Abstract
Although significant progress has been made in understanding the importance of Wnt signaling in the initiation of colorectal cancer, less is known about responses that accompany the reversal of oncogenic Wnt signaling. The aim of this study was to analyze in vivo and in vitro responses to an ‘ideal’ Wnt pathway inhibitor as a model for the therapeutic targeting of the pathway.
A tetracycline-inducible transgenic mouse model expressing truncated β-catenin (ΔN89β-catenin) that exhibited a strong intestinal hyperplasia was analysed during the removal of oncogenic β-catenin expression both in 3D ‘crypt culture’ and in vivo. Oncogenic Wnt signaling was rapidly and completely reversed. The strongest inhibition of Wnt target gene expression occurred within 24 hours of doxycycline removal at which time the target genes Ascl2, Axin2 and C-myc were down-regulated to levels below that in the control intestine. In vitro, the small molecule Wnt inhibitor CCT036477 induced a response within 4 hours of treatment. By 7 days following doxycycline withdrawal, gene expression, cell proliferation and tissue morphology were undistinguishable from control animals.
In conclusion, these results demonstrate that the reversal of Wnt signaling by inhibitors should ideally be studied within hours of treatment. The reversible system described, involving medium throughput in vitro approaches and rapid in vivo responses, should allow the rapid advance of early stage compounds into efficacy models that are more usually considered later in the drug discovery pipeline.
Keywords: Wnt pathway, reversal, colorectal cancer, crypt culture, organoids
Introduction
The intestinal epithelium is organised into two functionally distinct compartments: the invaginating crypts of Lieberkuhn that contain stem cells and villus protrusions formed primarily from differentiated enterocytes.
The Wnt/ß-catenin signaling pathway drives differentiation and proliferation within the crypt compartment.1 In the absence of Wnt ligand, the cytoplasmic ß-catenin protein is targeted for ubiquitin-mediated degradation by a multi-protein complex including the adenomatous polyposis coli (APC) tumor suppressor protein.2 Wnt signaling inhibits ß-catenin turnover, leading to its accumulation and translocation to the nucleus where it complexes with the T-cell factor/lymphoid enhancing factor (TCF/LEF) transcription factors to induce transcription of genes including ascl2, axin2 and c-myc.3
The importance of Wnt signaling for intestinal development has been demonstrated using both mouse models and organoid culture models.4-7 Inducible deletion of ß-catenin led to a rapid and complete destruction of the crypt compartment that correlated with a reduction in cell proliferation and the terminal differentiation of stem cells.4 The loss of intestinal crypts was similarly observed in transgenic mice lacking Tcf4 and in animals over-producing the extracellular Wnt signaling inhibitor Dickkopf-1 (Dkk-1).5, 6 In vitro, the Wnt pathway activator R-spondin1 was required for the survival and development of intestinal organoids in culture.7
Mutations that constitutively activate canonical Wnt signaling constitute the most prevalent genetic abnormality in human colon cancer where more than 90% of tumours contain mutations that activate the pathway.8 Mutations to ß-catenin prevent the phosphorylation of N-terminal serine and threonine residues (S33, S37, T41, S45) that are required for turnover leading to ligand-independent expression of the Wnt target genes.9
Harada et al. showed that Cre-loxP deletion of exon 3 of ß-catenin (including S33, S37, T41, S45 sequences) induced the formation of adenomatous intestinal polyps.10 Wong et al. failed to identify tumour formation following expression of a ß-catenin transgene lacking the 89 N-terminal amino acids (ΔN89ß-catenin), but did observe increased crypt cell proliferation and apoptosis accompanied by a slowing of the cell migration within the crypt/villus axis.11 Romagnolo et al. created a transgenic mouse model expressing a ΔN131 ß-catenin protein and identified a similar increase in apoptosis along with multifocal dysplastic regions in the small intestine.12 Taken together, these results confirm the importance of the Wnt pathway and suggest that stabilization of ß-catenin can be an initial step leading to colorectal cancer. While the initiation of colorectal cancer following ß-catenin mutation has been studied in detail, the more clinically important process of blocking oncogenic signaling has not been followed.
