated the preferred direction of stable units as an additional measure of recording stability (see below).
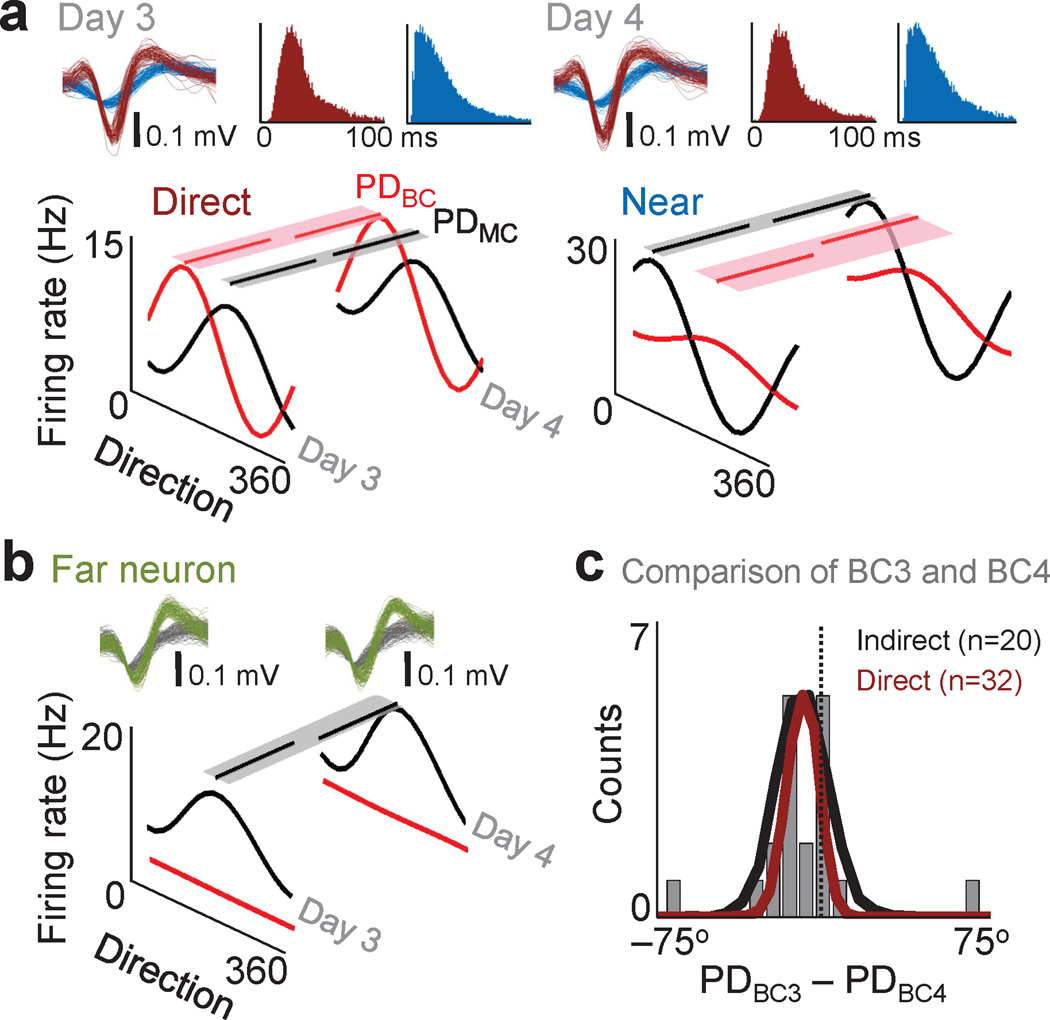
Fig. 5. Stability of neural properties across consecutive days of brain control.
a. Average directional modulation relationship for a direct and near unit during manual control and brain control on two consecutive days. Partial lines above each tuning curve represent the respective preferred direction for each daily brain control (PDBC) and manual control (PDmC) session. Shaded region is the respective variance of the bootstrap distributions of PDBC and PDMC. Waveforms and interspike interval distributions from a direct (red) and near (blue) unit on consecutive days are also shown. b. Directional modulation of a far unit on two consecutive days. PDBC could not be estimated because of a lack of modulation. c. Population distribution of preferred direction changes for indirect and direct neurons (PDBC3–PDBC4). For indirect units, the actual (grey bars) and bootstrap (black line) distributions are shown. The dark red line is the bootstrap distribution for direct units. Gray vertical line represents a ΔPD of 0.
Electromyography (EMG)
Surface gold disc electrodes (Grass Technologies) were mounted on medical adhesive tape and placed on the skin overlying muscle groups. Muscle groups tested included pectoralis major, biceps, deltoid, triceps, trapezius, and forearm extensors and flexor muscles. EMG signals were amplified 10,000× (Grass Technologies). Offline, signals were high-pass filtered, rectified, and smoothed by convolution with a 25 ms triangular kernel (MATLAB Software, R2009b). Directional activation was estimated using the activity in a 300 ms window after movement onset.
Experimental setup and behavioral training
Monkeys were trained to perform a center-out delayed reaching task using the Kinarm exoskeleton (BKIN Technologies, Kingston, ON). The behavioral task consisted of hand movements from a center target to one of eight targets distributed over a 14 cm diameter circle (i.e. Manual Control). Target radius was 0.75 cm. Trials were initiated by entering the center and holding for a variable period (500–1000 ms). The GO cue (center changed color) was provided after the hold period. A liquid reward was provided after a successful reach. Visual feedback of hand position was provided by a cursor precisely co-located with the center of the hand (radius of 0.5 cm).
Decoding parameters from neural ensembles
We used linear regression to map neural activity to kinematic parameters23,26. This was performed using functions available in the MATLAB software (R2009b). For a subset of experiments (brain control task #2), the neural activity ‘predicted’ joint position. These values were then converted into Cartesian coordinates. The cursor position was updated on the Kinarm projection screen at 10 Hz. We also tested direct prediction of hand velocity in Cartesian coordinates (brain control task #1, shown in Fig. 1a). Neural activity was streamed over a local intranet via the PLEXNET client-server application (Plexon Inc, Dallas, TX) and converted into 100 ms bins of spiking activity.
