Abstract
The effect of 2- pyridine aldoxime methyl chloride (2-PAMCl) and atropine with or without curcumin was investigated in dichlorvos (2,2-dichlorovinyl dimethyl phosphate; DDVP) induced toxicity in rats. Rats were exposed to DDVP (2 mg/kg sub-cutaneously) once daily for the period of 21 days. Post DDVP exposure, rats were further treated with 2-PAMCl (50 mg/kg intramuscular, once daily) + atropine (10 mg/kg, i.m. once daily) with or without curcumin (200 mg/kg; oral; once daily) for further 21 days. We observed a significant increase in lipid peroxidation (LPO), reactive oxygen species (ROS), oxidized glutathione (GSSG), while there was a significant decrease in antioxidant enzymes, brain acetylcholinesterase (AChE) and 5-hydroxy tryptamine (5-HT) activity on DDVP exposure of rats. These alterations were restored significantly by co-administration of 2-PAMCl + atropine in DDVP exposed rats. Curcumin when co-supplemented with 2-PAMCl + atropine also significantly protected serum aspartate aminotransferase (AST) and restored brain AChE activity and 5-HT level in animals sub-chronically exposed to DDVP. Histopathological observations along with biochemical changes in rat blood and tissues revealed significant protection offered by 2-PAMCl + atropine against DDVP. The results indicate that DDVP-induced toxicity can be significantly protected by co-administration of 2-PAMCl + atropine individually, however, curcumin co-supplementation with 2-PAMCl + atropine provides more pronounced protection, concerning particularly neurological disorders.
Keywords: Dichlorvos toxicity, 2-PAM.Cl, curcumin, atropine, oxidative stress
Introduction
Organophosphorous compounds (OP) have been used as pesticides (chlorpyrifos, paraoxon, DDVP, etc.) and as chemical warfare nerve agents (sarin, tabun, agent VX, etc.). The irreversible binding to and subsequent inactivation of acetylcholinesterase, an enzyme that normally catalyzes hydrolysis of acetylcholine (ACh) at neuromuscular junctions and other cholinergic synapses, is generally believed to be the major mechanism of their toxicity (Shenouda et al., 2009).The subsequent accumulation of ACh in the cholinergic clefts causes overstimulation of the peripheral as well as the central cholinergic nervous system resulting in clinical manifestations in the form of acute cholinergic crisis (Taylor, 2001; Savolainen, 2001). Dichlorvos has been widely and effectively used throughout the world in agriculture for controlling insects that damage crops. For the treatment of nerve agent/OP poisoning several antidotes have been evaluated and the currently recommended drugs are atropine and pralidoxime chloride. Pralidoxime chloride is an oxime, which reactivates the inhibited AChE and restore function at the neuromuscular junction (Taylor, 2001). Pralidoxime has therapeutic efficacy against acute toxicity of dichlorvos (DDVP) (Zhu et al., 2005). The clinical experience with the use of PAM-2 iodide given with atropine and diazepam was extremely favorable in the treatment of the victims of the Tokyo Sarin attack in 1995 (Stojiljkovic and Jokanovic, 2005). However, PAM-2 should not be recommended as the drug of choice in poisoning with warfare nerve agents due to its lack of efficacy against Tabun and Soman (Kassa, 2005). Certain oximes when used in combination with atropine were found to be more effective than atropine alone in organophosphate toxicity (Weissman and Raveh, 2008). Atropine is usually prepared following extraction from the plants Atropa belladonna (deadly nightshade), Datura stramonium (Jimson weed) or Duboisia myoporoides (McEvoy 2002). Atropine blocks the effect of excess acetylcholine at muscarinic receptors in the brain and peripheral tissues due to inhibition of the enzyme acetylcholinesterase (AChE).
Besides the aforesaid manifestations of its toxicity, OP induced oxidative stress further contributes to tissue damage. However, reports on anti-oxidative effects of 2-PAMCl and atropine in OP induced toxicity are scanty. In recent years, accumulating evidence has supported the protective effects of phenolic antioxidants from medicinal plants against oxidative stress mediated disorders (Soobratee et al. 2005). Several studies have indicated a beneficial role of curcumin in terms of its antioxidant, anti-tumorigenic, and anti-inflammatory action (Bengmark, 2006). It is also known to scavenge free radicals. The beneficial effects of Curcuma longa have been postulated to be due to the phenolic yellow pigments of turmeric, curcuminoids, along with the major compound curcumin (Miquel et al. 2002).
Despite the above-mentioned beneficial effects of curcumin, its efficacy in OP toxicity particularly in dichlorvos induced toxicity, has not yet been studied. The present study was designed to evaluate (i) DDVP-induced oxidative stress in blood and soft tissues in rat, (ii) beneficial effects of curcumin in combination with 2-PAMCl and atropine in preventing DDVP-induced oxidative stress, and (iii) histopathological alterations in liver and brain of rats.
Materials and methods
Experiments were performed on male Wistar rats weighing 120±10 g. The animals were obtained from the animal facility of Defence Research and Development Establishment. All animals received humane care in compliance with the guidelines of the “Institutional Animal Ethics Committee (IAEC)”. The Animal Ethics Committee of DRDE, Gwalior, India, approved the protocols for the experiments. The animals were housed in polypropylene cages on dust-free and autoclaved paddy husk and provided with standard pellet diet (Ashirwad Feeds, India) and water ad libitum. The animals were maintained under standard conditions of temperature and humidity with alternating 12 h light/dark cycles.
Forty male Wistar rats were equally divided into four groups of 10 animals each. For the first 3 weeks, DDVP was administered subcutaneously once daily and post-treatment with 2-PAMCl and atropine alone and in combination with curcumin was carried out for further 3 weeks. Details of groups and different treatments are given below.
