Abstract
Context
Purpose in life is associated with a substantially reduced risk of Alzheimer disease (AD), but the neurobiologic basis of this protective effect remains unknown.
Objective
To test the hypothesis that purpose in life reduces the deleterious effects of AD pathologic changes on cognition in advanced age.
Design
A longitudinal, epidemiologic, clinicopathologic study of aging was conducted that included detailed annual clinical evaluations and brain autopsy.
Participants
Two hundred forty-six community-based older persons from the Rush Memory and Aging Project participated.
Main Outcome Measures
Purpose in life was assessed via structured interview, and cognitive function was evaluated annually and proximate to death. On postmortem examination, 3 indexes of AD pathologic features were quantified: global AD pathologic changes, amyloid, and tangles. The associations of disease pathologic changes and purpose in life with cognition were examined using linear regression and mixed models.
Results
Purpose in life modified the association between the global measure of AD pathologic changes and cognition (mean [SE] parameter estimate, 0.532 [0.211]; P=.01), such that participants who reported higher levels of purpose in life exhibited better cognitive function despite the burden of the disease. Purpose in life also reduced the association of tangles with cognition (parameter estimate, 0.042 [0.019]; P=.03), and the protective effect of purpose in life persisted even after controlling for several potentially confounding variables. Furthermore, in analyses examining whether purpose in life modified the association between AD pathologic effects and the rate of cognitive decline, we found that higher levels of purpose in life reduced the effect of AD pathologic changes on cognitive decline (parameter estimate, 0.085 [0.039]; P=.03).
Conclusion
Higher levels of purpose in life reduce the deleterious effects of AD pathologic changes on cognition in advanced age.
Alzheimer disease (AD) is one of the most significant public health challenges of the 21st century. Despite intense research focus, disease-modifying agents remain elusive and AD is poised to overwhelm health care systems as its burden increases in the coming decades. Given the lack of effective therapies, the identification of factors that promote cognitive health in elderly persons is urgently needed to decrease the burden of AD.1 Although relatively few such factors have been identified, compelling evidence indicates that positive psychological and experiential factors are associated with maintenance of cognitive function.2–5 Furthermore, novel neuroimaging and clinicopathologic findings have shown that some of these factors confer protective benefit (ie, provide neural reserve) by reducing the deleterious effects of AD pathologic changes on cognition.1,6–10 Purpose in life, the psychological tendency to derive meaning from life’s experiences and possess a sense of intentionality and goal directedness, is a related psychological factor and component of well-being that has long been hypothesized to be associated with positive health outcomes.11–15 Indeed, studies (mostly cross-sectional) have shown that purpose in life is associated with cognitive and psychological health in elderly persons,15–21 but the neurobiologic basis of the beneficial effect of purpose in life remains unknown.
In recent years, systematic examination21–23 has shown that purpose in life is associated with a substantially reduced risk of incident AD, mild cognitive impairment, disability, and death. In this study, we sought to extend these findings by examining the neurobiologic basis of the protective effect of purpose in life on cognition. Thus, we tested the hypothesis that higher levels of purpose in life reduce the deleterious effects of AD pathologic changes on cognition in older persons. Participants were 246 persons from the Rush Memory and Aging Project, a longitudinal, epidemiologic, clinicopathologic study of aging.24 We first examined the association of purpose in life with a global measure of AD pathologic changes and then evaluated whether purpose in life modified the relationship of those changes with level of cognition proximate to death. Next, we examined the potential modifying effects of purpose in life on the relationship of molecularly specific measures of amyloid and tangles with cognition and several potential confounders of the protective effect of purpose in life. Finally, we conducted analyses using repeated measures of cognitive function collected over time to examine whether purpose in life reduced the deleterious effects of AD pathologic changes on the rate of cognitive decline.
METHODS
PARTICIPANTS
Participants were from the Rush Memory and Aging Project.24 All provided written informed consent and an anatomical gift act, and the study was approved by the institutional review board of Rush University Medical Center. To date, more than 1400 persons have completed the baseline assessment. The overall annual follow-up rate of survivors exceeds 90% and the autopsy rate exceeds 80%. At the time of these analyses, complete postmortem data were available from 246 persons without dementia at baseline for whom valid measurements of purpose in life and cognition were available.
CLINICAL ASSESSMENT AND DIAGNOSIS
Participants undergo detailed annual clinical evaluations that include medical history, complete neurologic examination, and cognitive testing.24 Follow-up evaluations, identical to the baseline evaluation in all essential details, are conducted annually by examiners blinded to previous data. The diagnosis of dementia and probable AD follow the recommendations of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association, which require evidence of cognitive decline in memory and at least one other cognitive domain.25 Prior research26 showed that approximately 90% of participants in the Rush Memory and Aging Project who met clinical criteria for AD had the diagnosis confirmed at autopsy.