Here, we have characterised for the first time the regression of an intestinal hyperplasia using a doxycycline-dependent mouse model expressing mutant ß-catenin. Timecourse analyses showed a surprisingly rapid restoration of the normal intestinal phenotype. In crypt organoids in vitro, mutant ß-catenin was required for Wnt gene expression and cell survival. Transcriptional responses to the inhibition of Wnt signaling were extremely rapid. Loss of mutant ß-catenin in vivo resulted in an active inhibition of target gene expression within 24 hours while a small molecule inhibitor of Wnt signaling blocked targets within 4 hours. The combined in vitro/in vivo models we describe should allow the early assessment of compound efficacy in drug discovery programmes and emphasizes the importance of studying anti-Wnt signaling therapeutics at very early times after administration.
Results
Expression of the tetracycline-inducible ΔN89ß-catenin in vitro and in vivo
A doxycycline-responsive promoter regulating expression of ΔN89-βcatenin was targeted downstream of the collagen 1a1 locus by FLP-FRT site directed recombination.13 The targeted ES cells already contained the doxycycline-regulated transactivator M2-rtTA gene under the control of the constitutively active ROSA26 promoter (Figure 1A).13 Three out of three analyzed clones were found to have integrated the transgene correctly and expression of the ΔN89ß-catenin protein was dependent on doxycycline addition (Supplementary figure 1A and B). Mice were generated by diploid blastocyst injection. Following 10 days treatment with 2mg/ml doxycycline in the drinking water, the expression of ΔN89ß-catenin protein was noted in different tissues in mice, including skin, liver, lung, stomach and intestine (Supplementary figure 1C). The global tissue transgene expression didn’t affect the animals over the time-scale of the assays since the mice were viable after 15 days of treatment.
Figure 1.
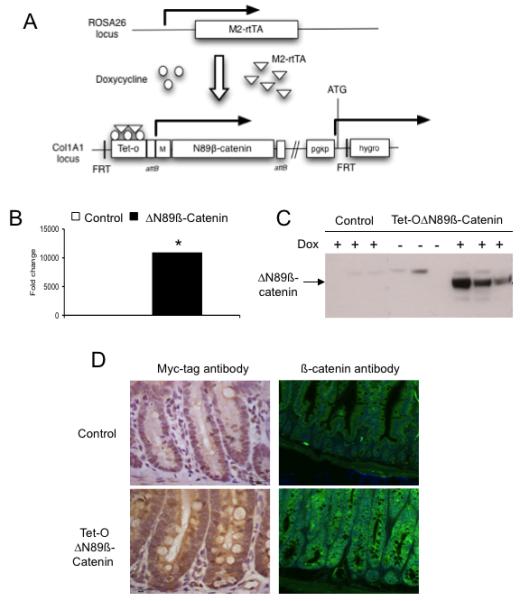
Expression of ΔN89β-catenin following doxycycline treatment in vivo. (A) Schematic representation of the system used to express ΔN89β-catenin in ES cells and mice. A cassette containing ΔN89-βcatenin under control of the doxycycline-responsive promoter was flipped downstream of the collagen locus. Tet-o, tetracycline/doxycycline responsive operator; attB, gateway recombination site; M, myc protein tag; FRT, flippase recognition target site; pgkp, hygro, hygromycin resistance gene; ATG, transcriptional initiation codon; M2-rtTA, M2 reverse tetracycline transactivator. (B) ΔN89β-catenin mRNA level in the intestine of control and Tet-OΔN89β-catenin mice after 10 days doxycycline treatment (*, p<0.05, Mann Whitney, n=4). (C) Western blot analysis of myc tagged ΔN89β-catenin protein in the intestine after 10 days doxycycline treatment. (D) Detection of myc tagged ΔN89β-catenin by immunohistochemistry (left) and total β-catenin by immunofluorescence (right) following 10 days doxycycline treatment. Dox: doxycycline.