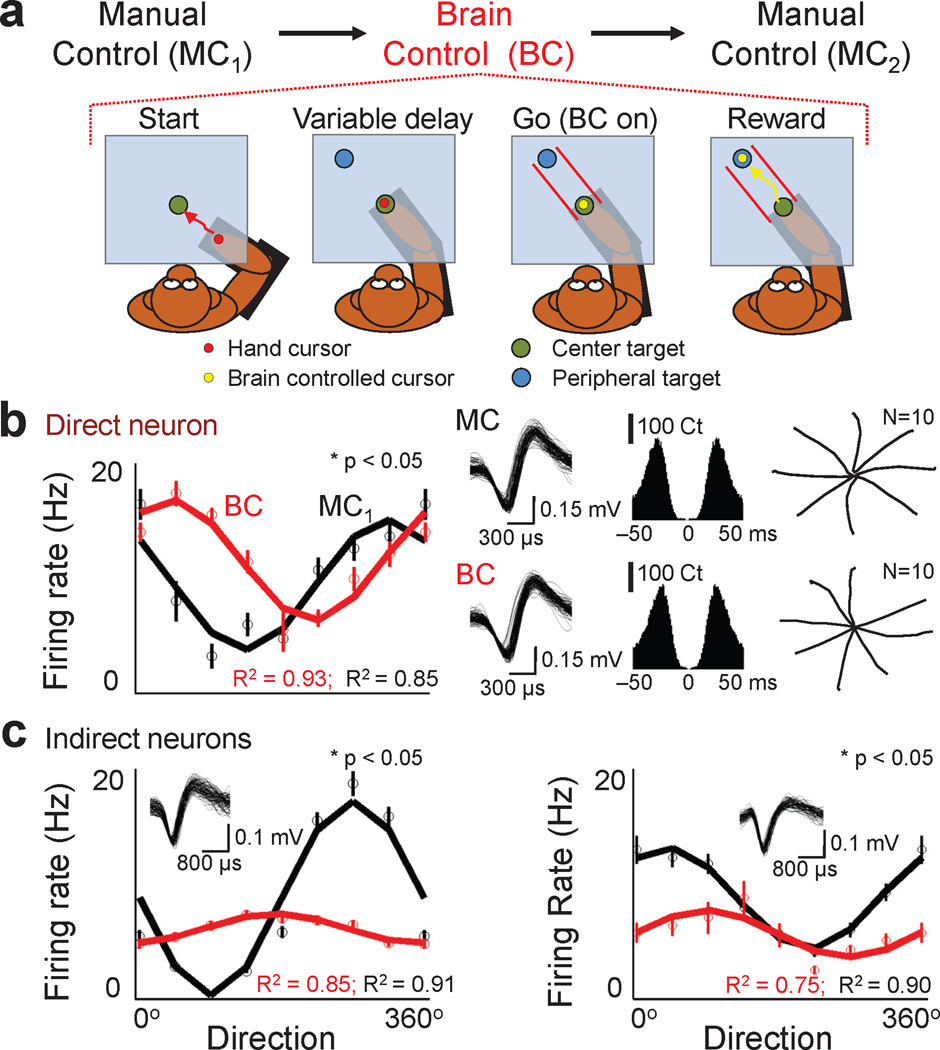
Fig. 1. Modification of neural firing properties during brain control.
a. For each daily session, subjects were required to serially perform a delayed center-out task in manual control (MC1), brain control and then manual control (MC2). In the brain control task shown, visual guides (i.e. lines shown in red) enforced straight trajectories. Trials were started by the animal physically moving to the center target. After a hold period, brain control (i.e. absence of any movements) was initiated. b. Changes in the preferred direction of a direct neuron. Solid lines are the cosine fit (R2 is the percent of variance accounted for by the fit). Circles and bars (s.e.m.) show the directional modulation of the firing rate. Panels on the right show the waveform, crosscorrelograms (0.1% of spikes in a window < 1.5 ms) and the mean trajectories during manual control and brain control. Statistics performed with boostrap analysis. c. Changes in the preferred direction of indirect neurons. The directional modulation relationships are arranged similarly to b. Insets show waveforms of the respective indirect neurons.
The number of neurons incorporated ranged from 10 to 45. This variability was a function of several factors. Primarily, we were limited by availability of well-isolated neurons. The yield slowly decreased over time after implantation. The second limitation was the stability of an ensemble. Prior to each experiment, we monitored the activity in one array over days to identify possible stable units. While such monitoring increased the probability of recording a stable ensemble, it did not guarantee stability. Thus, there were numerous failed experiments resulting from an inability to record the ensemble over the desired time period. The other factor was the need to monitor both direct and indirect neurons during the course of learning brain control.
We ensured that well-isolated units were part of both the direct and indirect populations. Supplementary Figure 8 compares the properties of the two groups from one experiment (same experiment as shown in Fig. 2a). Using the linear decoder described above, we also compared the ability of randomly selected neurons from either the direct or indirect population to predict limb movement parameters. After running a 100 such comparisons, we found that the mean correlation between the actual and predicted limb positions was very similar (direct population: R=0.77 ± 0.06 and indirect population: R=0.74 ± 0.05, mean ± 2 sd).
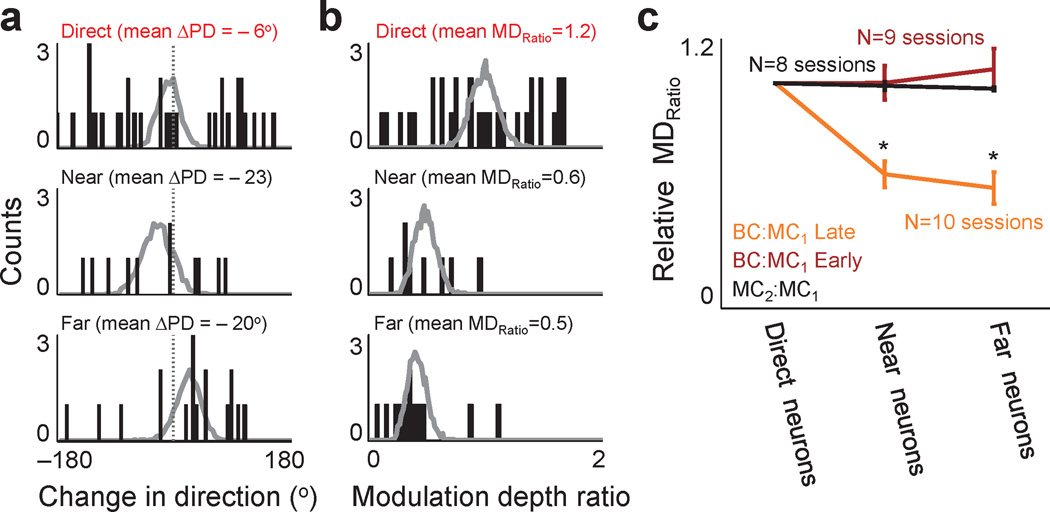
Fig. 2. Differential modulation of neuronal populations during brain control.
a. Distribution of shifts in preferred directions (ΔPD) between manual control and brain control. Each bar shows the number of neurons (i.e. ‘counts’) with a corresponding ΔPD. The labels above indicate the mean ΔPD for each population. Superimposed in gray is the bootstrap distribution. b. Distribution of changes in modulation depth ratio (MDratio) for BC:MC for the three neural populations. This panel is arranged similarly to a. c. Ratio of relative modulation depths. To compare multiple experiments and experimental conditions, we normalized each session to the mean MDratio for direct neurons. ‘Early’ and ‘Late’ represent brain control sessions respectively from days #1–2 and day ≥ 3 of training. MC1:MC2 is the ratio of modulation depths of the manual control sessions before and after brain control. Error bars show s.e.m.