ASSESSMENT OF COGNITION
Cognitive function is assessed annually via 21 tests, as previously described.24,27,28 This battery includes the Mini-Mental State Examination, but the scores from this test are used only to describe the cohort.29 A composite measure of global cognitive function is derived from the scores on 19 cognitive tests; these include immediate and delayed recall of story A from Logical Memory, immediate and delayed recall of the East Boston Story, Word List Memory, Word List Recall, Word List Recognition, a 15-item version of the Boston Naming Test, Verbal Fluency, a 15-item reading test, Digit Span Forward, Digit Span Backward, Digit Ordering, Symbol Digit Modalities Test, Number Comparison, a modified version of the Stroop Neuropsychological Screening Test, a 15-item version of Judgment of Line Orientation, and a 16-item version of Standard Progressive Matrices.
To compute the composite measure of global cognitive function, raw scores on each of the tests are converted to z scores using the baseline mean and standard deviation of the entire cohort, and the z scores of all 19 tests are averaged. Psychometric information has been reported.28
ASSESSMENT OF PURPOSE IN LIFE
Purpose in life refers to the tendency to derive meaning from life’s experiences and possess a sense of intentionality and goal directedness that guides behavior. Purpose in life was assessed at baseline using a 10-item scale derived from Ryff’s Scales of Psychological Well-Being, as previously described.22,30 For each of the 10 items, participants rated their level of agreement using a 5-point scale; scores on this measure are averaged to yield a total score, with higher scores indicating higher levels of purpose in life. The Cronbach coefficient α on the scale used in this study indicated a moderate level of internal consistency.31
OTHER COVARIATES
Other variables used in the analyses included age at death, sex, and educational level. Depressive symptoms were assessed with a 10-item Center for Epidemiologic Studies–Depression scale.24,32 Neuroticism, the tendency to experience psychological distress, was measured using the Neuroticism subscale of the NEO Five-Factor Inventory.33,34 Social network size was quantified with standard questions regarding the number of children, family, and friends the participants had and how often they interacted.10 Self-reported history of diabetes mellitus, heart disease, hypertension, thyroid disease, cancer, head injury, and stroke24 was summarized as an index of chronic illness. Physical activity was assessed using questions adapted from the 1985 National Health Interview Survey24 and a composite measure of participation in physical activity expressed as hours per week, as previously described.24 All participants undergo genotyping, and those with 1 or more copies of the apolipoprotein ε4 allele were considered positive for ε4 for these analyses.
QUANTIFICATION OF AD PATHOLOGIC CHANGES
To quantify global AD pathologic characteristics, tissue blocks from the midfrontal gyrus, the middle or superior temporal gyrus, the inferior parietal gyrus, the entorhinal cortex proper, and the hippocampus (CA1/subiculum) were embedded in paraffin, sliced at 6 μm, and stained with a modified Bielschowsky silver stain. Neuritic plaques, diffuse plaques, and neurofibrillary tangles were counted. A composite measure of global AD pathologic features was created on the basis of silver-stained neuritic plaque, diffuse plaque, and neurofibrillary tangle counts.24,35
For quantification of molecularly specific measures of amyloid and tau tangles, multiple tissue blocks from the entorhinal cortex proper, hippocampus (CA1/subiculum), superior frontal cortex, dorsolateral prefrontal cortex, inferior temporal cortex, angular gyrus cortex, anterior cingulate cortex, and calcarine cortex were embedded in paraffin and cut into 20-μm slices.35,36 Amyloid-β was labeled with an N-terminus–directed monoclonal antibody (10D5, 1:1000; provided by Elan Pharmaceuticals), and immunohistochemical analysis was performed. Quantification of the amyloid-β load was performed via an automated, multistage computational image analysis protocol that uses ImageJ 1.42g (http://rsbweb.nih.gov/ij/). The mean fraction area occupied by amyloid was computed for each region/subject; values for all regions then were averaged to yield a composite measure of amyloid deposition. The paired helical filament tau was labeled with an antibody specific for phosphorylated tau, AT8 (1:1000; Innogenetics), and the density of tangles (per square millimeter) was averaged within and subsequently across regions. The use of these composites is supported by Cronbach coefficient α, factor analysis, and intercorrelations.36,37
STATISTICAL ANALYSIS
Regression analyses were used to first examine the extent to which purpose in life was related to each of the 3 measures of AD pathologic changes. We then constructed a linear regression model that examined the level of cognition proximate to death as a function of the global measure of AD pathologic changes and purpose in life. To test our primary hypothesis, we constructed a second model that included terms for the main effects for disease pathologic changes and purpose in life as well as their interaction. The interaction term directly tests the hypothesis that purpose in life reduces the effect of a unit of damage on the level of cognitive function. We repeated these models separately for amyloid and tangles to determine whether the effects were present for one type of AD pathology but not the other. Next, we examined the robustness of the findings after controlling for several potential confounders. Finally, using all available longitudinal cognitive data collected during the course of the study, we applied linear mixed models to examine whether purpose in life modified the association between AD pathologic changes and the rate of cognitive decline. The rate of cognitive decline was estimated from repeated annual cognitive assessments, and the 3-way interaction between purpose in life, pathologic changes, and the slope of cognitive decline represents the effect of a 1-unit increase in purpose in life on the association between AD pathologic changes and the rate of cognitive decline. All models were adjusted for age, sex, and educational level and were validated graphically and analytically. Analyses were done with commercial software (SAS/STAT, version 9.2; SAS Institute, Inc).38
RESULTS
The mean age of the participants in this study was 88.2 years (median,89.0 years) at the time of death, the mean score on the purpose in life measure was 3.5 (median, 3.5), and the mean Mini-Mental State Examination score at baseline was 27.5 (median, 28.0); additional descriptive data are provided in Table 1. The mean (SD) interval between the assessment of purpose in life and the cognitive assessment proximate to death was 3.3 years (1.95; range, 0–8 years), the mean interval from assessment of purpose in life to death was 3.9 years (1.99; range, 0–8 years), and the mean interval from the last cognitive assessment to death was 0.6 year (0.40; range,0–2 years).