Group I: Normal Control (Drinking water)
Group II: DDVP (2 mg/kg body weight subcutaneously) once daily for three weeks
Group III: DDVP (2 mg/kg body weight subcutaneously) once daily for three weeks and intramuscular administration of 2-PAMCl and atropine (50 and 10 mg/kg body weight, respectively) once daily for further three weeks (4 to 6 weeks)
Group IV: DDVP (2 mg/kg body weight subcutaneously) once daily for three weeks and 2-PAMCl + atropine (as in group III) and oral administration of Curcumin (200 mg/kg body weight dissolved in groundnut oil) once daily for three weeks (4 to 6 weeks).
Rats were weighed weekly throughout the experimental period. After administration of the last dose, the animals were given 24 hours of rest and were then sacrificed under light ether anesthesia. Blood was collected in heparinized tubes. For the collection of serum, blood was collected in non-heparinized tubes. Liver and brain were dissected out, rinsed in cold saline, blotted, weighed and used for various biochemical variables. Liver and brain from each rat were processed immediately for biochemical estimation as well as for histopathology. Five animals from each group were used for biochemical and histopathological observations.
Analysis of blood GSH concentration was performed with the method described by Ellman et al. (1959) and modified by Jollow et al. (1974). The generation of ROS was performed as described by Socci et al. (1999). TBARS was measured by the method of Ohkawa et al. (1979). Alanine amino transferase (ALT) and aspartate amino transferase (AST) activities in serum were assayed according to the method of Reitman and Frankel. (1957). Liver GSH and GSSG levels were measured as described by Hissin and Hilf. (1976). Liver SOD activity was assayed by the method of Kakkar et al. (1984). Catalase activity was assayed following the procedure of Aebi (1984). The frozen brain tissue samples were weighed and homogenized in acidified butanol. 5-hydroxytryptamine (5-HT) was estimated according to the procedure of Jacobwitz and Richardson (1978). A 10% brain homogenate (w/v) was prepared in 0.25 M sucrose. Activity of acetylcholinesterase (AChE) in brain was determined according to the method of Ellman et al. (1961) using acetylthiocholine as substrate. The activity of AChE was measured at 412 nm and its unit is expressed as nmol/min/mg protein.
Liver and brain tissues fixed in Bouin's fluid were dehydrated through a graded series of alcohol clearing in toluene using automatic tissue processor (Leica TP 1020) and embedded in paraffin. Liver tissues were sectioned at 5 μm thickness and brain sections were taken at 12 μm, which was followed by hematoxylin and eosin staining (H&E stain), and observed under light microscope (Leica, DMLB).
All data were expressed as means±SEM. Data were analyzed statistically using analysis of variance followed by Bonferroni test. Statistical significance was set at p<0.05. Data analysis was performed with GraphPad InStat (GraphPad Software Inc., 5755 Oberlin Drive, #110, San Diego, CA 92121, USA).
Results
Effect of 2-PAMCl and atropine with or without curcumin on hematological variables in DDVP exposed rats
Table 1 shows the effect of co-administration of curcumin, 2-PAMCl and atropine after DDVP exposure of rats. There were no significant changes in any of the hematological variables in rats from the treated groups.
Table 1.
Effect of co-administration of 2-PAMCl, atropine and curcumin on hematological variables in DDVP intoxicated rats.
| Parameters | Control | DDVP | DDVP + 2-PAMCl + Atropine | DDVP + 2-PAMCl Atropine + Curcumin + |
|---|---|---|---|---|
| WBC | 22.7±1.28 | 27.3±2.52 | 26.6±3.23 | 21.9±3.27 |
| RBC | 8.3±0.10 | 8.4±0.10 | 8.3±0.10 | 16.5±1.05 |
| HGB | 15.2±0.13 | 14.8±0.33 | 14.7±0.21 | 14.1±0.21 |
| HCT | 45.6±0.26 | 45.4±0.57 | 45.3±1.11 | 44.1±0.64 |
| MCV | 54.6±0.85 | 54.4±1.23 | 55.0±0.97 | 53.8±0.49 |
| MCH | 18.2±0.27 | 17.6±0.59 | 17.6±0.31 | 17.2±0.18 |
| MCHC | 33.3±0.25 | 32.5±0.68 | 32.1±0.37 | 32.0±0.20 |
| PLT | 736±78.2 | 703±39.78 | 784±53.49 | 710±24.49 |
Abbreviation used and units: WBC-white blood cells (×103 μl); RBC-red blood cells (×106 μl); Hemoglobin (g/dl); hematocrit (%); MCV-mean cell volume (fl); MCH-mean cell hemoglobin (pg); MCHC-mean cell hemoglobin concentration (g/dl); PLT – platelets (×103μl). Values are mean ±S.E; n=5.
