Abstract
The main barrier to a broader clinical application of umbilical cord blood (UCB) transplantation is its limiting cellular content. Thus, the discovery of hematopoietic progenitor cells in murine placental tissue led us investigate whether the human placenta contains hematopoietic cells, sites of hematopoiesis, and to develop a procedure of processing and storing placental hematopoietic cells for transplantation. Here we show that the human placenta contains large numbers of CD34-expressing hematopoietic cells, with the potential to provide a cellular yield several-fold greater than that of a typical UCB harvest. Cells from fresh or cryopreserved placental tissue generated erythroid and myeloid colonies in culture, and also produced lymphoid cells after transplantation in immunodeficient mice. These results suggest that human placenta could become an important new source of hematopoietic cells for allogeneic transplantation.
Introduction
As a source of hematopoietic cells, the utilization of umbilical cord blood (UCB) for transplantation is expanding (1, 2). The ability to conduct UCB transplantation using human leukocyte antigen (HLA) disparate donors with a reduced risk of severe graft-versus-host disease compared to established sources of hematopoietic cells such as bone marrow effectively extends the possibility of transplantation to those who lack a suitable HLA-matched family- or unrelated donor. The principal limitation of UCB transplantation is its cellular content, and with donor-recipient HLA compatibility represents the most important determinant of outcome after UCB transplantation. This limitation has prompted the development of clinical protocols using 2 UCB units for transplantation of larger adult recipients (3, 4). An alternate approach is to develop methods to increase the cellular yield of hematopoietic cells similar to those in UCB. It was shown that the mouse placenta is a source for hematopoietic cells (5, 6). Here, for the first time we show the presence of significant amounts of viable hematopoietic cells in human term placenta. These placental hematopoietic cells (PHCs) can be isolated before and after cryopreservation and storage. The colony-forming unit (CFU) activity of PHCs was confirmed, and xenotransplantation assays in immunocompromised mice demonstrated the potential of these placental cells for engraftment. Together, these results strongly suggest that the human term placenta has the potential to become a novel source of hematopoietic cells for transplantation.
Materials and Methods
Placenta Perfusion and Cryostorage
After informed consent, placentas were harvested from healthy females undergoing elective Cesarean section at Alta Bates Hospital (Berkeley, CA). UCB was collected from the placentas using standard cord blood collection techniques, and stored. Placentas were rinsed from the outside with saline and infused with 30 mL of an anticoagulant/ vasodilator solution (Heparin 30 U/mL, Papaverin hydrochloride, 1 mg/mL) at room temperature. The arteries and vein of the umbilical cord were subsequently cannulated, and connected to a perfusion circuit, containing a heat exchange unit, roller pump and perfusion reservoir. Constant temperature of the perfusate was maintained, perfusion procedures were performed similarly as described by us before (7), and the placentas were first perfused with phosphate buffered saline (PBS) to remove UCB remaining in the placental tissue. Long-term perfusions (6 hours) were performed with 500–1000 mL of alfa-MEM medium containing 5% bovine serum albumin (Sigma, St. Louis, MO), 10 U/mL heparin and 0.1 mg/mL Papaverin hydrochloride. For cryopreservation, placentas were perfused with a mixture of 15% propylene glycol, 14% DMSO, 14% Formamide, and 57% PBS with Penicillin 100 U/mL/ Streptomycin 100 µg/mL/Fungisone 0.25 µg/mL (PSF). The arterial and venous lines were then closed, placentas were placed into a −80°C freezer for 12 hours, and subsequently placed into liquid nitrogen vapor at −190°C for long-term storage.
Immunostaining
Tissues were fixed with 4% paraformaldehyde, paraffin-fixed and cut, deparaffinized in Xylenes, rehydrated in alcohols, permeabilized with cold Methanol (−20°C) and 1% Triton X-100 for 5 minutes. Slides were incubated with blocking buffer (3% BSA in 4x SCC, 2% goat serum, 3% FCS, 0.1% Tween 20) for 60 minutes at 37°C and incubated with primary antibody (1:10–100 dilution) overnight at 4°C. Slices were washed, incubated with blocking solution for 20 minutes and then incubated with secondary antibody (1:500) labeled with FITC- or Alexa Fluor-633 for 60 minutes at 37°C, washed, and mounted on slides with Gold Antifade reagent (Molecular Probes, Eugene, OR). The following antibodies were used: mouse anti-human CD34 (BD Pharmingen, San Jose, CA, Cat# 555820), mouse anti-human CD31 (Acris Antibodies GmbH, Germany, Cat# BM4047), rabbit anti-human CD133 (Abcam, Cambridge, MA, Cat# ab16518), mouse anti-human CD90 (BD Pharmingen, Cat# 550402), mouse anti-human CD38 (BD Pharmingen, Cat# 555458), mouse anti-human KDR (R&D Systems, Cat# MAB3571), rat anti-human HLA-DR (Serotec, Cat# MCA71F). Isotype anti-mouse and anti-rabbit antibodies were from Zymed (S. San Francisco, CA), secondary anti-mouse and anti-rabbit FITC- or Alexa Fluor 633-labeled antibodies were from Molecular Probes (Invitrogen). Microscopic images were obtained with an Olympus IX70 inverted microscope (Olympus), CoolSNAPfx camera (Roper Scientific) and QED Acquisition software (Media Cybernetics). Merged images were prepared using Photoshop CS3 software (Adobe).