Table 1.
Descriptive Characteristics of the Cohort
| Variablea | Mean (SD) [Range] |
|---|---|
| Age at death, y | 88.2 (6.0) [67.4 to 101.5] |
| Educational level, y | 14.5 (2.7) [5 to 22] |
| Female sex, No. (%) | 161 (65.5) |
| White, non-Hispanic race/ethnicity, No. (%) | 234 (95.1) |
| Purpose in life score | 3.5 (0.46) [2.4 to 5.0] |
| Depressive symptoms, No. | 1.6 (1.9) [0 to 7] |
| Global cognitive function | −0.05 (0.54) [−3.0 to 1.2] |
| Neuroticism | 15.3 (7.1) [0 to 40] |
| Social network size, No. | 6.0 (4.8) [0 to 29] |
| Chronic medical conditions, No. | 1.3 (1.1) [0 to 4] |
| Physical activity | 2.5 (3.1) [0 to 25] |
| Presence of the apolipoprotein ε4 allele, No. (%) | 54 (22.0) |
Measures of global cognitive function, neuroticism, and physical activity are explained in the “Assessment of Cognition” and “Other Covariates” subsections of the “Methods” section.
RELATIONSHIP OF PURPOSE IN LIFE WITH AD PATHOLOGIC CHANGES
Although we hypothesized that purpose in life provides reserve by reducing the deleterious effects of AD pathologic changes on cognitive function, it is possible that purpose in life could have a direct association with those changes. Thus, we first examined the relationship of purpose in life with the 3 indexes of AD pathologic changes in regression models adjusted for age, sex, and educational level. Purpose in life was not directly related to the global AD pathologic measure (mean [SE] parameter estimate, −0.015 [0.055]; P =.79) or the more specific measures of amyloid (parameter estimate, 0.054 [0.157]; P =.73) and tangles (parameter estimate, −0.010 [0.088]; P = .91).
GLOBAL COGNITION AS A FUNCTION OF PURPOSE IN LIFE AND AD PATHOLOGIC CHANGES
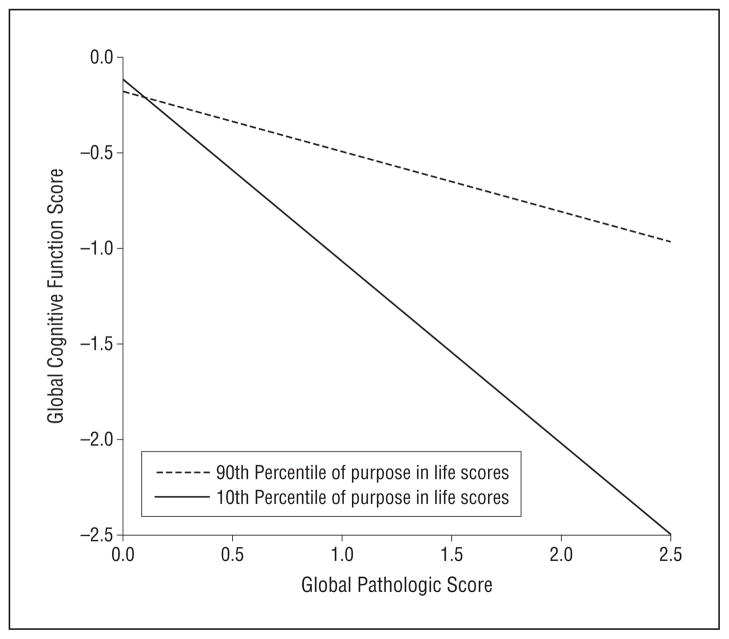
In an initial analysis of global cognition as a function of AD pathologic changes and purpose in life, the mean (SE) global cognitive score was 0.601 (0.096) units (P <.001) lower for each unit of global AD pathologic factors, but purpose in life was not significantly related to the level of cognition (Table 2, main effects, model 1). To test the hypothesis that purpose in life reduced the association of AD pathologic changes with the level of cognition, we repeated this analysis with an additional term for the interaction between global AD pathologic changes and purpose in life. The interaction was significant (mean [SE] parameter estimate, 0.532 [0.211]; P = .01; Table 2, purpose × global AD pathologic change interaction term, model 1). To illustrate this effect, we plotted the estimated relationship between AD pathologic factors and the global cognitive function score for participants at the 90th percentile vs those at the 10th percentile of purpose in life (Figure 1). The figure shows that participants with higher levels of purpose in life exhibited better cognitive function even at more severe levels of AD.