Effect of 2-PAMCl and atropine with or without curcumin on serum biochemical variables in DDVP exposed rats
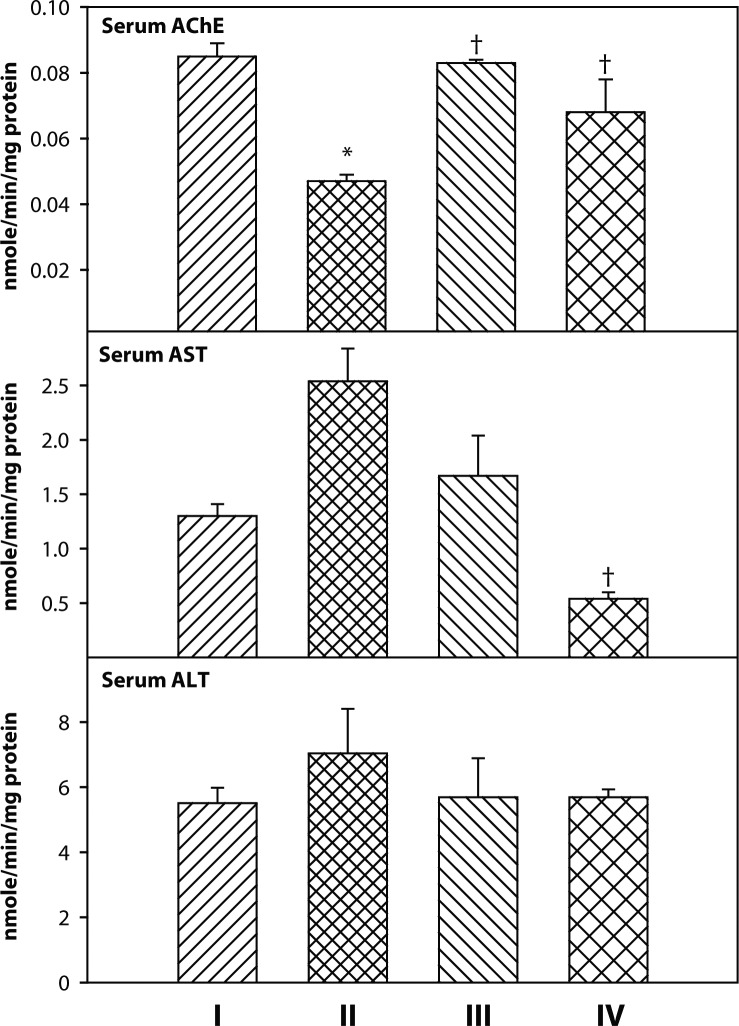
Exposure to dichlorvos alone significantly reduced serum AChE level compared to control rats (Figure 1). There was a significant increase in serum AChE activity following co-administration of 2-PAMCl + atropine or 2-PAMCl + atropine + curcumin with DDVP compared to the DDVP alone group. Serum AST activity recovered significantly in rats treated with 2-PAMCl + atropine + curcumin post DDVP exposure. There was no change in serum ALT level in any of the experimental groups.
Figure 1.
Effect of co-administration of 2-PAMCl, atropine and curcumin on serum biochemical variables in DDVP intoxicated rats. I: Control, II: DDVP, III: DDVP + 2-PAMCl and atropine, IV: DDVP + 2-PAMCl and atropine + curcumin. Abbreviations used: AChE-Acetylcholinesterase, AST-Aspartate transaminase; ALT-Alanine transaminase. Values are mean±S.E.; n=5. *Significantly (p<0.05) different as compared to control rats. †Significantly (p<0.05) different as compared to DDVP exposed rats.
Effect of 2-PAMCl and atropine with or without curcumin on blood ROS and GSH level in DDVP exposed rats
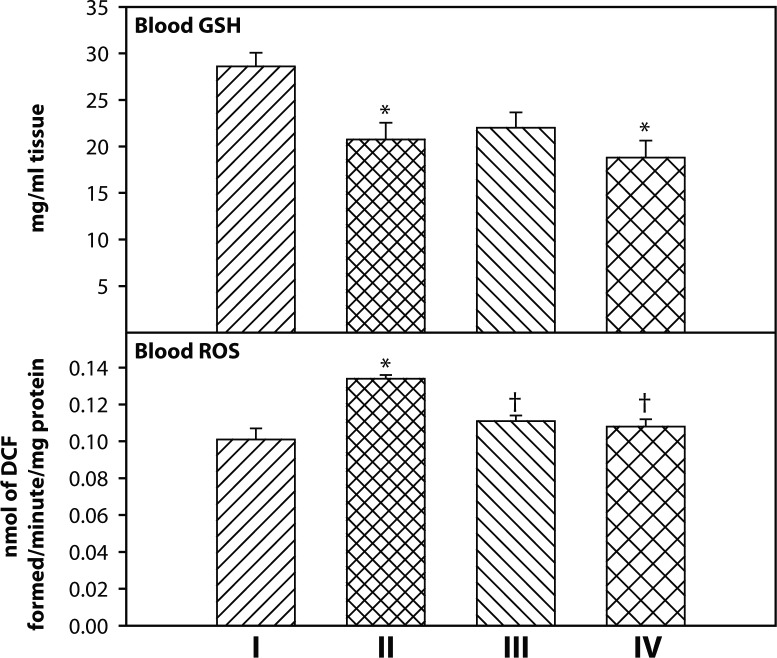
Blood ROS and GSH levels in different groups are presented in Figure 2. Blood ROS level was significantly (p<0.05) higher in the DDVP group compared to the control group. However, blood ROS level decreased significantly (p<0.05) after treatment with 2-PAMCl + atropine and 2-PAMCl + atropine + curcumin. There was a significant (p<0.05) decrease in blood GSH level in DDVP alone and DDVP along with 2-PAMCl + atropine + curcumin treatment groups compared to the control group.
Figure 2.
Effect of co-administration of 2-PAMCl, atropine and curcumin on blood ROS and GSH level in DDVP exposed rats. I: Control, II: DDVP, III: DDVP + 2-PAMCl and atropine, IV: DDVP + 2-PAMCl and atropine + curcumin. Abbreviations used: ROS-reactive oxygen species; GSH-reduced glutathione. Values are mean±SE. n=5. *Significantly (p<0.05) different as compared to control rats. †Significantly (p<0.05) different as compared to DDVP exposed rats.