Placental Cell Isolation
Cells were isolated from placental tissue by either enzyme digestion or perfusion with AMD 3100. Portions of the perfused and washed placenta were infused with 50 mL PSF containing 2.5 U/mL Dispase, Trypsin (0.5 mg/mL) for 20 minutes at 37°C, and tissue samples were digested with 0.1% Collagenase I, and 2.5 U/mL Dispase. Alternatively, following perfusion as described above with phosphate buffered saline (PBS) to remove UCB remaining in the placental tissue, the specific blocker of the CXCR-4 receptor (8, 9), 1,1′-[1,4-Phenyl-enebis(methylene)] bis-1,4,8,11-tetraazacyclotetradecane octahydrochloride (AMD 3100, Sigma-Aldrich, St. Louis, MO, USA) was added to the perfusate at concentration 300 microgram per liter. Perfusion was continued up to 6 hours. Following centrifugation, the cells from tissue digest or placental perfusate were re-suspended in growth medium in Petri dishes or analyzed by flow cytometry.
Hematopoietic Differentiation Assays
Colonies of cells were scored for CFU-E, BFU-E, CFU-GM, and CFU-GEMM after incubation for 2 weeks with complete MethoCult® Methylcellulose-based media (Stem Cell Technologies, Vancouver, Canada).
FACS Analysis
The Procount Progenitor Cell Enumeration Kit (BD Biosciences) containing fluorochrome-conjugated monoclonal antibodies directed against CD34 and CD45, in combination with the viability stain ToPro-3 iodide (Molecular Probes, Eugene, OR), was used to determine live CD34+ CD45+dim cells in cord blood, tissue digests and placental perfusate using a FACSCalibur flow cytometer (BD Biosciences) and FlowJo analysis software (Tree Star, Inc., Ashland, OR). Antibodies against KDR (R&D Systems, Cat# MAB3571) and CD31 (AbCam, Cat# ab59251) and CD133 (AbCam, Cat# ab16518) were used to identify cells in this population positive for these endothelial markers. In addition, fluorochrome-conjugated monoclonal antibodies directed against CD3 (PE-conjugated, Miltenyi Biotec, Cat# 130-091-374), CD25 (PE-conjugated, Miltenyi Biotec, Cat# 120-001-311), CD45 (APC-conjugated, Caltag Laboratories, Cat# MHCD4505), CD51/61 (BD Pharmingen, Cat# 550037) and CD235 (Dako, Denmark, Cat# R7078) were used to characterize cells in cell culture of mouse tissue. From the concentration in the weighed and digested fractions of placental tissue, the total number of these cells present in the total placenta was estimated by extrapolation to the total weight of the placental tissue. Similarly, the concentration of nucleated and viable CD34+ CD45+dim expressing cells was determined in the unit of UCB collected from the same placenta. The total number of these cells in the unit of UCB was estimated by extrapolation from the concentration in the measured volume to the total volume of UCB collected.
Chimeric Mice
Nonirradiated NOD-Cg-Prkdcscid Il2rgtm1Wjl/SzJ, and 2.5 Gy total-body irradiated NOD-Cg-Rag1tm1Mom Prf1tm1Sdz/Sz mice (The Jackson Laboratory, Bar Harbor, Maine) were injected via the intra-peritoneal (IP) route with 106 nucleated cells prepared from digested placental tissue. After 3 months and weekly IP injection with erythropoietin (1 U), IL-3 (5 ng), stem cell factor (25 ng) and GM-CSF (5 ng) IP, the animals were sacrificed, and blood, bone marrow, and spleen cells were immunostained for human CD45 (pan-leukocyte), CD3 (lymphocyte), CD25 (lymphocyte/monocyte), and CD51–CD61 (platelet). Blood was analyzed by flowcytometry, and tissue by fluorescent microscopy. Non-transplanted mouse tissue and human blood cells were used as negative and positive controls, respectively.
Results and Discussion
Paraffin sections of the chorion of term human placentas were stained with 3,3-diaminobenzidine for the CD34 antigen. As shown in Figure 1, we found CD34-expressing cells in the vessels and vessel walls as well as in the tissue stroma. These cells are indicated in Figure 1 by white and dark arrows, respectively. This data suggested the presence of CD34-expressing endothelial cells in the vessel wall, as well as non-endothelial CD34-expressing cells in tissue not related to vessels.
Figure 1.

CD34 in placental tissue. 3,3-diaminobenzidine staining for CD34 antigen in paraffin sections of human placenta. White arrows (Fig. 1A and B) indicate CD34+ cells in the vessel walls (endothelial cells) and dark arrows (Fig. 1C) point at CD34+ cells in tissue at ×10 (A) or ×40 (B) or ×20 (C) magnification.
As shown in Figure 2, significant numbers of CD34-expressing cells are also positive for other hematopoietic precursor markers such as CD90, CD38 and CD133. These data observed using a microscope are evidence of the presence of multiple clusters of CD34-expressing cells, not associated with the fetal or maternal circulation.