Table 2.
Global Cognition as a Function of Purpose in Life, 3 Indexes of AD Pathologic Changes, and the Interaction Between Each Pathologic Index and Purpose in Lifea
| Main Effects
|
With Interaction Term
|
|||
|---|---|---|---|---|
| Parameter Estimate (SE) | P Value | Parameter Estimate (SE) | P Value | |
| Model 1 | ||||
| Global AD pathologic changes | −0.601 (0.096) | <.001 | −0.366 (0.135) | .007 |
| Purpose in life | 0.229 (0.123) | .06 | −0.052 (0.165) | .76 |
| Purpose in life × global AD pathologic changes | 0.532 (0.211) | .01 | ||
| Model 2 | ||||
| Amyloid load | −0.082 (0.013) | <.001 | −0.062 (0.020) | .002 |
| Purpose in life | 0.265 (0.124) | .03 | 0.132 (0.161) | .41 |
| Purpose in life × amyloid load | 0.045 (0.035) | .20 | ||
| Model 3 | ||||
| Neurofibrillary tangles | −0.072 (0.008) | <.001 | −0.048 (0.014) | .001 |
| Purpose in life | 0.206 (0.115) | .08 | 0.002 (0.147) | .99 |
| Purpose in life × neurofibrillary tangles | 0.042 (0.019) | .03 | ||
Abbreviation: AD, Alzheimer disease.
Derived from linear regression models controlled for age, sex, and educational level.
Figure 1.
Predicted association between Alzheimer disease (AD) pathologic changes and global cognitive function. The illustrated association was derived from a linear regression model with terms for age, sex, educational level, main effects for purpose in life and global AD pathologic changes, and the interaction of purpose in life with pathologic changes.
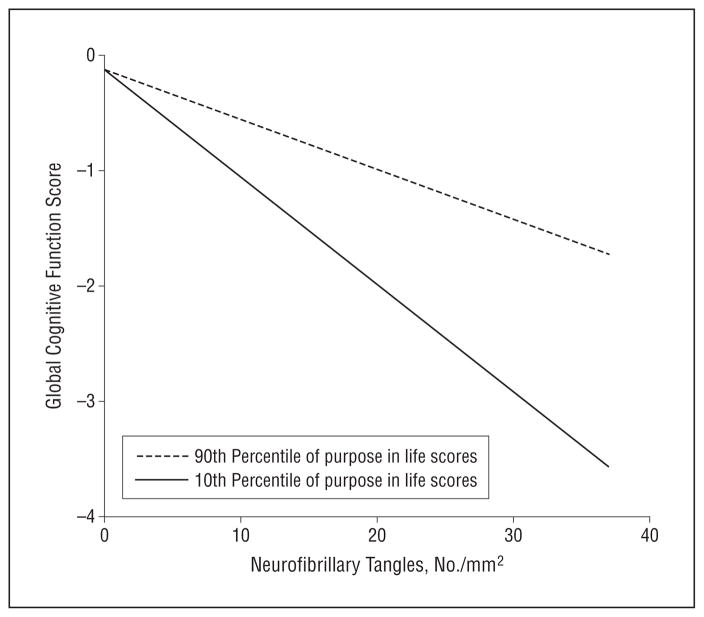
Next, to determine whether the modifying effects of purpose in life were specific to a particular type of AD pathology, we conducted a similar series of analyses in which we replaced the global measure of AD pathologic changes with the more molecularly specific measures of amyloid and tangles. In the analyses for amyloid, the average cognitive function score was 0.082 unit lower (P<.001; Table 2, main effect, model 2) for each 1% increase in amyloid. However, the interaction between amyloid and purpose in life was not significant (P=.20). In analyses for tangles, the average cognitive score was 0.072 unit lower for each neurofibrillary tangle per square millimeter (P<.001, Table 2, main effect, model 3). Furthermore, the interaction between tangles and purpose in life was significant (P=.03; Table 2, interaction term, model 3). To illustrate this effect, we plotted the predicted relationship between tangles and the global cognitive score for participants at the 90th vs 10th percentile of purpose in life (Figure 2). Again, the figure shows that participants with higher levels of purpose in life exhibited better cognitive function even at more severe levels of tangle involvement.
Figure 2.