Effect of 2-PAMCl and atropine with or without curcumin on liver biochemical variables in DDVP exposed rats
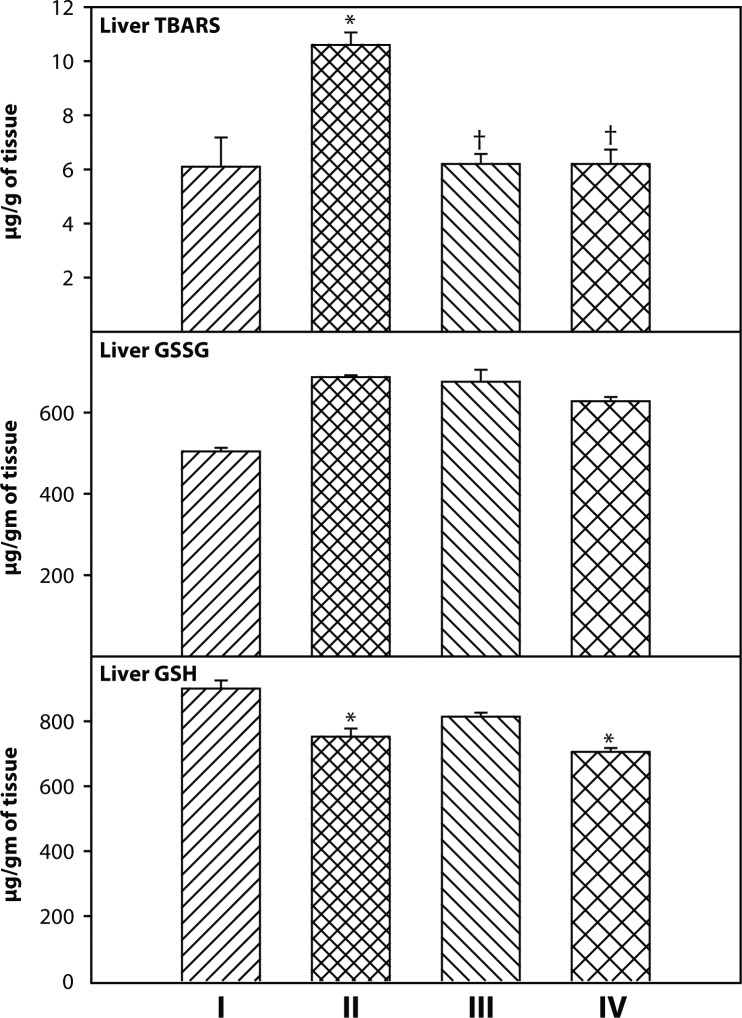
The level of TBARS was significantly elevated in DDVP exposed groups as compared to the control group. However, treatment with 2-PAMCl + atropine alone and in combination with curcumin restored TBARS level towards normal as shown in Figure 3. Liver GSH level was significantly depleted in groups exposed to DDVP and supplementation of curcumin with 2-PAMCl + atropine recovered the GSH level. There were no changes in liver GSSG levels in any of the groups studied (Figure 3).
Figure 3.
Effect of co-administration of 2-PAMCl, atropine and curcumin on liver GSH, GSSG and TBARS levels in DDVP intoxicated rats. I: Control, II: DDVP, III: DDVP + 2-PAMCl and atropine, IV: DDVP + 2-PAMCl and atropine + curcumin. Abbreviations used: GSH-reduced glutathione; GSSG-oxidized glutathione; TBARS-thiobarbituric acid reactive substances. Values are mean±SE; n=5. *Significantly (p<0.05) different as compared to control rats. †Significantly (p<0.05) different as compared to DDVP exposed rats.
Effect of 2-PAMCl and atropine with or without curcumin on liver SOD and catalase levels in DDVP exposed rats
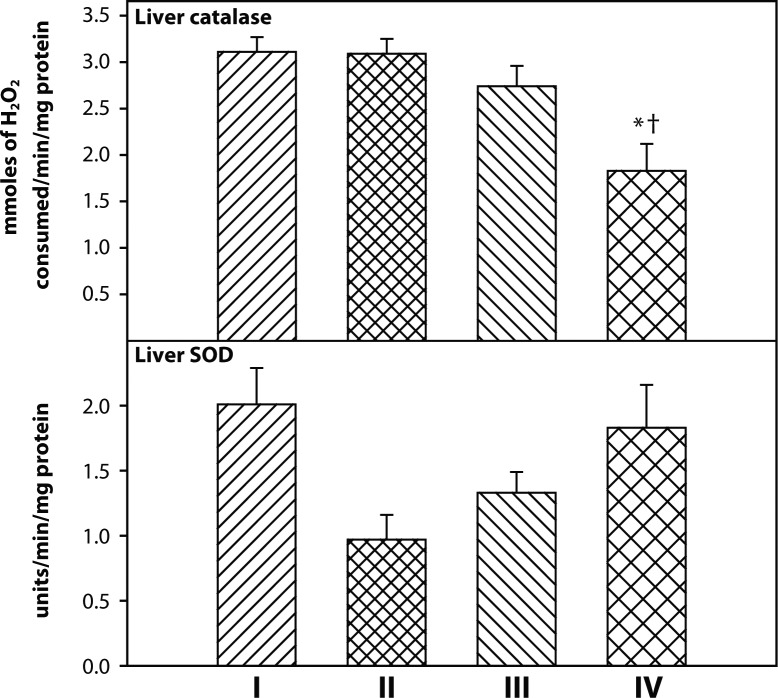
Liver catalase activity in rats exposed to DDVP and treated with 2-PAMCl + atropine + curcumin was significantly lower as compared to control and DDVP alone exposed groups (Figure 4). There were no significant changes in liver SOD activity.