Figure 2.

Hematopoietic cells in human placenta. Multiple clusters of CD34+cells, not associated with fetal or maternal circulation, are present in placental tissue. Significant numbers of CD34 cells are also positive for hematopoietic precursor markers such as CD90, CD38 and CD133. Paraffin sections of human placenta stained for CD34, CD90, CD38, and CD133 are shown. A1: CD90 (green), A2: CD34 (red), A3: merged image of A1 and A2 with nuclear co-staining (DAPI blue), size indication bar is 10 µm. The white arrow points to CD34+CD90+cells. B1: CD38 (green), B2: CD34 (red), B3: merged image of B1 and B2 with nuclear co-staining (DAPI blue), size indication bar is 100 µm. The white arrows point to CD34+ CD38+ cells. The green arrow points at a CD34+ capillary. C1: CD133 (green), C2: CD34 (red), C3: merged image, showing staining for CD34 (red), CD133 (green), and nuclei (DAPI blue), size indication bar is 20 µm. The white arrows point to CD34+CD133+cells not associated with structures of the circulation (capillary walls). The green arrow points at the CD34+CD133+capillary. D: Negative control staining. D1: merged image of staining with isotype anti-mouse antibody and isotype anti-rabbit antibody, secondary FITC (green) anti-mouse and Alexa Fluor 633 anti-rabbit antibody (red), co-stained for nuclei with DAPI (blue). No signals from cell markers other than nuclei are present. D2: Merged image of staining with mouse anti-CD34 antibody and isotype anti-rabbit antibody, secondary FITC anti-mouse (green) and Alexa Fluor 633 anti-rabbit (red) antibody, co-stained for nuclei with DAPI (blue). Only signals from the CD34 cell marker and nuclei are present. D3: Isotype anti-mouse antibody and anti-CD133 rabbit antibody, secondary FITC anti-mouse and Alexa Fluor 633 anti-rabbit antibody, co-staining DAPI (blue), merged image. Only a signal for CD133+cells is observed, no signals from CD34 cell markers are present. The size indication bar is 50 µm.
The white arrow in Figure 2A3 indicates a cell positive for both CD34 and CD90. Figure 2B3 shows cells that express both CD34 and CD38, and Figure 2C3 indicates the presence of CD34-expressing cells positive for CD133. Because CD34 and CD133 antigens are expressed by endothelial cells as well as by hematopoietic cells, we investigated whether CD34 and CD133-expressing cells present in the mesenchyme of human placenta were also positive for the endothelial markers CD31 and KDR (also known as the vascular endothelial growth factor receptor VEGFR2, or CD309). Hematopoietic cells are positive for CD34 and or CD133, but do not express CD31 or KDR.
Figure 3 shows dual staining for CD34 and CD133, as well as CD31, and KDR. Distinct populations that express both CD34 and CD31 (indicative of endothelial cells), and distinct populations expressing CD34 but not CD31 (indicative of hematopoietic cells) were found. Similarly cells that express CD34, but not KDR (indicative of hematopoietic cells), were found to be present. Also cells were present that express CD133 but not CD31 (indicative of hematopoietic cells). Figure 3A and B shows cells that express CD34 but not CD31, as well as cells that express both CD34 and CD31. Similarly Figure 3C shows cells that express CD34 but not KDR, as well as cells that express both CD34 and KDR. Co-staining for CD31 and CD133 shows some cells that express CD133 but not CD31, but also cells that express only CD31, and not CD133. This is further illustrated in supplemental Figure 1 (available in the online version).
Figure 3.
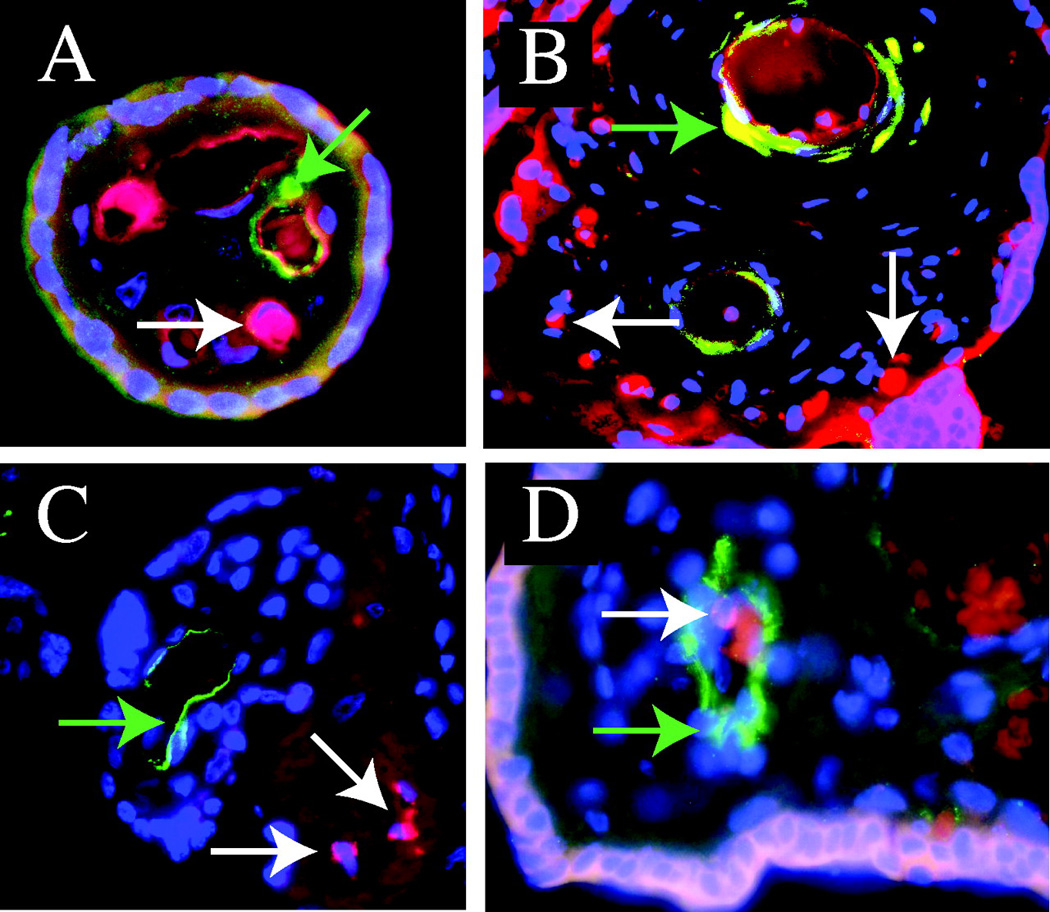