Online brain control
As noted above, these animals had been previously trained to perform brain control task #223. The task structure for brain control Task #1 was new to them. New brain control experiments were performed over short periods of time (typically 3–6 days). There was consistent evidence of improvements in performance with practice (also see Supplementary Fig. 2). Animals were permitted to use a ‘fixed decoder’ (i.e. held constant after initial training on day #1) and stable recordings from a neural ensemble23,27. Each ‘experiment’ consisted of a new set of stable neurons and a decoder that was fixed after training on day #1. Each experiment consisted of multiple daily ‘sessions’.
During brain control task #1, the animal kept his right upper limb in the exoskeleton. As shown in Figure 1, the animals were required to move their hand to the center target to start a trial and to keep it in the center target at all times. A new cursor (under brain control) appeared at the start of the trial. The animals were required to move the cursor to the target by modulation of motor cortex activity (under velocity control). Hand position was continuously monitored during brain control. The trial was aborted with any change in hand position. To start a new trial, the animal had to move out of the center target and reposition his hand. Thus, in essence, the ‘brain controlled cursor’ was reset to the center target for each trial. During selected sessions, we concurrently performed video and surface EMG recordings from proximal and distal muscle groups (Supplementary Fig. 1). As also shown in Supplementary Figure 3, Monkey R was trained to perform this task in a single trial randomized fashion (switching between manual control and brain control trials).
For brain control task #2, the cursor was continuously under brain control. The task-related hand was removed from the exoskeleton and restrained on the side during brain control. The cursor was under continuous volitional control. Subjects were required to self-initiate each trial by moving the brain controlled cursor to the center. A trial was considered incorrect if the cursor failed to reach the target within 10 seconds after a GO cue. To start a trial, the cursor had to be held over the center target for 250–300ms. The ‘chance’ level of self-initiation was ~0.5 per minute. This value was determined through experiments where the task was performed by spontaneous neural activity (i.e. the computer monitor was turned off while the cursor was controlled by spontaneous activity). In contrast, while engaged in the task, each subject self-initiated trials at a rate of 3–10/min.
Data analysis
The majority of analysis was performed on ‘Late’ sessions (defined as sessions ≥ day 3 in an experiment). Sessions from Day #1 and #2 were labeled ‘Early’.
Preferred Direction
Directional tuning was estimated by comparing the mean firing rate as a function of target angle during movement execution. In manual control, the time to target was relatively constant (~700ms). In brain control, this period was variable and decreased with learning. For the analysis, a 500 ms window was used (starting 200 ms prior to movement). As shown in Supplementary Figures 6 and 7, our results did not depend on the specific time window.
The tuning curve was estimated by fitting the firing rate with a sine and a cosine as:
| (1) |
where θ corresponds to reach angle and f corresponds to the firing rate across the different angles. Linear regression was used to estimate the B coefficients. The preferred direction was calculated using the following: preferred direction = tan−1 (B2/B3), resolved to the correct quadrant44. For units with changes in preferred direction, we ensured that regression captured ≥ 50% of the variance. Thus, the unit shown in Fig. 5b was not included in the analysis of preferred direction changes.
Modulation Depth
Modulation depth was calculated as the peak-to-peak amplitude of the tuning curve. For Figure 2c, we ensured that the tuning fit was appropriate for the manual control trial. The modulation depth of the brain control was computed regardless of the fit. This ensured that units no longer modulated in brain control were included (e.g. Fig. 5b). The MDratio was calculated by dividing the modulation depth under two conditions (e.g. comparison of brain control/manual control). To compare multiple experiments and experimental conditions, we normalized each experiment to the mean MDratio for direct neurons. We also tested a non-parametric metric (difference between highest and lowest firing rate). We reached the same conclusion of a relative decrease for the indirect population.
Changes in directional tuning
A bootstrap resampling procedure was used to test significance of modulation depth and preferred direction changes 23,28. By repeating this 2000 times, we created a distribution corresponding to the null hypothesis (i.e. no change in preferred direction). The confidence intervals were based on the specified ‘p value’ using a percentile bootstrap. Comparison of modulation depth and firing rates were performed in an analogous manner.
Changes in mean preferred direction and mean modulation depth
A bootstrap statistic was also used to compare differences between populations (e.g. Fig. 2b). The experimental values for each population were sampled with replacement 2000 times. By taking the mean of each resample, we created a distribution of values. For comparison between conditions, we sampled one value from the respective zero mean distributions to create a distribution of absolute differences. We did not note any bias and the corresponding distributions were symmetric (Fig. 2b).
We also tested an alternative tuning measure23. Specifically, we reexamined the tuning analysis based on the actual path of the cursor (as opposed to the intended direction). A similar percentage of units experienced a change in preferred direction (path taken: 69 ± 8% vs intended direction: 67 ± 12% mean ± std, n=10 sessions, p > 0.05). Moreover, a related hypothesis is that changes in individual preferred directions could also manifest as changes in ensemble firing patterns. A preliminary analysis of the reversible changes in firing patterns when switching from manual control to brain control are shown in Supplementary Figure 945−46.
Modifications during a session
Neurons with a significant change were selected. To estimate values over the course of MC1/BC/MC2 trials, a moving window of trials (2 sets of trials to each of 8 targets) was used. Each individual parameter was then plotted over time (Fig. 4a & c). To calculate the baseline preferred direction change, we first determined the mean preferred direction during MC1 for each neuron. This value was subtracted from all values during MC1/BC/MC2. Thus, the baseline was ‘zeroed’ for ease of comparison. As we were interested in examining the rapidity and stability of shifts, we took the absolute value of this. The traces in Figure 4b & d were the overall average.