The intestinal expression of ΔN89ß-catenin mRNA in Tet-OΔN89β-catenin-rtTA mice was strongly induced (11,000-fold) 10 days after doxycycline treatment (p=0.03, Mann Whitney, n=4; Figure 1B). Mutant β-catenin mRNA was undetectable in the intestine samples from untreated mice (Supplementary figure 2A). Western blot analysis of intestinal tissues using the Myc epitope tag antibody showed the presence of ΔN89β-catenin protein in doxycycline treated Tet-OΔN89β-catenin-rtTA mice, but not in control or untreated mice (Figure 1C). Immunolocalisation showed cytoplasmic, but not nuclear, ΔN89β-catenin expression within both the crypt and villus compartments of doxycycline-treated Tet-OΔN89β-catenin-rtTA mice (Figure 1D and Supplementary figure 2B). Nuclear accumulation of β-catenin was noted in the crypts of doxycycline-treated Tet-OΔN89β-catenin-rtTA mice (Figure 3C) and was similarly observed in Cre+APCfl/fl mice following β-napthoflavone intra-peritoneal injections (Supplementary figure 2C).
Figure 3.
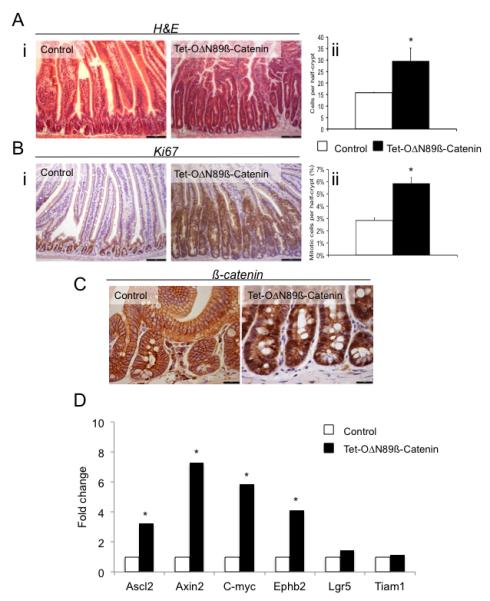
Wnt pathway up-regulation in the intestine induces crypt compartment enlargement and proliferation. All samples were taken following 10 days of doxycycline treatment (Ai) Hematoxylin and Eosin (H&E) stained sections of intestine from Tet-OΔN89β-catenin mice. (Aii) Number of cells, counted using H&E stained slides, in the crypt compartment. (Bi) Ki67 stained sections of intestine from Tet-OΔN89β-catenin mice. (Bii) Number of mitotic cells, scored using H&E stained sections in the crypt compartment. (C) Immunohistochemistry for total β-catenin in Tet-OΔN89β-catenin mice. (D) qRT-PCR analysis of Wnt target genes in the intestine of control and Tet-OΔN89β-catenin mice. The level of ΔN89β-catenin transcript is expressed relative to that in control. *Significant difference compared to control (p<0.05, Mann-Whitney, n=4).
Taken together, these observations show low background levels and a strong induction of ΔN89β-catenin expression following doxycycline treatment.
Loss of ΔN89ß-catenin expression after doxycycline removal
The timecourse of responses to the removal of ΔN89β-catenin was determined by removing doxycycline from the water of Tet-OΔN89β-catenin mice that had been induced for 10 days. mRNA and protein expressions were assessed after 1, 2, 7 or 14 days of doxycycline removal. ΔN89ß-catenin mRNA reverted to control levels within 1 day of doxycycline removal (p=0.11, Mann Whitney, n=4) (Figure 2A). The rapid loss of ΔN89β-catenin mRNA expression was not unexpected since the half-life of doxycycline in mouse serum is estimated to be 3 hours, suggesting that at least 99% of serum doxycycline would be excreted after 1 day.14 Western blot analyses also showed a rapid loss of ΔN89β-catenin protein since protein was not detectable 1, 2, 7 or 14 days following doxycycline withdrawal (Figure 2B). The rapid loss of ΔN89β-catenin, despite it being a stabilized form of ß-catenin, was compatible with the previous work since the half-life of truncated ß-catenin in melanoma cells has been estimated to be approximately 4.5 hours by comparison with 2 hours for the wild-type protein.15 The rapidity of the biological response is clear, but finer details of the dyamics are dependent on a complex interplay between the ΔΝ89β-catenin mRNA decay rate, intestinal doxycycline levels and the threshold for Tet-O-promoter activity in each intestinal cell type.