Dual staining for CD31, CD34, CD133 and KDR in human placenta. Distinct populations of CD34+/CD31+and CD34+/CD31− cells as well as CD34+/KDR− and CD133+/CD31− are present on paraffin sections of human placenta, stained for CD34 (red), CD31 (green), CD133 (green), KDR (green) and nuclei (DAPI, blue). A: Merged image for CD34 (red), and CD31 (green). The white arrow points at a CD34+, CD31− cell and the green arrow points at a CD34+, CD31+cell. B: Merged image for CD34 (red), and CD31 (green). The white arrows point at a CD34+, CD31− cells not associated with capillaries, and the green arrow points at a CD34+, CD31+ cells in vessel walls. C: Merged image for CD34 (red), and KDR (green). The white arrows point at CD34+KDR− cells not associated with vessels, and the green arrow points at a CD34+KDR+ cell in vessel wall. D: Merged image for CD31 (green), and CD133 (red). The white arrow points at a CD133+ CD31− cell and the green arrow points at a CD133−, CD31+ endothelial cell.
The compilation of these data shows that the chorion of the human term placenta contains clusters of hematopoietic cells that are not associated with fetal or maternal circulation, and are separate from the CD34-expressing endothelial cells. Having shown the presence of these cells in the placental chorion, we pursued different approaches to harvest these cells, define their characteristics by flow cytometry, and test their viability in cell culture and a murine transplant model. Enzymatic digestion of placental tissue generated cell suspensions that could be analyzed by flow cytometry. The measurement of CD34 in combination with CD45 is routinely used to characterize cord blood. Using the Procount Progenitor Cell Enumeration Kit (BD Biosciences) in combination with the viability stain ToPro-3 iodide (Molecular Probes, Eugene, OR), we identified live CD34+CD45+dim cells in the suspension of random chorion samples from 20 human placentas before and after cryopreservation. Perfusion of the placenta before enzyme digestion minimized residual cord blood in the placenta.
A similar analysis was performed on cord blood collected from the same placentas. Forward/side scatter and ToPro-3 labeling was used to select live cells, and CD34+ CD45+dim cells were identified and enumerated by the inclusion of fluorescent beads in the suspension. A typical example of such an analysis is shown in Figure 4A. The total placental CD34+ CD45+dim cells content was estimated based on the total placenta weight and the concentration of CD34+ CD45+dim cells after digestion of the placenta chorion fractions. Similarly, the total number of CD34+CD45+dim cells in the cord blood unit was estimated based on the concentration of these cells in the analyzed volume, and then extrapolated to estimate the number of these cells in the total amount of UCB collected. The placenta averaged a total of 2 × 107 CD34+CD45+dim cells, approximately 10-fold more than a typical cord blood collection (10).
Figure 4.

Flow cytometry of placenta tissue digest samples stained for viable CD34+CD45+dim cells. A: Dot plot of placental-derived cells selected for live (ToPro3 negative) nucleated cells, stained with the antibodies against CD34 and CD45. Events in the upper right of the plot are positive for binding of both antibodies: CD34+ CD45+dim. Beads included in the analysis allow enumeration of the cells in the suspension. B: Dot plot of the perfusate indicating the CD34+ CD45+dim population of cells (gated box). C: Dot plot of the CD34+ CD45+dim population shown in the gated box in B, labeled with both KDR and CD31. The percentages of events in each quadrant are indicated. Virtually all CD34+ CD45+dim cells collected from placental perfusate are negative for CD31 and KDR.