Predicted association between neurofibrillary tangles and global cognitive function. The illustrated association was derived from a linear regression model with terms for age, sex, educational level, main effects for purpose in life and tangle pathologic changes, and the interaction of purpose in life with pathologic changes.
We repeated the series of models just described after adding terms for depressive symptoms, neuroticism, social network size, presence of the apolipoprotein ε4 allele, chronic medical conditions, the interval between assessment of purpose in life and death, and each of their interactions with AD pathologic factors separately to ensure that these variables did not confound or mediate the protective effect of purpose in life on cognition. The parameter estimates were essentially unchanged (Table 3), although the association was reduced to a trend when controlling for neuroticism; this is likely because 22 persons had missing data in neuroticism and because we had less power given the reduced sample size for that analysis.
Table 3.
Examination of Potential Confounders of the Modifying Effect of Purpose In Lifea
| Confounder | Interaction of Purpose in Life With Global AD Pathologic Changes
|
|
|---|---|---|
| Parameter Estimate (SE) | P Value | |
| Depressive symptoms (n = 246) | 0.647 (0.230) | .005 |
| Neuroticism (n = 224) | 0.464 (0.282) | .10 |
| Social network size (n = 246) | 0.566 (0.021) | .009 |
| No. of chronic medical conditions (n = 246) | 0.540 (0.213) | .009 |
| Physical activity (n = 246) | 0.584 (0.020) | .009 |
| No. of persons with an apolipoprotein ε4 allele (n = 241) | 0.459 (0.218) | .04 |
| Time to death (n = 246) | 0.570 (0.204) | .006 |
Abbreviation: AD, Alzheimer disease.
Derived from separate linear regression models adjusted for age, sex, educational level, purpose in life, and AD pathologic characteristics and the interactions of purpose and pathologic characteristics and each of the covariates and pathologic characteristics.
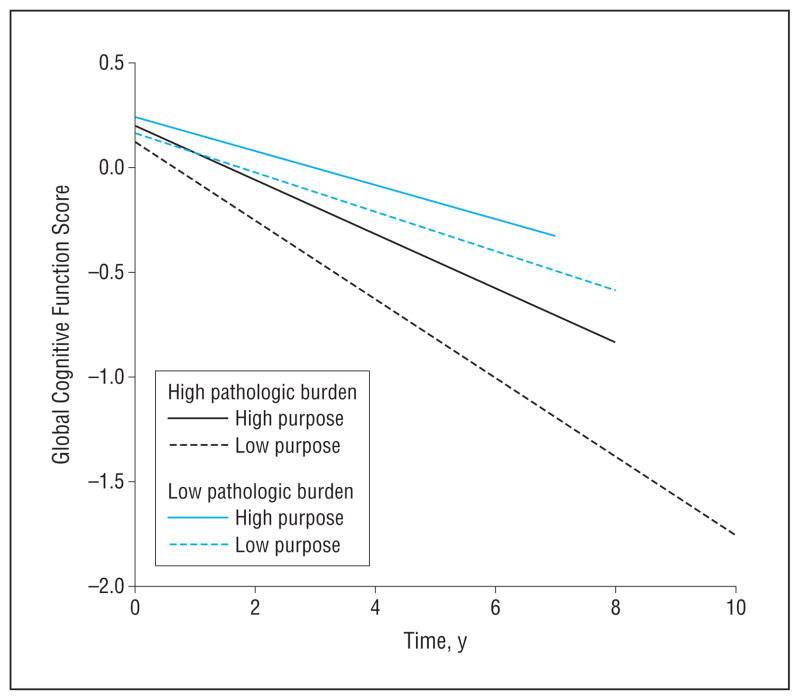
Finally, because cognitive decline is the primary manifestation of AD pathologic changes and ultimately we are interested in identifying factors that modify the course of cognitive decline in aging, we used a linear mixed model to examine the potential modifying effect of purpose in life on the association between AD pathologic changes and the rate of cognitive decline; this analysis used all available longitudinal cognitive data from persons with at least 2 cognitive assessments (n=225). The mean change in global cognition was −0.07 (0.022) unit per year. Results indicated that, for a 1-unit increase in AD pathologic factors, the rate of cognitive decline was 0.06 unit faster (mean [SE] parameter estimate, −0.06 [0.024]; P=.02). Furthermore, a 1-point increase in purpose in life reduced the effect of AD pathologic changes on cognitive decline by 0.09 unit (parameter estimate, 0.085 [0.039]; P = .03). This is illustrated in Figure 3, which shows that, among persons with a relatively low burden of pathology (25th percentile), cognitive function in persons with higher levels of purpose in life (75th percentile) declined more slowly than in those with lower purpose in life (25th percentile). The protective effect of purpose in life was similar but much more striking among persons with a high burden of pathology (75th percentile).
Figure 3.