Figure 2.
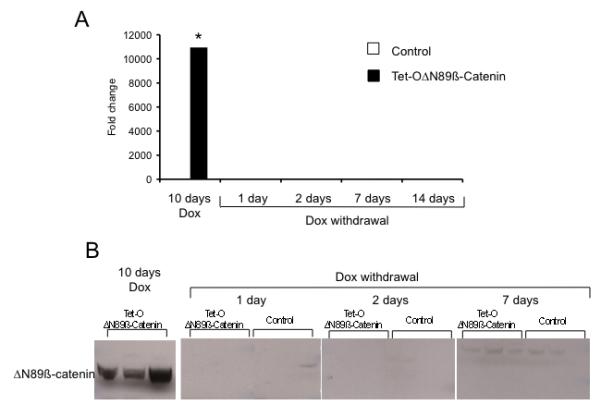
Loss of ΔN89β-catenin expression following doxycycline removal in the intestine. (A) ΔN89β-catenin mRNA levels after 10 days doxycycline treatment and 1,2,7 and 14 days following doxycycline removal. The level of the transcripts is expressed as fold of control (*, p<0.05, Mann Whitney, n=4). (B) ΔN89β-catenin protein expression as detected by western analysis for the myc tag after 10 days doxycycline treatment and 1,2 and 7 days following doxycycline withdrawal. Dox: doxycycline.
Up-regulation of stabilized β-catenin strongly modifies the crypt compartment
Histological analysis of the intestine samples from 10 day-induced ΔN89β-catenin mice revealed an enlarged crypt compartment (Figure 3Ai). The number of cells per half-crypt was 29.0±4.0 in induced versus 15.4±0.4 in control animals (p=0.03, Mann Whitney, n=4) (Figure 3Aii). The expansion of the crypt compartment was associated with increased cell proliferation. Crypts from induced Tet-OΔN89β-catenin-rtTA mice showed raised levels of the S-phase marker Ki-67 and higher number of mitotic figures (5.84±0.49% versus 2.83±0.2% in control half-crypts p=0.03, Mann Whitney, n=4; Figure 3B and supplementary figure 3). Apoptotic cell death as followed by active caspase-3 staining trended higher in doxycycline treated crypts, but this was not statistically significant (38.8±22.0 positive cells per 50 half crypts for Tet-OΔN89β-catenin-rtTA mice and 5.6±1.8 for control mice; p=0.19, Mann Whitney, n=5; Figure 5A and 5B). Active caspase-3 cells in mutant crypts were localized all along the enlarged crypt axis, in contrast with restricted apoptosis in the bottom of control crypts (Supplementary figure 4). Intestinal differentiation processes were partly altered following doxycycline treatment in Tet-OΔN89β-catenin-rtTA mice. While the percentage of goblet and paneth cells per crypt was not changed (Supplementary figure 5), the cellular localization of paneth cells within the crypt compartment was strongly modified and was now distributed throughout the crypt-like area rather than being confined to their normal position at the crypt base (Supplementary figure 6).
Figure 5.