Cord blood units are routinely cryopreserved in liquid nitrogen until they are used for transplant. A similar approach may be needed to store placental tissue for future use. We explored, therefore, if viable cells could be harvested from placental tissue that is properly cryopreserved. We developed a perfusion technique of placenta with a cryopreservative solution using the fetal circulation, as described in the Method section. After perfusion, the placentas were stored in liquid nitrogen, together with cord blood units. Following storage, placentas were thawed, perfused, to remove cryopreservation solution, and CD34+ CD45+dim cells were analyzed in chorionic tissue as indicated above. Post-thaw yields of CD34+ CD45+dim cells from placentas that were frozen as long as 12 months were indistinguishable from yields in fresh placentas. Thus, both microscopic and flow cytometric techniques demonstrated an abundance of cells expressing CD34 and CD45dim in the human placenta.
The estimated total number of CD34+CD45+dim cells in placental tissue suggests that harvest of these cells should be considered as an additional source of hematopoietic stem cells for bone marrow transplant. Enzymatic tissue digestion, however, is not preferable for cells to be used for transplant. Furthermore, such preparations may contain cells that are positive for CD34, but are not of hematopoietic origin, as shown in Figures 2 and 3. To demonstrate the human placenta as a source of hematopoietic cells and to evaluate an alternative way to harvest these cells, we used AMD 3100, a bicyclam, which is an antagonist of the CXCR-4 Chemokine Receptor. This compound was used in humans (8) and non-human primates (9) to mobilize hematopoietic stem cells into peripheral blood with long-term repopulating capacity. We proposed that this specific blocker of the CXCR-4 receptor could have a similar affect on mobilizing hematopoietic stem cells from human placenta.
Four separate placentas were drained of cord blood and perfused with buffer, to remove cord blood remnants. Subsequently they were perfused with a set volume of buffer, and the cell content in the perfusate buffer was measured by flow cytometry as shown in Figure 4. Similar to the analysis of digested placental tissue, forward/side scatter and ToPro-3 labeling was used to select live cells (not shown). In this live cell population, CD34+ CD45+dim cells were identified and enumerated by the inclusion of fluorescent beads in the suspension, using the same approach as shown for placental digest in Figure 4A. After 30–60 minutes of perfusion and before the addition of AMD 3100 to the perfusate buffer, we measured approximately 1 × 106 nucleated cells per mL in the perfusate buffer, a fraction of which were live CD34+/CD45dim cells (Table 1). Six hours after addition of 300 µg/L AMD 3100 to the perfusate buffer, the concentration of live CD34+/CD45dim cells increased from 600 to 2500 per mL to a total of 16 × 105 of these cells in perfusate. A 6-hour perfusion of three placentas with buffer devoid of AMD 3100 did not change CD34+/CD45dim concentrations (not shown), indicating that the presence of AMD 3100 was needed for the release of these cells into the perfusate. Further analysis of the CD34+/ CD45dim population in the perfusate showed that virtually all CD34+/CD45dim cells released from placental tissue with AMD 3100 were negative for the endothelial markers KDR and CD31.
Table 1.
Placental-Derived Cells in Perfusate of Human Term Placentas Before and After 6 Hours Perfusion with 300 µg/L AMD 3100a
| TNC × 106/mL |
CD34+ CD45di m per mL |
CF U-E per mL |
CF U- GM per mL |
CFU-Total per mL |
|
|---|---|---|---|---|---|
| Perfusate before AMD 3100 treatment | 1.0 ± 0.3 | 600 ± 500 | 90 ± 70 | 90 ± 30 | 240 ± 10 |
| Perfusate 6 hours after AMD 3100 treatment | 4.7 ± 3.0 | 2500 ± 100 | 600 ± 200 | 500 ± 200 | 1800 ± 700 |
Total nucleated cell count (TNC), and live CD34+/CD45dim collected in 750 ± 100 mL perfusate are indicated as well as the number of colony forming units (CFU) generated by these cells in cell culture (n = 4, ± SD).
Figure 4B and C illustrates flow cytometric analysis of cells in perfusate labeled with CD34, CD45, CD31, and KDR. Figure 4B shows the total population of cells in perfusate labeled with antibodies against CD34 and CD45. The CD34+/CD45dim population is indicated in the selection box. In this population, more than 99% of the cells are negative for CD31 and KDR (Fig. 4C). Similar results are found for the colocalization of CD133 and KDR (not shown). These data indicate that perfusion of the human term placenta with a compound that is currently used to release donor hematopoietic cells into the peripheral blood can be used to harvest hematopoietic CD34+/CD45dim cells from tissue. The total amount of these cells harvested compares favorably with reported data on cord blood CD34+ cells (10).
The ability to form blood cell-forming colonies in cell culture is an essential characteristic of the potential of CD34+/CD45dim cells to be used in transplant. We performed standard colony-forming analyses in MethoCult® medium to evaluate the viability and ability of placental-derived cells to differentiate, after placenta digestion or perfusion. The placental cells generated a large number of colonies, including CFU-E (colony forming unit erythroid), BFU-E (burst-forming unit erythroid), CFU-GM (colony forming unit-granulocyte, macrophage), CFU-GEMM (colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte). Colonies generated from placenta and UCB showed a similar microscopic appearance (Fig. 5A), and expressed hemoglobin (Fig. 5B).
Figure 5.