Predicted association between Alzheimer disease pathologic changes and rate of decline in global cognitive function. The graph illustrates the predicted associations among persons with high (75th percentile) and low (25th percentile) purpose in life and high (75th percentile) and low (25th percentile) pathologic changes derived from a mixed model controlled for age, sex, and educational level.
COMMENT
It has been established that purpose in life is related to cognitive function and predicts cognitive decline in elderly individuals in analyses based on almost 1000 persons with up to 12 follow-up assessments of cognition.21–24 Here, we sought to examine the neurobiologic basis of this association. In a cohort of almost 250 older persons with detailed cognitive testing and autopsy data, we found that higher levels of purpose in life reduced the deleterious effects of AD pathologic changes on cognitive function. This protective effect was observed for a global measure of the changes as well as a more molecularly specific measure of tangle pathologic changes. In addition, the protective effect of purpose in life on cognition persisted after controlling for various potentially confounding variables. Moreover, higher levels of purpose in life reduced the deleterious effect of AD pathologic changes on the rate of cognitive decline. These findings suggest that purpose in life provides neural reserve by protecting against the harmful effects of AD pathologic changes on cognitive function in elderly persons.
The concept of reserve applies to many physiological systems; that is, most systems can sustain extensive organ damage before clinical manifestation of deficits. The brain also can tolerate the accumulation of pathologic changes without expressing them clinically. This is evident from numerous clinicopathologic and, more recently, neuroimaging studies1,9,36,39–45 that have demonstrated the nearly ubiquitous accumulation of AD pathology even among older persons without overt cognitive deficits. Identifying determinants of neural reserve has proved to be challenging, in part because reserve is multifactorial and few studies have the specimen data needed to directly test neurobiologic hypotheses. However, recent evidence6–10,37 suggests that psychosocial variables, such as educational level and social networks, may provide reserve by reducing the deleterious effects of AD pathologic changes on cognition. It is now widely recognized that AD has a long preclinical phase during which the pathologic changes accumulate and cognitive function declines, and the identification of factors that protect against the deleterious effects of this process may help combat the large and rapidly increasing public health challenge posed by AD.46
Purpose in life, an indicator of human thriving, has long been hypothesized to be an important determinant of health outcomes.11,15,20,30 In recent years, prospective studies19,21 have shown that purpose in life and related aspects of well-being are associated with a substantially reduced risk of adverse cognitive health outcomes, including the risk of AD and its precursor, mild cognitive impairment, as well as a slower rate of cognitive decline even among older persons without AD or mild cognitive impairment. Furthermore, purpose in life is associated with a reduced risk of incident disability and death.22,23 Initial evidence suggests that purpose in life may also be modifiable, rendering it a potential treatment target.47 To date, however, we are not aware of any study that has examined the neurobiologic basis of the protective effect of purpose in life.
Purpose in life is a complex and multifaceted trait like construct, and it is likely that purpose in life works via complex mechanisms to provide reserve. The ability to find meaning in life’s experiences and develop a sense of direction and intentionality requires self-reflection, synthesis of diverse experiences into a narrative, awareness of one’s role and potential within the broader context, establishment of goals and priorities, and focus. Furthermore, having a sense of purpose is thought to generate motivation to behave in ways consistent with one’s purpose and work toward goals.11,48,49 Purpose in life is related to aspects of psychological health, including happiness, satisfaction, personal growth, and better sleep,14,17,18,50 and associations between purpose in life and aspects of personality (ie, neuroticism, extraversion, and conscientiousness), as well as depressive symptoms, have been reported.23,48,50 Although few studies have examined the extent to which purpose is related to engagement in health-promoting behaviors, results of a meta-analysis48 showed that a higher level of purpose in life was associated with better health, everyday competence, social integration, participation in the labor force, and socioeconomic status among middle-aged and older persons. Purpose in life also is associated with better treatment outcomes for persons with addiction.51 Taken together, the available data suggest that persons with higher levels of purpose tend to be goal-oriented and resilient, and their active pursuit of goals likely enhances the strength and efficiency of neural systems. Furthermore, although purpose in life may be most beneficial in aging (when cognitive and other resources are diminishing), elderly persons who report higher levels may have acquired over their lifespan an expanded repertoire of behaviors that facilitate neurocognitive development.21 Although one could speculate from this that purpose in life may somehow prevent the accumulation of AD pathologic changes, we did not find evidence of a direct association with the changes. This may indicate that, instead of preventing the accumulation of pathologic changes, purpose in life contributes to the development of efficient neural systems that allow one to maintain cognition even in the face of accumulating characteristics of AD.