Figure 4.
Effect of co-administration of 2-PAMCl, atropine and curcumin on liver SOD and catalase levels in DDVP intoxicated rats. I: Control, II: DDVP, III: DDVP + 2-PAMCl and atropine, IV: DDVP + 2-PAMCl and atropine + Curcumin. Abbreviation used: SOD-superoxide dismutase. Values are mean±SE; n=5. *Significantly (p<0.05) different as compared to control rats. †Significantly (p<0.05) different as compared to DDVP exposed rats.
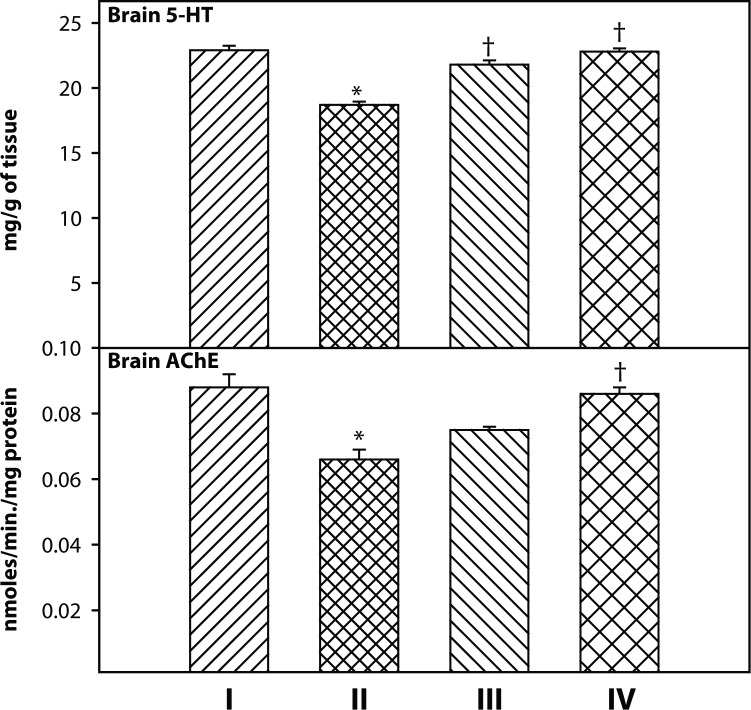
Effect of 2-PAMCl and atropine with or without curcumin on brain AChE activity and 5-HT level in DDVP exposed rats
A significant decrease in brain AChE and 5-HT level was observed in DDVP exposed rats compared to the control group (Figure 5). However when compared with DDVP exposed rats, there was a significant recovery in brain AChE activity in the group treated with 2-PAMCl + atropine + curcumin. 5-HT levels decreased marginally in the DDVP alone group and recovered significantly when treated with 2-PAMCl + atropine alone and in combination with curcumin after DDVP exposure.
Figure 5.
Effect of co-administration of 2-PAMCl, atropine and curcumin on brain biochemical variables in DDVP intoxicated rats. I: Control, II: DDVP, III: DDVP + 2-PAMCl and atropine, IV: DDVP + 2-PAMCl and atropine + curcumin. Abbreviations used: AChE-acetylcholinesterase; 5-HT-5-hydroxytryptamine. Values are mean±SE; n=5. *Significantly (p<0.05) different as compared to control rats. † Significantly (p<0.05) different as compared to DDVP exposed rats.
Effect of 2-PAMCl and atropine with or without curcumin on histopatholocurcumin on histopathological changes in liver and brain of rats intoxicated with DDVP
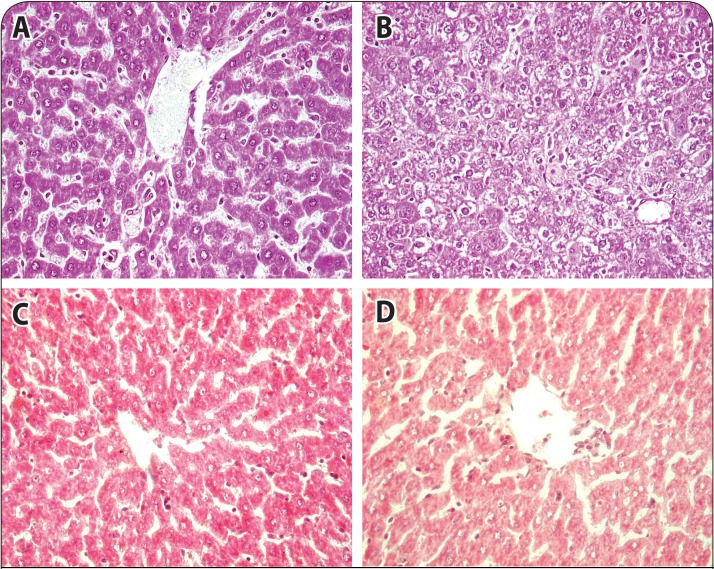
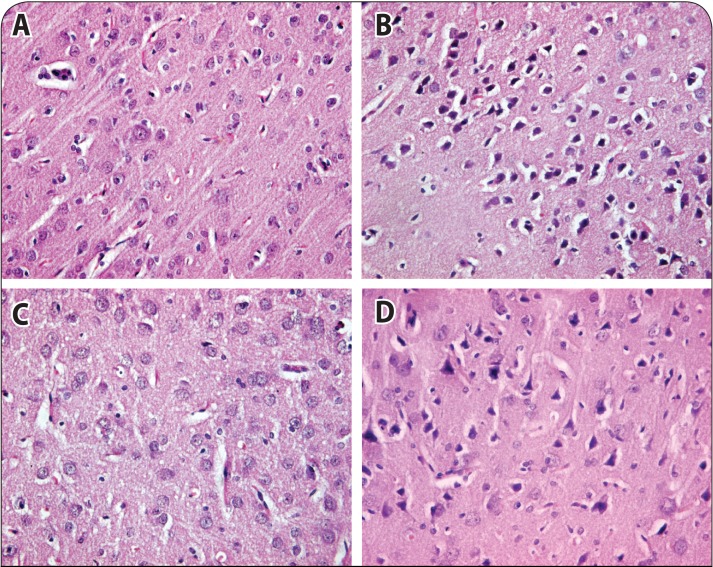
The histopathological findings in rat liver following DDVP exposure are given in Table 2 and shown in Figure 6. The liver of control rats showed normal lobular architecture with hepatocytes arranged in cords encircling the central canal. Liver sections of DDVP exposed rats showed cytoplasmic clumping, hyperactivation of Kupffer cells with foamy cytoplasm, hepatocellular vacuolation, necrosis of hepatocytes, basophilia in nuclei representing karyorrhexis and hepatocytes showing karyolysis along with ballooning. Rats treated with 2-PAMCl + atropine post DDVP exposure showed a few hyperactive Kupffer cells and necrotic cells in the hepatic parenchyma. However, mild cytoplasmic clumping, hyperactive Kupffer cells, hepatocyte vacuolation, necrosis, and inflammatory foci were observed in sections of the liver of rats treated with 2-PAMCl + atropine + curcumin after DDVP exposure. Neuronal integrity was assessed in the cortex (Table 3; Figure 7) and hippocampus of the brain in control and treated rats. The cortex region of control rat showed various types of normal neuroglial cells arranged in several layers. The brain of rats treated with DDVP showed moderate to severe neuronal degeneration and proliferation of glial cells along with moderate chromatolysis and inflammation of glial cells. Rats exposed to DDVP and treated with 2-PAMCl + atropine showed minimal to mild lesions of neuronal tissue. However mild gliosis and chromatolysis along with minimal