Placenta-derived cells in cell culture. Placenta and cord blood (UCB)-derived cells gave rise to multiple hematopoietic lineages following 14 days culture in MethoCult® medium (Stem Cell Technologies, Canada). A: Bright light microscopic appearance of colonies is shown (magnification ×50) derived from placental tissue or cord blood. CFU-E (colony forming unit erythroid), BFU-E (burst-forming unit erythroid), CFU-GM (colony forming unit-granulocyte, macrophage), CFU-GEMM (colony forming unit-granulocyte, erythroid, macrophage, megakar-yocyte) are indicated. B: Bright light microscopic appearance of colonies obtained following 14 days culture in MethoCult® medium with EPO stained with Benzidine for hemoglobin (blue). C, D: Following cryopreservation and thawing, placenta-derived cells gave rise to multiple hematopoietic lineages upon 21-day culture in MethoCult® medium. Bright light microscopic appearance of these colonies is shown. C: CFU-GEMM from placenta-derived cells under high magnification (×200). D: CFU-GEMM from cord blood (×200). Note presence of flat fibroblast-like cells. E. Following 2-week culture in Methocult medium®, cells isolated from placental tissue differentiated into myeloid and erythroid lineages. Representative flow cytometry diagrams of cells isolated from culture medium and double-stained for human surface antigens are shown. E1: Negative control. E2: Double staining for human CD45 (pan leukocyte marker, abscissa) and CD25 (lymphocyte/monocyte marker, ordinate). E3: Double staining for CD45 (pan leukocyte marker, abscissa) and CD51/CD61 (megakaryocyte/platelet marker). E4: Staining for CD235 (blue histogram, human glycophorin, grey histogram presents negative control).
To ensure that these colonies were of fetal and not maternal origin, we analyzed colonies generated from male placentas for the presence of the Y chromosome. The cultured colonies were more than 90% positive for the Y-chromosome, as measured using FISH with Y-chromosome paint (Cambio, UK). This is the upper limit of detection by this technique, and indicated that the colonies grown originate from cells harvested from the fetal side of the placenta. Cells derived from placentas after cryopreservation generated similar results in cell culture (Fig. 5C, D).
Following a 2-week culture in Methocult medium®, cells isolated from placental tissue were further characterized by flow cytometry and showed the presence of myeloid and erythroid lineages. Representative flow cytometry diagrams of cells isolated from culture medium and stained for human CD45 (pan leukocyte marker), CD25 (lymphocyte/monocyte marker), CD51/CD61 (megakaryocyte/platelet marker), and CD235 (human glycophorin A) are shown in Figure 5E. Double staining for human CD45 and CD25 shows the white cell lineage (Fig. 5E2), double staining for CD45 and CD51/CD61 indicates the platelet lineage (Fig. 5E3), and staining for CD235 indicates the erythroid lineage (Fig. 5E4). Together these data indicate that cells from placental tissue differentiated into all hematopoietic lineages in vitro.
To enumerate colony forming units generated from placental cells, we determined the number of colonies formed from cells collected from perfusates before and after AMD 3100 treatment. The colonies were counted 14 days after the start of the culture, and Table 1 indicates the total CFU count as well as the CFU-E and CFU-GM count in these cultures per mL of perfusate used. The appearance of these colonies was as indicated in Figure 5A. The total CFU count generated from 1 mL of perfusate increased approximately 9-fold (from 240 to 1800 per ml) before and 6 hours after AMD 3100 treatment, respectively. Together, these data show that perfusion of human placentas with AMD 3100 leads to the release of CD34+/CD45dim cells in perfusate, that these cells are negative for endothelial markers, and that perfusate contains an abundant number of colony forming cells.
It was reported that in human stem cell donors, infusion of 40–80 µg AMD 3100/kg body weight leads to an increase in hematopoietic stem cells in the peripheral blood that can be harvested and used for stem cell transplant (8). Similarly primate studies showed the use of 1000 µg/kg body weight (9) for the release of these cells into the circulation. While a direct comparison between perfusate volume, placental weight, body weight and circulating blood volume is complex, we attempted to use concentrations of this compound that approximated those used in human and primate studies (8, 9). Our data shows that AMD 3100 at 300 µg/L will release cells into the perfused circulation of the placenta in a timeframe similar for the release of such cells from bone marrow into peripheral blood of human or primate (8, 9). We, therefore, conclude that stem cell niches in placental circulation may act similarly as compared to bone marrow with respect to the ability of AMD 3100 to mobilize hematopoietic stem cells. The use of this compound was recently approved by the FDA for routine use in humans (11). Our data indicate that the use of this compound offers a viable alternative to harvest hematopoietic stem and progenitor cells from placenta to be used in transplant protocols.
To show the potential of placenta-derived cells to engraft after transplantation, immunodeficient mice were injected with the same placental-derived cells that showed the potential to form colony forming units in cell culture. We did not select subpopulations. We argued that the selection of specific subpopulations may have excluded phenotypically yet uncharacterized hematopoietic stem and progenitor cells from placenta. We followed, therefore, a similar approach as pursued with human cord blood transplants in immunodeficient mice (12), and injected an unseparated cell population. Injection of more than 5 × 105 cells/animal IV from digested placental tissue caused lung embolism and death in some animals (data not shown). To avoid this, we used well tolerated IP injections of these cell preparations. Three mice, not exposed to radiation treatment, were injected with cell suspensions derived from human placental chorion. In one mouse, the bone marrow showed the presence of 0.2% human CD45-expressing cells 80 days post-transplant; the two other mice showed no human CD45 expressing cells in either bone marrow or blood 80 days post-transplant.