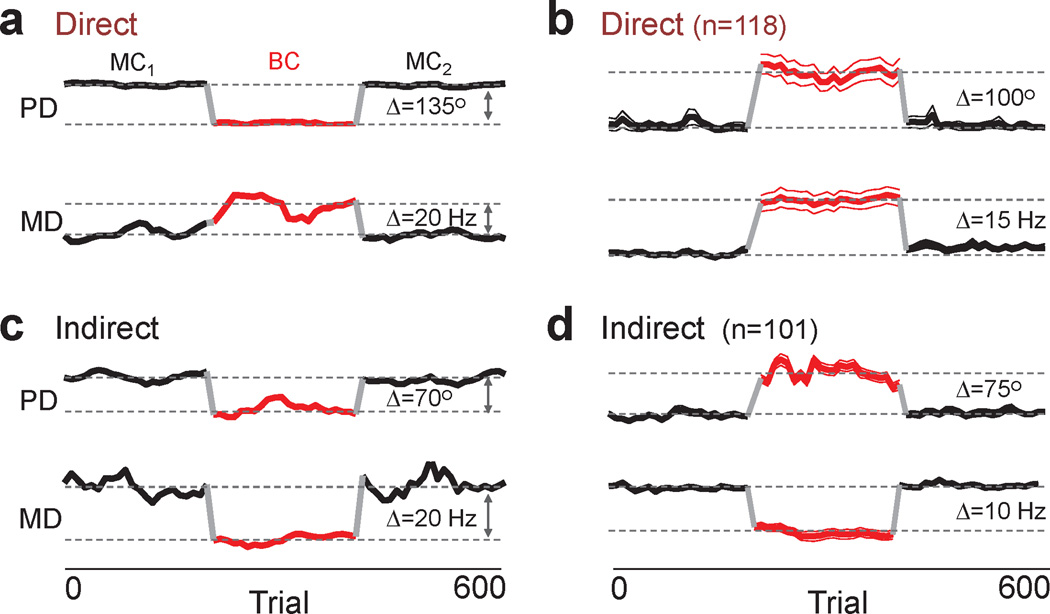
Fig. 4. Stability of state-dependent changes in neural properties during a session.
a. Traces show the preferred direction and modulation depth for a moving window of trials (window of 16 trials) for a direct unit. Each segment is color coded and labeled (MC1, brain control or BC, MC2). b. Average of multiple direct units from both animals. To illustrate the time course at the population level, the respective mean MC1 value was subtracted from each individual trace and the absolute value was used for the average. n=number of units included in the average. Each plot shows the mean (thick line) ± 2 s.e.m. (thin line). c,d. Individual example and average responses of indirect units. Arranged as in a,b.
Results
Two macaque monkeys were trained to perform center-out reaching movements using a robotic exoskeleton that constrained movements to the horizontal plane (i.e. Manual Control). Following implantation of microelectrodes, a small ensemble of neurons, typically from the contralateral M1, was randomly selected to be ‘directly linked’ to BMI control. The remaining neurons were recorded but not linked to the BMI (i.e. indirect neurons). The spiking activity of the ‘direct’ ensemble was transformed to motor commands with a linear decoder optimized to predict upper limb movements11,13,18,23,25. The animals learned brain control using stable recordings of the direct ensemble across days and a decoder that was held constant after the initial training23,26–27. Stability of recordings across days was assessed by stationarity of spike waveforms and the interspike interval (ISI) distribution23,28–31. As an additional measure, we frequently monitored the directional modulation of each unit during manual control sessions.
The animals were trained to perform two tasks in brain control during separate experiments. Task 1 was structured to equate initial conditions for manual and brain control and to minimize changes in posture and workspace (Fig. 1a) 32–33. The right upper limb remained in the exoskeleton under both conditions (Fig. 1a). During manual control, the animal made physical movements to initiate and complete trials. During brain control, in contrast, the animal first made physical movements to the ‘Center’ target. After a variable ‘Hold’ period, a brain control trial started. During brain control of the computer cursor, the animal was required to hold its arm stationary with the hand in the center target. Arm kinematics were monitored continuously and the trial was aborted if any motion occurred. We also performed electromyogram (EMG) recordings to rule out muscle contractions during brain control (Supplementary Fig. 1). We ensured that the trajectories were comparable using ‘guide’ lines (Fig. 1a). If the cursor moved outside the lines, the trial was aborted. In contrast to Task 1, the second task was similar to past experiments11–13,19,23, where the animal’s arm was taken out of the exoskeleton and restrained during brain control.
The animals typically developed proficient brain control over time (usually days ≥ 3 in each experiment, Supplementary Fig. 2). It is important to note that while both of these animals had extensive experience with brain control, they required practice to achieve skilled control with a new set of neurons and a given decoder. Task performance during ‘late’ sessions (i.e. ≥ day 3 of practice) was 86 ± 2 % mean ± sem in Monkey P and 83 ± 2 % in Monkey R, with a mean time to target of 2.4 ± 0.3 s and 2.8 ± 0.25 s respectively in Monkey P (16 ‘late’ sessions from 4 experiments) and R (9 ‘late’ sessions from 3 experiments).
Modification of preferred directions
We first analyzed changes in the preferred direction of direct neurons during Task 1 (Fig. 1b). We found that a significant proportion experienced a change in preferred direction during brain control in comparison to manual control (56 ± 8% mean ± sem with a significant change; 3 sessions with 10 neurons each from 1 experiment in Monkey P and 3 sessions with 15 neurons each from 1 experiment in Monkey P; bootstrap analysis with p<0.05 and a correction for multiple comparisons was used). When animals were further trained to rapidly switch between brain control and manual control on a single trial basis (Supplementary Fig. 3), there was still a significant shift in preferred directions (11 of 20 neurons modified, 2 sessions in Monkey R). Moreover, consistent with past experiments12–14, similar modifications were present during Task #2 (61 ± 5% mean ± sem, 8 sessions from 4 experiments, 10–45 neurons per session, p< 0.05 bootstrap analysis). Thus, changes in limb posture and workspace do not exclusively account for the changes in preferred direction after transition to brain control. For subsequent analysis, we combined the datasets from the two tasks.
We next analyzed the indirect neurons (Fig. 1c). Interestingly, in both animals we found that indirect neurons also experienced a similar change in their preferred direction (Monkey P: n=6 sessions with 18–25 units per session, 60 ± 6% mean ± sem; Monkey R: n=4 sessions, 63 ± 10% mean ± sem with 10–18 units, p< 0.05 bootstrap analysis). To assess specific differences among population of neurons, we subdivided the indirect neurons (Fig. 2a). Indirect neurons recorded on a BMI channel (i.e. microwire with a direct neuron) were labeled as ‘near’ (see Supplementary Fig. 4). The remaining indirect neurons were labeled as ‘far’ (i.e. recorded on a microwire ~500–700µm from a BMI channel). We did not find a significant difference between the percentage and the extent of changes in the preferred direction of these two groups (p > 0.05, bootstrap analysis). Together, our results indicate that there were large scale changes in the preferred direction of both direct and indirect neurons after the transition to brain control.