lesions of neuronal degeneration were observed in brain section of rats treated with 2-PAMCl + atropine + curcumin after DDVP exposure. The hippocampal region of control rats showed normal cellular composition in all three layers (Figure 2). However subcutaneous DDVP exposure of rats resulted in severe chromatolysis and necrosis of Purkinje fibers of the hippocampal region. The severity of lesions in DDVP exposed rats treated with 2-PAMCl + atropine was minimized compared to DDVP exposed rats, whereas in 2-PAMCl + atropine + curcumin treated animals mild neuronal lesions were seen (Table 3).
Table 2.
Effect of co-administration of 2-PAMCl, atropine and curcumin on liver histopathological changes in rats intoxicated with DDVP.
| Lesions | Control | DDVP | DDVP + 2PAMCl + atropine | DDVP + 2PAMCl + atropine + curcumin |
|---|---|---|---|---|
| Clumping of cytoplasm | + | + + + + | − | + + |
| Hyperactivation of Kupffer cells | − | + + | + + | + + |
| Hepatocyte vacuolation | − | + + + + | − | + + |
| Necrosis/Apoptosis | + /− | + + + | + | + + |
| Infiltration of inflammatory cells | − | + + + + | − | + + |
| Karyorhexis/Karyolysis | − | + + + | − | + |
| Ballooning of hepatocytes | + /− | + + | − | + + |
N=6. – Nil; + , Minimal (<12%); + + , Mild (<22%); + + + , Moderate (<45%) and + + + + , Severe (>45%).
Figure 6.
Photomicrographs of liver sections of control, DDVP exposed and treated rats, H&E 40×. A: Control liver showing normal lobular architecture with hepatocytes arranged in cords encircling the central canal. B: DDVP exposed rat liver showing cytoplasmic clumping, hyperactivation of Kupffer cells, vacuolation, necrosis and ballooning of hepatocytes, C and D: 2-PAMCl and atropine and 2-PAMCl and atropine + curcumin (200 mg/kg) treatment after DDVP exposure showing few mild hyperactive Kupffer cells and necrotic cells in hepatic parenchyma.
Figure 7.
Photomicrographs of brain histopathology of control, DDVP exposed and treated rats, H&E 40×. A: Control rat brain showing normal neuroglial cells arranged in several layers. B: DDVP exposed rat brain showing chromatolysis of nuclear material, gliosis and pyknotic neurons in cortex. C: Rat brain with DDVP + 2-PAMCl and atropine showing mild lesions of neuronal damage with moderate gliosis. D: 2-PAMCl + atropine + curcumin (200 mg/kg) treatment after DDVP exposure showing mild gliosis and chromatolysis along with minimal lesions of neuronal degeneration.
Discussion
The widespread use of pesticides produces a number of serious health effects affecting both humans and animals. The results of the present study suggests a beneficial role of curcumin with or without 2-PAMCl + atropine in DDVP exposed rats by providing effective protection to a number of biochemical variables and parameters indicative of oxidative stress, particularly in rat blood and tissues. Liver and brain are the major target organs of DDVP exposure as its metabolism occurs in the liver and the brain is particularly susceptible to oxidative damage due to the high utilization of inspired oxygen.
The liver plays an imperative role in the metabolism and biotransformation by which toxic compounds get converted into less harmful products (Hodgson, 2004).