This low level of engraftment confirms reported data that engraftment in xenotransplants in non-irradiated mice is very low (12). Mice exposed to 2.5 Gy total body irradiation one day before injection with human placenta-derived cells showed engraftment of human cells. Microscopic analysis of murine spleens 80 days post-injection showed cells positive for human CD45 and human HLA-DR (Figure 6A–C). Mouse blood, bone marrow and spleen were further analyzed by flow cytometry for the presence of human cells. Typical flow cytometric results of engraftment in the mouse spleen are shown in Figure 6D. Similarly flow cytometric analysis indicated the presence of human cells in blood and bone marrow.
Figure 6.

Transplantation of human placenta-derived cells in mice. Following transplantation in NOD/SCID mice, human placenta-derived cells gave rise to human cells in murine tissue. A–C: Human cells are visible as lighter cells in this black and white micrograph in which all cells are stained for nuclei. A: Labeling with anti-human HLA-DR antibody, B: labeling with anti-human CD45 antibody, C: negative control (isotype antibody staining). D: Representative flow cytometry diagrams of cells isolated from mouse spleen and double-stained for human surface antigens. D1: Negative control. D2: Double staining for human CD45 (pan leukocyte marker, abscissa) and CD3 (lymphocyte marker, ordinate). D3: Double staining for CD45 (pan leukocyte marker, abscissa) and CD25 (lymphocyte/monocyte marker, ordinate). D4: Double staining for CD45 (pan leukocyte marker, abscissa) and CD51/CD6 (megakaryocyte/platelet marker).
Table 2 shows the typical results of presence of cells that express human CD45 in three individual mice. Blood, bone marrow and spleen were double-positive for CD45/ CD3, CD45/CD25, and CD45/CD51/CD61 cells. While bone marrow of these mice showed the presence of erythroid precursors (positive for CD235), human erythrocytes were not observed in peripheral blood, confirming similar results reported for cord blood transplant (12). Apparently the microenvironments in the hematopoietic organs or the circulation of these mice are not compatible with human adult red cells. Thus, NOD/SCID mice demonstrated chimerism for human blood cells following transplantation of placenta-derived cells similar as reported for transplantation with human cord blood (13). Importantly, our data do not allow direct comparison with cord blood transplants or provide optimized transplant conditions. The experiments were designed to prove the principle that human placental-derived cells are able to engraft immunodeficient animals.
Table 2.
Flow Cytometric Analysis of Immunodeficient NOD/SCID Mice Two Months After Transplant with Human Placental-Derived Cells*
| 1 | 2 | 3 | |
|---|---|---|---|
| Experiment | Percentage of total |
||
| Blood | |||
| hCD45/hCD3 | 0.4 | 0.6 | 3.2 |
| hCD45/hCD51/61 | 1.2 | 0.8 | 0.2 |
| hCD45/hCD25 | 1.0 | 1.0 | 0.3 |
| Bone marrow | |||
| hCD45/hCD3 | 1.4 | 1.0 | 2.9 |
| hCD45/hCD51/61 | 1.0 | 0.9 | 0.2 |
| hCD45/hCD25 | 1.0 | 0.8 | 0.2 |
| Spleen | |||
| hCD45/hCD3 | 3.0 | 1.2 | 3.0 |
| hCD45/hCD51/61 | 3.0 | 1.7 | 1.7 |
| hCD45/hCD25 | 2.8 | 0.7 | 1.1 |
Mice were exposed to 2.5 Gy total body irradiation. The percentage of cells that tested positive for antibodies against human CD45, CD3, CD51/61, or CD25 is indicated.