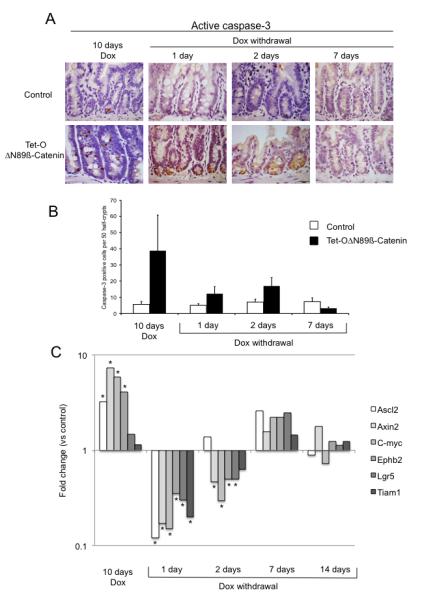
Restoration of the normal intestinal phenotype is associated with raised levels of apoptosis and down-regulation of Wnt target gene expression. (A) Apoptosis in the intestine as assessed by active caspase-3 staining (B) Number of apoptotic cells, scored using active caspase-3 stained sections in the crypt compartment from control and Tet-OΔN89β-catenin mice after 10 days doxycycline treatment and 1, 2 and 7 days following doxycycline withdrawal. (C) qRT-PCR analysis of Wnt target genes in the intestine of control and Tet-OΔN89β-catenin mice treated with doxycycline for 10 days and 1, 2 and 7 days following doxycycline withdrawal. The level of the transcripts is expressed relative to that in control animals. *Significant difference compared to control (p<0.05, Mann-Whitney, n=4-5). Dox: doxycycline.
After 10 days doxycycline treatment, the body weight of Tet-OΔN89β-catenin-rtTA mice was reduced by 13.8% compared to control mice (26.1±0.6g for control, 22.5±0.8g for mutant; p<0.01, Mann Whitney, n=22-25; Supplementary figure 7).
To confirm the involvement of the Wnt pathway in these profound changes, the accumulation of ß-catenin in the nucleus of crypt cells was analysed by immunohistochemistry using an antibody recognizing both endogenous and truncated β-catenin and the expression of known Wnt target genes was studied by qRT-PCR. As expected, after 10 days doxycycline treatment, a high number of cells in hyperproliferative crypts showed nuclear accumulation of ß-catenin in Tet-OΔN89β-catenin-rtTA mice (Figure 3C). In addition, the majority of the Wnt target genes (Ascl2, Axin2, c-myc and Ephb2 but not Lgr5 and Tiam1) were up-regulated (Figure 3D). The level of endogenous β-catenin mRNA was not altered following 10-days doxycycline treatment in Tet-OΔN89β-catenin-rtTA mice (data not shown).
In contrast to the phenotype seen in intestinal crypts, we did not observe any changes in the villus compartment 10-days after doxycycline treatment of Tet-OΔN89β-catenin-rtTA mice even although ΔN89β-catenin protein was expressed in the villus (Figure 1D). The number of cells per half-villus was similar in control (86.7±4.3) and Tet-OΔN89β-catenin-rtTA (98.7±4.8) mice (p=0.09, Mann Whitney, n=5) (data not shown) and the villus cell marker alkaline phosphatase was expressed at similar levels in both groups of mice (data not shown). Lastly, no Ki-67 stained proliferating cells were detected in ΔN89β-catenin expressing or control villus tissues (Figure 3B).
Reversal of the Wnt pathway induces rapid regression of the crypt compartment
The effect of reversal of the Wnt pathway was rapid. After one day of doxycycline withdrawal, the crypt compartment of Tet-OΔN89β-catenin-rtTA mice was reduced in size and by day 7, crypts from mutant mice were histologically indistinguishable from control crypts (Figure 4A). No statistically significant differences in cell numbers were noted between control and mutant mice after 1, 2, 7 or 14 days of doxycycline removal (Figure 4B).
Figure 4.