Transaminases (ALT and AST) were used as important biomarkers for determining hepatotoxicity. ALT is a cytosolic enzyme, more specific for the liver. While AST is the mitochondrial enzyme, predominantly found in the liver, skeletal muscles and kidneys. The increase in their activity occurs mainly due to leakage of transaminases from liver cytosol into the blood stream, indicating liver damage or dysfunction. This increase may occur due to the formation of reactive oxygen species after dichlorvos exposure. Our results showed a pronounced increase in serum AST and ALT activity indicating hepatotoxicity. These results coincide with previous studies (Dwivedi & Flora, 2011; Dwivedi et al., 2010; Manal et al., 2008) that showed significant increases in the activity of transaminases in rats and humans exposed to organophosphate pesticides (OP). Treatment with 2-PAMCl and atropine had no effect on liver AST level, but co-administration of curcumin along with 2-PAMCl and atropine resulted in decreased liver AST activity, indicative of hepato-protecitve action of curcumin in DDVP toxicity (Ataman et al., 2010). Fu et al. (2008) reported hepatoprotective action of curcumin in various animal models of liver injury.
Acetylcholinesterase (AChE) inhibition is a well known mechanism of organophosphate pesticide toxicity. Organophosphates inhibit AChE by phosphorylating serine residues at its active site resulting in accumulation of acetylcholine (ACh) in the synapse that produces cholinergic effects. In the present study, AChE activity was significantly inhibited by dichlorvos treatment, indicating OP poisoning (Ojha et al., 2011). Celik and Isik (2009) also demonstrated that administration of sub-acute dichlorvos inhibited AChE and butylcholinesterase activities in various tissues of rats.
Biogenic amines are known to be highly targeted by organophosphates in the developing brain (Slotkin, 2004, 2005). The presented results revealed that 5-HT level decreased non-significantly in DDVP exposed rats. However, the level of brain 5-HT and AChE recovered marginally towards normal in both treated groups. The most pronounced beneficial effect was observed in rats treated with 2-PAMCl + atropine + curcumin suggesting their neuroprotective property.
Oxidative stress is reported to be one of the important mechanisms involved in OP toxicity (Llopis et al., 2003). Being lipophilic in nature, OP interact with cells through biomembranes rich in polyunsaturated fatty acids, thus oxidatively damaging them, known as lipid peroxidation (Mittal and Flora, 2006). We observed increased TBARS level in the present study predominantly in animals given DDVP (Dwivedi et al., 2010). The effect of curcumin on lipid peroxidation has been widely studied in various models. It was found to inhibit lipid peroxidation in rat liver microsomes, erythrocyte membrane and brain (Reddy and Lokesh, 1994).
Oxidative stress occurs usually with excessive generation of free radicals followed by a parallel depletion of antioxidant enzymes like GSH. Reduced GSH and its metabolizing enzymes provide the major defense against ROS induced cellular damage (Celik and Suzek, 2008).Organophosphate appears to disturb this key cellular pathway perhaps by disturbing mitochondrial metabolism, as suggested by Delgado et al. (2006). In the present study, we observed depleted levels of antioxidant enzymes and elevated levels of ROS and TBARS following DDVP exposure in rats. Curcumin is known to exhibit a strong antioxidant activity and is a potent scavenger of free radicals such as superoxide anion radicals, hydroxyl radicals and nitrogen dioxide radicals (Maheshwari et al., 2006; Anand et al., 2008). Curcumin treatment improved the levels of antioxidant enzymes, mainly GSH, SOD and catalase (Tirkey et al., 2005).
In the present study, histopathological findings supported the results of biochemical variables. Histology of the liver showed signs of hepatocellular degeneration, vacuolation, inflammation and pyknosis. Histology of the brain showed severe degenerative and necrotic changes, similarly as reported by Abdollahi et al. (2004). Both 2-PAMCl + atropine in combination with curcumin exhibited hepatoprotective and neuroprotective mechanisms
Thus the present study provides some interesting new observations for possible therapeutic combination upon exposure to DDVP. The study is one of the first few investigating the protective efficacy of curcumin with or without 2-PAMCl and atropine.
Acknowledgement
The authors thank Dr. R. Vijayaraghavan, Director for his support and encouragement. Financial assistance from the Defence Research and Development Organization supporting the project is gratefully acknowledged.
REFERENCES
- Abdollahi M, Rainba A, Shadnia S, Nikfar S, Rezaie A. Pesticide and oxidative stress: a review. Med Sci Monitor : R. 2004;10:A141–RA147. [PubMed] [Google Scholar]
- Aebi H. Catalase,in Methods in Enzymol, Vol. 105. In: Packer L, editor. Orlando, FL: Academic Press; 1984. pp. 125–126. [Google Scholar]
- Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harik-umar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Ataman Kose, Nurullah Gunay, Beril Kose, Ali Ocak R., Ozcan Erel, Abdullah Demiryurek T. Pestic. Biochem Physiol. 2010. Effects of atropine and pralidoxime pretreatment on serum and cardiac oxidative stress parameters in acute dichlorvos toxicity in rats; pp. 249–255. [Google Scholar]
- Bengmark S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr. 2006;30:45–51. doi: 10.1177/014860710603000145. [DOI] [PubMed] [Google Scholar]
- Celik I, Isik I. Neurotoxic effects of subacute exposure to dichlorvos and methyl parathion at sublethal dosages in rats. Pestic. Biochem Physiol. 2009;94:1–4. [Google Scholar]
- Celik I, Suzek H. Effects of subacute exposure to dichlorvos at sublethal dosages on erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Fd. Chem. Toxicol. 2008;46:2796–2801. [Google Scholar]