Together our data shows that the human term placenta harbors hematopoietic cells similar as was reported for murine placenta. Studies performed in mice demonstrated that during development, hematopoietic stem cells originate in aorta-gonad-mesonephros region of the embryo (14–16), and could also be derived from embryonic arteries (17). These hematopoietic stem cells colonize the liver, which subsequently becomes the major site of hematopoiesis at the later stages of gestation in the mouse. The aorta-gonad-mesonephros was considered the primary site of origin of hematopoietic stem cells (18) until placenta was discovered to harbor multiple multi-potent progenitors of blood cells and primitive hematopoietic stem cells in mice (19–21). Alvarez-Silva et al. (19) reported that mouse placenta is several-fold richer in hematopoietic stem cells than liver early in murine development. The early appearance of hematopoietic stem cells suggests that placenta is a hematopoietic organ, and the most likely origin of these cells is the mesoderm of allantois. The rapid proliferation of hematopoietic stem cells suggests unique conditions for hematopoiesis in placenta (5). It is yet unknown whether placenta is the site at which these cells reside temporarily on their journey to the liver, or if placenta is the site of hematopoietic stem cell proliferation (6). Later during mouse development, the numbers of hematopoietic stem cells decline in mouse placenta, while they increase in liver (5), and they express the same phenotype in either tissue. While erythropoiesis in mouse placenta is less pronounced than in liver, precursors of B-lymphocytes appear in murine placenta before they can be found in liver indicating that murine placenta is a myeloid organ (5, 22). Our data show that the human term placenta, similar to the mouse placenta, contains hematopoietic stem- and progenitor cells. We show that it is possible to cryopreserve and thaw whole human placentas, and recapitulate hematopoiesis with cells obtained from human placental tissue. We show that AMD 3100, a compound used to mobilize hematopoietic stem cells in humans, also mobilizes such cells from placenta by perfusion. We believe therefore that the placenta has the potential to serve as a source of hematopoietic cells for transplantation to augment the cellular yield of UCB collections. This approach would be an alternative to protocols that use two unrelated UCB units for allogeneic hematopoietic cell transplantation (3, 4). Several important additional assessments must be completed before a clinical trial using placenta cells becomes practical. Therefore, we have initiated efforts to study the sterility, potency, and reproducibility of large-scale placental harvest and storage. The ability to process placental hematopoietic cells has the potential to transform the clinical application of fetal hematopoietic stem cell transplantation, making this therapeutic option available to many more who might benefit from a transplantation, especially larger children, adolescents and adults.
Acknowledgments
The authors like to thank Bertram H. Lubin for critical review of the manuscript. A short report of our findings was published in April 2008 (23).
This work was supported in part by grants from Philip Morris, Inc., and Philip Morris International to V.S., by funds from the Jean J. Deleage Ph.D. and Josette Deleage Foundation, CHORI Ventures and Grant HL-070583 from the National Institutes of Health to F.A.K.
Footnotes
Note in press: During the printing process, Barcena et al. published an online report (24), which confirms our findings on the presence of hematopoietic cells in term human placenta. Based on their digestive collection procedure, which cannot exclude endothelial lineage, they estimate only 105 CD34+, CD45dim cells in term placenta. The viability of these cells was not tested in a transplant model. In this report we show that perfusion is able to generate an order of magnitude more CD34+, CD45+dim cells from term placenta, that these cells are not of endothelial lineage, and that these cells are viable both in cell culture and in a murine transplant model. Based on the numbers of hematopoietic cells harvested, our study indicates that the term human placenta should be considered as a practical resource for stem cell transplantation.
References
- 1.Ballen KK. New trends in umbilical cord blood transplantation. Blood. 2005;105:3786–3792. doi: 10.1182/blood-2004-10-4125. [DOI] [PubMed] [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 4.Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. Curr Opin Immunol. 2006;18:571–575. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Mikkola HK, Gekas C, Orkin SH, Dieterlen-Lievre F. Placenta as a site for hematopoietic stem cell development. Exp Hematol. 2005;33:1048–1054. doi: 10.1016/j.exphem.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 7.Serikov VB, Fleming NW, Talalov VA, Stawitcke FA. Effects of the ventilation pattern and pulmonary blood flow on lung heat transfer. Eur J Appl Physiol. 2004;91:314–323. doi: 10.1007/s00421-003-0966-4. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, Bridger G, Dunbar CE, Hematti P. AMD-3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–3778. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.M-Reboredo N, Diaz A, Castro A, Villaescusa RG. Collection, processing and cryopreservation of umbilical cord blood for unrelated transplantation. Bone Marrow Transplant. 2000;26:1263–1270. doi: 10.1038/sj.bmt.1702728. [DOI] [PubMed] [Google Scholar]
- 11.United States Food and Drug Administration. [Accessed 2008]; Available at: http://www.fda.gov/cder/foi/label/2008/022311lbl.pdf< http://ga4.org/ct/Cdq5gO61LXKc>.
- 12.Ueda T, Yoshino H, Kobayashi K, Kawahata M, Ebihara Y, Ito M, Asano S, Nakahata T, Tsuji K. Hematopoietic repopulating ability of cord blood CD34(+) cells in NOD/Shi-scid mice. Stem Cells. 2000;18:204–213. doi: 10.1634/stemcells.18-3-204. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, Nakahata T. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood. 2003;102:873–880. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- 14.Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 15.Godin I, Cumano A. The hare and the tortoise: an embryonic haematopoietic race. Nat Rev Immunol. 2002;2:593–604. doi: 10.1038/nri857. [DOI] [PubMed] [Google Scholar]
- 16.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 17.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130:5437–5444. doi: 10.1242/dev.00755. [DOI] [PubMed] [Google Scholar]
- 20.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Melchers F. Murine embryonic B lymphocyte development in the placenta. Nature. 1979;277:219–221. doi: 10.1038/277219a0. [DOI] [PubMed] [Google Scholar]
- 23.Serikov V, Kuypers F. Human term placenta as a source of hematopoietic stem cells. Cell Transplantation and Tissue Engineering. 2008;3:51–56. [Google Scholar]
- 24.Barcena A, Muench MO, Kapidzic M, Fisher SJ. A new role for the human placenta as a hematopoietic site throughout gestation. Repro Sci. 2009;16:178–187. doi: 10.1177/1933719108327621. [DOI] [PMC free article] [PubMed] [Google Scholar]