While the analysis described above focused on individual units, we also examined changes at the population level. For the direct group, while a majority of the individual shifts in preferred direction (ΔPD) were significant, the sum of the positive and negative shifts resulted in a non-significant net shift (Fig. 2a). There were no significant differences between the direct, near and far populations (Fig. 2a, p > 0.05, bootstrap analysis). A similar finding was also evident when considering all neurons in both animals (Supplementary Fig. 5). Thus, it appears that there is a relative remapping of the preferred directions without any significant systematic rotational shifts for each neural population.
Differential modification of modulation depths
We next examined for changes in modulation depth. For each neuron, we calculated the ratio of modulation depths between brain control and manual control (BC:MC MDratio). We initially focused on sessions with proficient task performance (i.e. ≥ day 3 of practice defined as ‘Late’). During these brain control sessions, indirect neurons were less modulated than during manual control (Fig. 1c and Fig. 2b,c). Similar to past reports22–23,34, there was some heterogeneity in the direct population responses. In contrast, both of the indirect populations experienced a consistent net relative reduction in MDratio (Fig. 2b).
We compared population means across multiple experiments. The mean BC:MC1 MDratio was 1.2, 0.6, 0.5 respectively for the direct, near and far populations (Fig. 2b). The median values were respectively 1.2, 0.5, and 0.5. Only the near and far groups demonstrated a significant decrease. Superimposed are the bootstrap distributions of each group. In addition, when we varied the time window for measurement of directional tuning, there were no significant changes in our conclusions (Supplementary Figs. 6 and 7).
Across six experiments in both animals, we observed a consistent difference between the relative mean modulation depths of the direct and indirect neuronal populations (Fig. 2c, BC:MC1 Late, 10 sessions from 6 experiments in Monkey P and R). Surprisingly, the units with close proximity to direct neurons behaved similarly to more distant neurons. These differences emerged upon stabilization of task performance (Fig. 2c, BC:MC1 Early versus BC:MC1 Late, p < 0.05 for near and far populations, 9 sessions taken from 6 experiments in both Monkey P and R). Together, our results indicate that differential modulation of the neuronal populations was specifically present during proficient neuroprosthetic control and not during the initial learning period.
In addition, as evident in the examples illustrated in Figure 1 (also see Supplementary Fig. 4), there were changes in the mean firing rate of individual neurons when comparing manual control to brain control. They appeared to be independent of the changes in modulation depth (e.g. compare near and far neurons in Supplementary Fig. 4). While some neurons experienced a combined decrease in the mean firing rate and the modulation depth (e.g. Fig. 1c left panel), other neurons experienced a change in the modulation depth while the mean firing rate remained unchanged (e.g. Supplementary Fig. 4). At the population level, however, there were no significant systematic differences in the mean firing rate between manual control and brain control for either the direct or the indirect populations (n=6 experiments, p > 0.05 bootstrap analysis).
State-dependent modification of neural properties
As described above, the subjects performed manual control both before and after brain control. Thus, comparison of modulation depth during MC1 and MC2 could assess for any lasting effects of the modifications during brain control. For example, studies of motor learning have documented the neural correlates of a ‘memory trace’ after motor learning4. Interestingly, there was no significant difference between the direct, near and far groups for this comparison (p>0.05, bootstrap analysis, Fig. 2c, MC1:MC2 MDratio). This indicates that the population modulation depth during manual control, both before and after the brain control, was very similar. Moreover, MC2:MC1 MDratio was significantly different from the BC:MC1 Late relationship for both the near and far neurons (p < 0.05, bootstrap analysis, 8 sessions taken from 6 experiments). This further implies that the population modulation depth reverts back to its original properties during the manual control task.
We subsequently assessed for differences at the level of individual units. The vast majority of units did not experience a significant change in preferred direction between MC1 and MC2 (Fig. 3a). Also shown is an example of a unit with a small, but significant, change. Figures 3b and c show respective examples of the distribution of individual changes in preferred direction and modulation depth (comparison of 64 neurons during a daily MC1 and MC2 session in Monkey P). All three neural populations were combined as no significant differences were evident for each separate comparison. In general, we found that the vast majority of neurons reverted back to their task-related firing patterns during MC2 in comparison to MC1 (89 ± 5% mean ± std and 83 ± 4.5% mean ± std without significant changes in preferred direction and modulation depth respectively, n=6 experiments). We also did not find evidence of significant differences for manual control sessions associated with ‘early brain control’ (87 ± 8% mean ± std and 80 ± 3% mean ± std without changes in preferred direction and modulation depth, n=6 experiments). Moreover, the presence of a unimodal distribution of changes (i.e. Fig. 3b and c) perhaps suggests a small degree of instability of the neuron-behavior relationship during the two sessions7,28,35–36. Alternatively, these changes could reflect subtle changes in task performance.
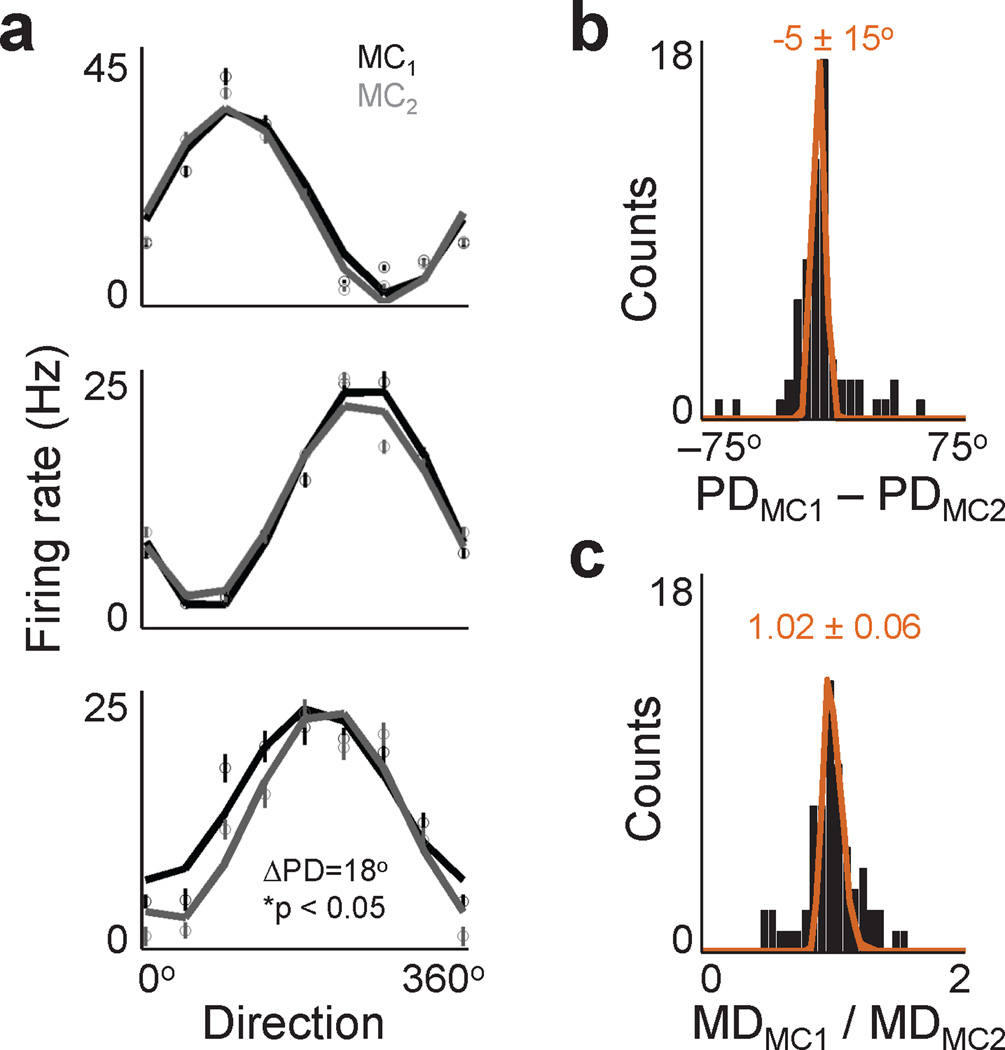
Fig. 3. Stability of neural properties.