- Delgado Eduardo HB, Streck EL, Quevedo JL, Dal-Pizzol F. Mitochondrial respiratory dysfunction and oxidative stress after chronic malathion exposure. Neurochemistry Research. 2006;31:1021–1025. doi: 10.1007/s11064-006-9111-1. [DOI] [PubMed] [Google Scholar]
- Dwivedi N, Bhutia YD, Kumar V, Yadav P, Kushwaha P, Swarnkar H, Flora SJS. Effects of combined exposure to dichlorvos and monocrotophos on blood and brain biochemical variables in rats. Human Exp. Toxicol. 2010;29:121–129. doi: 10.1177/0960327109357212. [DOI] [PubMed] [Google Scholar]
- Dwivedi N, Flora SJS. Concomitant exposure to arsenic and organophosphates on tissue oxidative stress in rats. Fd Chem Toxicol. 2011;49:1152–1159. doi: 10.1016/j.fct.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulphydryl groups. Arch Biochem. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Feaderstone RM. A new and rapid colorimetric determination of acetyl cholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol. Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- Hissin PJ, Hilf R. A fluorometric method for the determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–216. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Hodgson E. A Textbook of Modern Toxicology. 3rd edn. New Jersey: John Wiley and Sons, Inc; 2004. pp. 203–211. [Google Scholar]
- Jacobwitz DW, Richardson JS. Method for rapid determination of norepinephrine, dopamine and serotonin in the same brain region. Pharmacol Biochem Behav. 1978;8:515–519. doi: 10.1016/0091-3057(78)90380-5. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Zamppaglione Z, Gillette JR. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophy. 1984;21:130–132. [PubMed] [Google Scholar]
- Kassa J. The role of oximes in the antidotal treatment of chemical casualities exposed to nerve agents. In: Monov A, Dishovsky C, editors. Medical Aspects of Chemical and Biological Terrorism; Chemical Terrorism and Traumatism. Sofia, Bulgaria: Publishing House of the Union of Scientists in Bulgaria; 2005. pp. 193–208. [Google Scholar]
- Llopis SP, Ferrando MD, Pena JB. Fish tolerance to organophosphate induced oxidative stress is dependent on the glutathione metabolism and enhanced by N-acetylcysteine. Aquat. Toxicol. 2003;65:337–360. doi: 10.1016/s0166-445x(03)00148-6. [DOI] [PubMed] [Google Scholar]
- Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Manal E.A., Elhalwagy Nevine S., Darwish Enass, Zaher M. Prophylactic effect of green tea polyphenols against liver and kidney injury induced by fenitrothion insecticide. Pest. Biochem. Physiol. 2008;91(2):81–89. [Google Scholar]
- McEvoy G. Anticholinergic agents. AHFS drug information 2002. Bethesda, MD: American Hospital Formulary Service, Bethesda; 2002. pp. 1222–1228. [Google Scholar]
- Miquel J, Bernd A, Sempere JM, Diaz- Alperi J, Ramirez A. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Arch Geronto Geriatr. 2002;34:37. doi: 10.1016/s0167-4943(01)00194-7. [DOI] [PubMed] [Google Scholar]
- Mittal M, Flora SJS. Effects of individual and combined exposure to sodium arsenite and sodium fluoride on tissue oxidative stress, arsenic and fluoride levels in male mice. Chem. Biol. Inter. 2006;162:128–139. doi: 10.1016/j.cbi.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ojha A, Yaduvanshi SK, Srivastava N. Effect of combined exposure of commonly used organophosphate pesticides on lipid peroxidation and antioxidant enzymes in rat tissues. Pesticide Biochem Physiol. 2011;99:148–156. [Google Scholar]
- Reddy AC, Lokesh BR. Effect of dietary turmeric. (Curcuma longa) on iron-induced lipid peroxidation in the rat liver. Fd Chem Toxicol. 1994;32:279–283. doi: 10.1016/0278-6915(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Savolainen K. Understanding the toxic action of organophosphates. In: Krieger RI, editor. Handbook of Pesticide Toxicology, vol. 2. USA: Academic Press; 2001. pp. 1013–1043. [Google Scholar]
- Shenouda J, et al. An evaluation of the inhibition of human butyrylcholinesterase and acetylcholinesterase by the organophosphate chlorpyrifos oxon. Toxicol Appl Pharmacol. 2009;241:135–142. doi: 10.1016/j.taap.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. San Diego: Elsevier Academic Press; 2005. pp. 293–314. [Google Scholar]
- Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol. 1999;155:109–17. doi: 10.1006/exnr.1998.6969. [DOI] [PubMed] [Google Scholar]
- Soobrattee MA, Neergheen VS, Luximon- Ramma A, Aruoma OI, Bahorun T. Phenolic as potential antioxidant theraputic agents: Mechanism and actions. Mut Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Stojiljkovic MP, Jokanovic M. AUM shinrikyo and terrorist use of nerve agents in Japan. In: Monov A, Dishovsky C, editors. Medical Aspects of Chemical and Biological Terrorism; Chemical Terrorism and Traumatism. Sofia, Bulgaria: Publishing House of the Union of Scientists in Bulgaria; 2005. pp. 101–115. [Google Scholar]
- Taylor P. Anticholinesterase agents. In: Hardman GJ, Limbird LE, Gilman AG, editors. The Pharmacological Basis of Therapeutics, 10th edition. New York: McGraw-Hill; 2001. pp. 110–129. [Google Scholar]
- Tirkey N, Kaur G, Vij G, Chopra K. Curcumin, a diferuloylmethane, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol. 2005;5:15. doi: 10.1186/1471-2210-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman BA, Raveh L. Therapy against organophosphate poisoning: The importance of anticholinergic drugs with antiglutamatergic properties. Toxicol Appl Pharmacol. 2008;232:351–358. doi: 10.1016/j.taap.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Zhu QH, Huang JX, Lin Z. Therapeutic efficacy of pralidoxime chloride on acute dichlorvos poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2005;23:91–93. [PubMed] [Google Scholar]