Another possibility is that purpose in life may reduce the association of AD pathologic features with cognition by helping to invoke compensatory processes in the face of accumulating damage. The current study was motivated in part by a clinicopathologic study10 that showed that social networks modified the association of AD pathologic characteristics with cognition. Given that the development of social networks includes brain regions not involved in traditional aspects of cognition, such findings may indicate that social cognitive brain regions are brought online to maintain cognition in the face of accumulating AD pathologic changes in regions that support traditional cognitive abilities.52,53 It is noteworthy that higher levels of purpose in life are associated with less negative affect, more positive social relations, and better sleep and other health outcomes.15,17,47 Although controlling for covariates such as neuroticism, social networks, and depressive symptoms did not affect our findings, it seems intuitive that the noncognitive abilities that allow some people to readily derive meaning from life’s experiences and persist through challenging events might also provide reserve by increasing the availability of social cognitive or other noncognitive neural networks that can help preserve cognition even as AD pathologic changes accumulate.
Notably, the beneficial effect of purpose in life was most evident in analyses with tangles as compared with amyloid. Previous studies54 have shown that tangles are more strongly associated with cognitive function than amyloid. This generally was true in our data, and the modifying effects may have been most evident in analyses with tangles because of their relatively stronger association with cognition. Similar differential effects were reported in 2 studies; one of these55 showed that processing resources protected against the deleterious effects of AD pathologic changes on other cognitive systems, particularly in analyses of tangles, and the other10 showed a similar effect with social networks. These findings likely suggest that tangles are a major driver of cognitive impairment in old age and that the association of tangles with cognition is the predominant beneficiary of factors that provide reserve.
Our study has several strengths, including the ability to relate purpose in life to several alternative and highly specific measures of AD pathologic characteristics and detailed assessments of cognition conducted annually and proximate to death in a single cohort with very high rates of follow-up and brain autopsy; this allowed for testing of specific hypotheses about reserve with a high degree of internal validity. Furthermore, uniform structured procedures were followed, with blinding to previously collected data and of personnel collecting postmortem data to clinical data. The study also has limitations; in particular, data from observational studies do not allow for determination of causal effects. Further studies are needed to determine whether purpose in life may be useful as a target for interventions aimed to reduce the public health burden of cognitive decline in aging and AD.
Acknowledgments
Funding/Support: The study was supported by grants R01AG17917, R01AG34374, and R01AG33678 from the National Institute on Aging.
Footnotes
Author Contributions: Dr Boyle had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Additional Contributions: We are indebted to the participants and staff of the Rush Memory and Aging Project and the Rush Alzheimer’s Disease Center for their contributions to this work, as well as to Woojeong Bang, MS, for statistical programming. Ms Bang received no financial compensation for her work.
References
- 1.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355(9212):1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- 3.Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25(5):625–633. doi: 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, Marder KS, Bell KL, Sackeim HA, Van Heertum RL, Moeller JR, Stern Y. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60(3):359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry. 2007;64(10):1204–1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid but not tangles with cognitive function. Neurology. 2005;65(6):953–955. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 8.Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11–labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 11.Frankl VE. Man’s Search for Meaning. New York, NY: Washington Square Press, Simon & Schuster; 1963. [Google Scholar]
- 12.Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Singer BH, Ryff CD. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci U S A. 2005;102(51):18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott RA, Ploubidis GB, Huppert FA, Kuh D, Wadsworth ME, Croudace TJ. Psychometric evaluation and predictive validity of Ryff’s Psychological Well-Being items in a UK birth cohort sample of women. Health Qual Life Outcomes. 2006;4:76. doi: 10.1186/1477-7525-4-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryff CD, Singer B. Psychological well-being: meaning, measurement, and implications for psychotherapy research. Psychother Psychosom. 1996;65(1):14–23. doi: 10.1159/000289026. [DOI] [PubMed] [Google Scholar]
- 15.Ryff CD, Singer BH, Dienberg Love G. Positive health: connecting well-being with biology. Philos Trans R Soc Lond B Biol Sci. 2004;359(1449):1383–1394. doi: 10.1098/rstb.2004.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedberg P, Gustafson Y, Brulin C. Purpose in life among men and women aged 85 years and older. Int J Aging Hum Dev. 2010;70(3):213–229. doi: 10.2190/AG.70.3.c. [DOI] [PubMed] [Google Scholar]