a. Average directional modulation relationship during MC1 (black) and MC2 (gray) for three neurons. Neuron in lower panel experienced a significant change (bootstrap analysis, p<0.05). Error bars shown s.e.m. b. Actual (solid bars) and bootstrap (orange, mean ± std shown) distributions of changes in preferred direction during MC1 and MC2. All three neural populations were combined as they behaved similarly. c. Distributions of modulation depth changes. Arranged as in b.
What are the neural dynamics of switching (i.e. MC1 → brain control → MC2)? We measured the directional modulation relationship across sessions. For each transition, relatively rapid changes in preferred direction and modulation depth were evident for direct units (Fig. 4a, b). Similar dynamics were evident for indirect neurons, albeit with a reduction of modulation (Fig. 4c, d). Moreover, the properties of both direct and indirect neurons remained relatively stable during each state.
Stability of indirect neural properties across days
To further test the link between indirect units and brain control, we examined their properties across consecutive days of proficient brain control. For instance, if the properties of the indirect population remain constant across days of proficient brain control, it suggests that they play an active role. We selected a population of stable indirect neurons, all of which had stable waveform shapes, ISI distribution and preferred direction during manual control (Fig. 5a). The activities of these neurons were compared across two consecutive days of brain control (Task Performance, Day 3: 97% and Day 4: 98%). There was no significant difference in either preferred direction or modulation depth for these examples (p > 0.05, bootstrap analysis). There were also neurons that were robustly modulated during manual control but consistently not modulated during each daily brain control session (Fig. 5b). In general, individual indirect neurons maintained a relatively fixed neuron-behavior relationship for consecutive days of brain control (comparison of n=3 experiments, % of neurons with stable parameters: preferred direction 87 ± 4% and modulation depth 81 ± 2%, n= 16–20 indirect neurons). Strikingly, this was not significantly different from the neuron-behavior relationship for manual control described in the previous section (p > 0.05, bootstrap analysis).
We also compared the distribution of changes across days for both direct and indirect neurons at the population level (Fig. 5c). Interestingly, the indirect neuron distribution was also not significantly different from that for direct neurons (indirect −19 ± 12° and direct −15 ± 9°, mean ± std, p > 0.05). Across multiple experiments we also found that the population dynamics were very similar across consecutive days (indirect 0.6 ± 11° and direct –4 ± 10°, mean ± std, n=3 experiments). This was also evident for the MDratio distributions (indirect 1.0 ± 0.14 and direct 1.07 ± 0.15, mean ± std, n=3 experiments). Together, this indicates that indirect neurons maintained a relatively fixed neuron-behavior relationship during brain control. The similarity with the direct neurons further suggests that the indirect population may play an active role during brain control.
Discussion
This study demonstrates that large-scale modifications of the motor cortex network are associated with learning neuroprosthetic control. We consistently observed that learning brain control was linked to modifications of both direct and indirect neurons. While a similar fraction of both neural populations experienced a change in preferred direction, there were clear differences in their relative modulation. Thus, the process of learning neuroprosthetic control differentially modifies groups of neurons based on their causal relation to movements. Interestingly, these large-scale changes were remarkably stable over time and readily coexisted with the cortical activity patterns associated with actual upper limb movements.
Large-scale modifications associated with learning
One goal of the field of BMI is to allow skilled control of an external artificial actuator while minimizing the learning required12,23,37. A related hypothesis is that by tapping the existing cortical network for manual control, brain control can be achieved in a rapid and intuitive manner. In support of this possibility are studies demonstrating that: (1) motor cortex spiking can be dissociated from movements21,38, (2) that imagined movements result in patterns of activity in the absence of movement18, and (3) that an arbitrary activity pattern may be achieved through learning21,23. Past research has evaluated brain control with ‘biomimetic decoders’ that capture the relationship between neural activity and a movement parameter11–13,18. Multiple studies, however, have reported that learning is required to achieve skilled control12–13,18,23,26.
We also observed a requirement for learning when a new set of neurons and a decoder were introduced23,27. Moreover, we noted shifts in the preferred direction of both direct and indirect neurons. Our results also show for the first time that the surrounding indirect neurons are differentially modified. Interestingly, this was evident only after stabilization of performance and not during the initial learning process. Stability of recordings and the decoder are likely to be important for skill acquisition and the observed neural modifications23,27. It is important to note, however, the majority of BMI studies have not used such conditions, instead relying upon decoders that are retrained daily12–13,27,39. It remains unclear how the indirect neurons are modified under those conditions.
What is the role of indirect neurons? The stability of indirect neural properties suggests an active supportive role in neuroprosthetic control. Our analysis of individual neurons indicates that such stability is present over long daily sessions as well for sessions on subsequent days. Interestingly, the stability of both direct and indirect neural properties is quite similar. This implies that even while the decoders cannot ‘translate’ indirect neural activity, this activity may shape the direct activity. However, it also remains possible that indirect activity may play a negative role. In this viewpoint, our observed reduction in modulation depth may allow more efficient brain control by avoiding interference with the direct neurons22,24. As discussed below, this may be related to the ‘reweighting’ phenomenon after perturbations to the decoder22.
Changes in directional tuning with learning
Both indirect and direct populations experienced similar changes in preferred direction. Moreover, at the population level, there was no significant rotation. Interestingly, these widespread changes were closely linked to the process of learning. For example, both groups experienced an overall stabilization of tuning properties when task performance plateaued. This was also evident for neurons across days of brain control. This association suggests a link between learning neuroprosthetic control and the observed changes in preferred direction. After the initial switch to brain control during early sessions, performance is typically poor. This implies that the ensemble tuning properties (i.e. those present during manual control) are not sufficient. It is reasonable to assume that error-correction mechanisms are recruited over this period. It is thus possible that the observed shifts in preferred direction are the result of cortical mechanisms to minimize task-related errors. In support of this notion is our recent finding of a strong correlation between the extent of instability of direct neural tuning properties and task performance during long-term brain control23.
We also designed a novel task to closely approximate initial conditions for manual control and brain control (i.e. Task 1). This task minimized the possibility that changes in limb position and posture32–33 could by themselves result in widespread changes in preferred direction. With this task, we still noted large scale changes. Interestingly, even when initial conditions were such that the animal could not predict an upcoming trial (i.e. single trial switching between manual and brain control, Supplementary Fig. 3), changes in preferred direction were still evident. These results suggest that differences in initial conditions cannot completely account for the observed change in neural properties. It remains possible that differences emerging after the onset of movements (e.g. absence of limb dynamics and propioception) are triggers for the changes in neural properties. However, past studies have also shown that brain control in the presence of limb movements (i.e. where propioception and limb dynamics are likely to be present in some form) have also resulted in modifications13,40. Moreover, changes in sensory responses should affect both the indirect and direct neurons equally (i.e. a global change). Our observed differential modulation is not consistent with this possibility.
Differential modulation of direct and indirect populations
The distinction of direct versus indirect is an externally imposed causal link to cursor movements via the ‘decoder’. While prior to learning brain control these populations were similarly modulated, stable skill acquisition was associated with differential modulation. Thus, learning proficient control through error-correction processes and visual feedback appears to be capable of differentially modifying populations of units with a causal link to movements.
Our finding is closely related to the body of literature, albeit at the level of neural ensembles, on modifications of single neurons and pairs of neurons through operant conditioning21,24,41. Interestingly, studies of operant conditioning of single neurons found that non-conditioned adjacent neurons were largely correlated with the conditioned neurons24,41. Recent theoretical work suggests that spike-timing based plasticity could underlie changes in neural activity through operant conditioning42. Apparent differences in comparison to our findings may be the result of two factors. Firstly, differential modulation was only evident after several days of practice. Our analysis of ‘early’ sessions could be consistent with the correlated changes seen with daily conditioning of individual neurons. Secondly, learning neuroprosthetic control with larger ensembles may not be compatible with strategies that trigger correlated increases in neural activity.
Moreover, recent work on changes in neural activity in response to decoder perturbations suggest that error-correcting mechanisms can partially establish a link between neurons and their specific contributions to errors during brain control22. This finding may be related to our observation of differential modulation. Neural mechanisms of error correction are almost certainly recruited by this process. It seems reasonable to hypothesize that a common mechanism underlies both the initial establishment of proficient control as well as adjustments after a perturbation. Especially given the casual link between direct activity and cursor movements, direct neurons are more likely to contribute to errors than indirect neurons.
Reversibility of the modifications
The observed large-scale modifications were reversible in a state-dependent manner. While several studies have documented changes in neural properties during brain control 12–14,21–23, the time course and reversibility of such changes remained unclear. Here we show that modifications to both direct and indirect neurons were rapidly reversible. This indicates that proficient neuroprosthetic control is associated with the formation of a cortical state that readily coexists with the long-standing network for natural motor control. When switching between control states, the cortical network appears to rapidly switch, without interference, based on task requirements.
Such rapid reversibility may contrast with the network changes associated with adaptation to novel force fields4–5. In a previous study, after adaptation to a new dynamical environment, motor cortex appeared to retain a ‘memory trace’ evident at the level of single neurons5. While this may suggest a difference between motor adaptation versus neuroprosthetic learning, there are several factors that could account for the differences. Two such factors are the amount of time spent learning the task and the electrophysiological recording technique (e.g. acute single neuron recordings versus chronic recordings could target different neuronal populations). In general, the exact mechanisms that allow for apparently rapid changes to cortical properties when switching control states remain unclear. They may be related to existing cortical mechanisms for switching among states during natural motor control43. It also points to the general ability to maintain multiple neuron-behavior relationships without interference23,28,36.
In summary, our results demonstrate that learning neuroprosthetic control is associated with differential modulation of neuronal populations based on its causal link to movement control. Moreover, proficient control is linked to the formation of a stable large-scale set of neural activations.
Supplementary Material
Acknowledgements
The authors wish to thank Dragan Dmitrov for surgical assistance. The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development, and the American Heart Association/American Stroke Association to K.G.; the NINDS # NS21135 to J.D.W.; and the Alfred P. Sloan foundation, the Christopher and Dana Reeve Foundation, the National Science Foundation CAREER Award # 0954243, and the Defense Advanced Research Projects Agency contract N66001-10-C-2008 to J.M.C.
Footnotes
Author Contributions: KG and JMC designed the experiments. KG and JMC performed behavioral training. KG performed the experiments and analyzed the data. KG and JMC wrote the paper. JDW, JMC and KG performed surgical procedures. KG, JDW and JMC revised the paper.
Completing financial interest statement: The authors do not have any conflicts of interest.