- 17.Phelan CH, Love GD, Ryff CD, Brown RL, Heidrich SM. Psychosocial predictors of changing sleep patterns in aging women: a multiple pathway approach. Psychol Aging. 2010;25(4):858–866. doi: 10.1037/a0019622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood AM, Joseph S. The absence of positive psychological (eudemonic) well-being as a risk factor for depression: a ten year cohort study. J Affect Disord. 2010;122(3):213–217. doi: 10.1016/j.jad.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Gerstorf D, Lövdén M, Röcke C, Smith J, Lindenberger U. Well-being affects changes in perceptual speed in advanced old age: longitudinal evidence for a dynamic link. Dev Psychol. 2007;43(3):705–718. doi: 10.1037/0012-1649.43.3.705. [DOI] [PubMed] [Google Scholar]
- 20.Ryff CD, Dienberg Love G, Urry HL, Muller D, Rosenkranz MA, Friedman EM, Davidson RJ, Singer B. Psychological well-being and ill-being: do they have distinct or mirrored biological correlates? Psychother Psychosom. 2006;75(2):85–95. doi: 10.1159/000090892. [DOI] [PubMed] [Google Scholar]
- 21.Boyle PA, Buchman AS, Barnes LL, Bennett DA. Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Arch Gen Psychiatry. 2010;67(3):304–310. doi: 10.1001/archgenpsychiatry.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle PA, Barnes LL, Buchman AS, Bennett DA. Purpose in life is associated with mortality among community-dwelling older persons. Psychosom Med. 2009;71(5):574–579. doi: 10.1097/PSY.0b013e3181a5a7c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle PA, Buchman AS, Bennett DA. Purpose in life is associated with a reduced risk of incident disability among community-dwelling older persons. Am J Geriatr Psychiatry. 2010;18(12):1093–1102. doi: 10.1097/JGP.0b013e3181d6c259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 27.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67(3):441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11(4):400–407. [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Ryff CD, Keyes CL. The structure of psychological well-being revisited. J Pers Soc Psychol. 1995;69(4):719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- 31.Barnes LL, Wilson RS, Bienias JL, de Leon CF, Kim HJ, Buchman AS, Bennett DA. Correlates of life space in a volunteer cohort of older adults. Exp Aging Res. 2007;33(1):77–93. doi: 10.1080/03610730601006420. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RS, Schneider JA, Bienias JL, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, clinical AD, and cortical plaques and tangles in older persons. Neurology. 2003;61(8):1102–1107. doi: 10.1212/01.wnl.0000092914.04345.97. [DOI] [PubMed] [Google Scholar]
- 33.Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosom Med. 2007;69(1):47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 35.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18(3):691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 37.Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006;20(3 suppl 2):S63–S68. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- 38.SAS 9.2 Help and Documentation. Cary, NC: SAS Institute Inc; 2009. [Google Scholar]
- 39.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kemppainen NM, Aalto S, Karrasch M, Någren K, Savisto N, Oikonen V, Viitanen M, Parkkola R, Rinne JO. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008;63(1):112–118. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- 41.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62(11):1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 42.Mortimer JA, Borenstein AR, Gosche KM, Snowdon DA. Very early detection of Alzheimer neuropathology and the role of brain reserve in modifying its clinical expression. J Geriatr Psychiatry Neurol. 2005;18(4):218–223. doi: 10.1177/0891988705281869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51(5):567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 44.Rodrigue KM, Kennedy KM, Park DC. Beta-amyloid deposition and the aging brain. Neuropsychol Rev. 2009;19(4):436–450. doi: 10.1007/s11065-009-9118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roe CM, Xiong C, Grant E, Miller JP, Morris JC. Education and reported onset of symptoms among individuals with Alzheimer disease. Arch Neurol. 2008;65 (1):108–111. doi: 10.1001/archneurol.2007.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jicha GA, Abner EL, Schmitt FA, Kryscio RJ, Riley KP, Cooper GE, Stiles N, Mendiondo MS, Smith CD, Van Eldik LJ, Nelson PT. Preclinical AD Workgroup staging: pathological correlates and potential challenges. Neurobiol Aging. 2012;33(3):622.e1–622.e16. doi: 10.1016/j.neurobiolaging.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westerhof GJ, Bohlmeijer ET, van Beljouw IM, Pot AM. Improvement in personal meaning mediates the effects of a life review intervention on depressive symptoms in a randomized controlled trial. Gerontologist. 2010;50(4):541–549. doi: 10.1093/geront/gnp168. [DOI] [PubMed] [Google Scholar]
- 48.Pinquart M. Creating and maintaining purpose in life in old age: a meta-analysis. Ageing Int. 2002;27(2):90–114. doi: 10.1007/s12126-002-1004-2. [DOI] [Google Scholar]
- 49.McKnight PE, Kashdan TB. Purpose in life as a system that creates and sustains health and well-being: an integrative, testable theory. Rev Gen Psychol. 2009;13(3):242–251. doi: 10.1037/a0017152. [DOI] [Google Scholar]
- 50.Schmutte PS, Ryff CD. Personality and well-being: reexamining methods and meanings. J Pers Soc Psychol. 1997;73(3):549–559. doi: 10.1037//0022-3514.73.3.549. [DOI] [PubMed] [Google Scholar]
- 51.Martin RA, MacKinnon S, Johnson J, Rohsenow DJ. Purpose in life predicts treatment outcome among adult cocaine abusers in treatment. J Subst Abuse Treat. 2011;40(2):183–188. doi: 10.1016/j.jsat.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11 (2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 53.Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giannakopoulos PHF, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60(9):1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 55.Boyle PA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Processing resources reduce the effect of Alzheimer pathology on other cognitive systems. Neurology. 2008;70(17):1534–1542. doi: 10.1212/01.wnl.0000304345.14212.38. [DOI] [PMC free article] [PubMed] [Google Scholar]