References
- 1.Wise SP, Moody SL, Blomstrom KJ, Mitz AR. Changes in motor cortical activity during visuomotor adaptation. Exp Brain Res. 1998;121:285–299. doi: 10.1007/s002210050462. [DOI] [PubMed] [Google Scholar]
- 2.Paz R, Vaadia E. Learning-induced improvement in encoding and decoding of specific movement directions by neurons in the primary motor cortex. Plos Biol. 2004;2:E45. doi: 10.1371/journal.pbio.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paz R, Boraud T, Natan C, Bergman H, Vaadia E. Preparatory activity in motor cortex reflects learning of local visuomotor skills. Nat Neurosci. 2003;6:882–890. doi: 10.1038/nn1097. [DOI] [PubMed] [Google Scholar]
- 4.Gandolfo F, Li C, Benda BJ, Schioppa CP, Bizzi E. Cortical correlates of learning in monkeys adapting to a new dynamical environment. Proc Natl Acad Sci U S A. 2000;97:2259–2263. doi: 10.1073/pnas.040567097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30:593–607. doi: 10.1016/s0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 6.Padoa-Schioppa C, Li CS, Bizzi E. Neuronal correlates of kinematics-to-dynamics transformation in the supplementary motor area. Neuron. 2002;36:751–765. doi: 10.1016/s0896-6273(02)01028-0. [DOI] [PubMed] [Google Scholar]
- 7.Rokni U, Richardson AG, Bizzi E, Seung HS. Motor learning with unstable neural representations. Neuron. 2007;54:653–666. doi: 10.1016/j.neuron.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Arce F, Novick I, Mandelblat-Cerf Y, Vaadia E. Neuronal correlates of memory formation in motor cortex after adaptation to force field. J Neurosci. 2010;30:9189–9198. doi: 10.1523/JNEUROSCI.1603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapin JK, Moxon KA, Markowitz RS, Nicolelis MA. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999;2:664–670. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- 10.Birbaumer N, et al. A spelling device for the paralysed. Nature. 1999;398:297–298. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- 11.Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 13.Carmena JM, et al. Learning to control a brain-machine interface for reaching and grasping by primates. Plos Biol. 2003;1:193–208. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musallam S, Corneil BD, Greger B, Scherberger H, Andersen RA. Cognitive control signals for neural prosthetics. Science. 2004;305:258–262. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- 15.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. P Natl Acad Sci USA. 2004;101:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, Moran DW. A brain-computer interface using electrocorticographic signals in humans. J Neural Eng. 2004;1:63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 17.Santhanam G, Ryu SI, Yu BM, Afshar A, Shenoy KV. A high-performance brain-computer interface. Nature. 2006;442:195–198. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg LR, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 19.Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 20.Galan F, et al. A brain-actuated wheelchair: asynchronous and non-invasive Brain-computer interfaces for continuous control of robots. Clin Neurophysiol. 2008;119:2159–2169. doi: 10.1016/j.clinph.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–642. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarosiewicz B, et al. Functional network reorganization during learning in a brain-computer interface paradigm. P Natl Acad Sci USA. 2008;105:19486–19491. doi: 10.1073/pnas.0808113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. Plos Biol. 2009;7:e1000153. doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fetz EE. Volitional control of neural activity: implications for brain-computer interfaces. J Physiol. 2007;579:571–579. doi: 10.1113/jphysiol.2006.127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphrey DR, Schmidt EM, Thompson WD. Predicting measures of motor performance from multiple cortical spike trains. Science. 1970;170:758–762. doi: 10.1126/science.170.3959.758. [DOI] [PubMed] [Google Scholar]
- 26.Ganguly K, et al. Cortical representation of ipsilateral arm movements in monkey and man. J Neurosci. 2009;29:12948–12956. doi: 10.1523/JNEUROSCI.2471-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganguly K, Carmena JM. Neural correlates of skill acquisition with a cortical brain-machine interface. J Mot Behav. 2010;42:355–360. doi: 10.1080/00222895.2010.526457. [DOI] [PubMed] [Google Scholar]
- 28.Chestek CA, et al. Single-neuron stability during repeated reaching in macaque premotor cortex. J Neurosci. 2007;27:10742–10750. doi: 10.1523/JNEUROSCI.0959-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolelis MA, et al. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci U S A. 2003;100:11041–11046. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossman SE, Fontanini A, Wieskopf JS, Katz DB. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci. 2008;28:2864–2873. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberg PA, Wilson FA. Functional stability of dorsolateral prefrontal neurons. J Neurophysiol. 2004;92:1042–1055. doi: 10.1152/jn.00062.2004. [DOI] [PubMed] [Google Scholar]
- 32.Caminiti R, Johnson PB, Urbano A. Making arm movements within different parts of space: dynamic aspects in the primate motor cortex. J Neurosci. 1990;10:2039–2058. doi: 10.1523/JNEUROSCI.10-07-02039.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ajemian R, et al. Assessing the function of motor cortex: single-neuron models of how neural response is modulated by limb biomechanics. Neuron. 2008;58:414–428. doi: 10.1016/j.neuron.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Lebedev MA, et al. Cortical ensemble adaptation to represent velocity of an artificial actuator controlled by a brain-machine interface. J Neurosci. 2005;25:4681–4693. doi: 10.1523/JNEUROSCI.4088-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmena JM, Lebedev MA, Henriquez CS, Nicolelis MA. Stable ensemble performance with single-neuron variability during reaching movements in primates. J Neurosci. 2005;25:10712–10716. doi: 10.1523/JNEUROSCI.2772-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. J Neurophysiol. 1997;77:826–852. doi: 10.1152/jn.1997.77.2.826. [DOI] [PubMed] [Google Scholar]
- 37.Nicolelis MA, Lebedev MA. Principles of neural ensemble physiology underlying the operation of brain-machine interfaces. Nat Rev Neurosci. 2009;10:530–540. doi: 10.1038/nrn2653. [DOI] [PubMed] [Google Scholar]
- 38.Fetz EE. Operant conditioning of cortical unit activity. Science. 1969;163:955–958. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- 39.Green AM, Kalaska JF. Learning to move machines with the mind. Trends Neurosci. 2011;34:61–75. doi: 10.1016/j.tins.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Lebedev MA, et al. Cortical ensemble adaptation to represent velocity of an artificial actuator controlled by a brain-machine interface. Journal of Neuroscience. 2005;25:4681–4693. doi: 10.1523/JNEUROSCI.4088-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fetz EE, Baker MA. Operantly conditioned patterns on precentral unit activity and correlated responses in adjacent cells and contralateral muscles. J Neurophysiol. 1973;36:179–204. doi: 10.1152/jn.1973.36.2.179. [DOI] [PubMed] [Google Scholar]
- 42.Legenstein R, Pecevski D, Maass W. A learning theory for reward-modulated spike-timing-dependent plasticity with application to biofeedback. PLoS Comput Biol. 2008;4:e1000180. doi: 10.1371/journal.pcbi.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson AG, Chan V, O'Dell R, Schieber MH. Rapid changes in throughput from single motor cortex neurons to muscle activity. Science. 2007;318:1934–1937. doi: 10.1126/science.1149774. [DOI] [PubMed] [Google Scholar]
- 44.Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 45.Briggman KL, Abarbanel HD, Kristan WB., Jr Optical imaging of neuronal populations during decision-making. Science. 2005;307:896–901. doi: 10.1126/science.1103736. [DOI] [PubMed] [Google Scholar]
- 46.Churchland MM, Yu BM, Sahani M, Shenoy KV. Techniques for extracting single-trial activity patterns from large-scale neural recordings. Curr Opin Neurobiol. 2007;17:609–618. doi: 10.1016/j.conb.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.