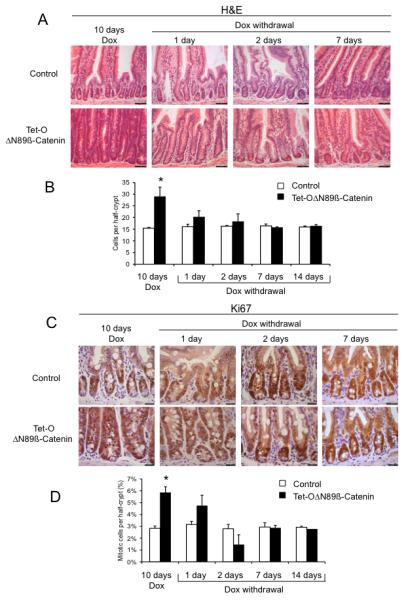
Reversal of the Wnt pathway restores a normal phenotype in the intestine. Tet-OΔN89β-catenin mice were doxycycline-treated for 10 days. Doxycycline was then withdrawn for the number of days shown. (A) Reversal of intestinal hyperplasia following doxycycline withdrawal as assessed by the histology of H&E stained sections. (B) Size of the crypt compartment as assessed by counting half-crypt size. (C) Cell proliferation in the intestine as assessed by Ki67 staining. (D) Mitotic cell number per half crypt. *Significant difference compared to control (p<0.05, Mann-Whitney, n=4). Dox: doxycycline.
The rapid reduction in crypt cell number was associated with an equally rapid reduction in crypt cell proliferation. After 1-day without doxycycline, the Ki67-positive compartment in Tet-OΔN89β-catenin-rtTA mice was still enlarged by comparison with controls (Figure 4C), but no significant difference were noted between the number of Ki67-positive cells and mitotic figures in crypts from control and Tet-OΔN89β-catenin-rtTA mice (Figure 4D and supplementary figure 3). Two days after doxycycline removal, no significant difference were observed between the number of Ki67-positive cells and mitotic figures in control and Tet-OΔN89β-catenin-rtTA mice (Figure 4C, 4D and supplementary figure 3). After 7 and 14 days without doxycycline, the number of Ki67-positive and mitotic cells in the intestine was similar between control and Tet-OΔN89β-catenin-rtTA mice (Figure 4C, 4D and supplementary figure 3). The rapid decrease of cell number in the crypt compartment of Tet-OΔN89β-catenin-rtTA mice following doxycycline removal was associated with raised levels of apoptosis (Figure 5A and 5B). The number of caspase-3 positive cells in 50 half-crypts, including data from both 1-day and 2-day doxycycline removal, was 14.6±3.1 for Tet-OΔN89β-catenin-rtTA mice and 6.0±1.1 for control mice (p=0.03, Mann Whitney, n=10; Figure 5B). The localization of active caspase-3 cells in mutant crypts was mainly observed at the bottom of the crypt following 1-day and 2-day doxycycline removal (Supplementary figure 4). The localization of paneth cells in mutant mice reverted into a normal position at the crypt base 7 days following doxycycline removal (Supplementary figure 6).
The removal of doxycycline was associated with profound alterations of gene expression in the intestine. Quantitative RT-PCR analysis showed that multiple Wnt target genes were actively down-regulated 1 day after doxycycline removal in Tet-OΔN89β-catenin-rtTA by comparison with controls (Figure 5C). After 2-days, Axin2, C-myc, Ephb2 and Lgr5 were still significantly repressed while expression levels were similar between mutant and control animals by 7 and 14 days following doxycycline withdrawal. The restoration of normal intestinal morphology by 7 days after doxycycline removal correlated with a recovery in body weight (Tet-OΔN89β-catenin-rtTA 26.4±0.4g versus control 27.0±0.5g, p=0.67, Mann Whitney, n=8) (Supplementary figure 7).
Doxycycline-dependent activation of Wnt signaling in intestinal organoids
To develop a system that allowed a kinetic analysis of early changes during oncogenic reversal, the tetracycline-inducible model was adapted for use with a recently described in vitro crypt culture technique.7 The potential toxicity of doxycycline was first analysed using Axin2-LacZ organoids in the presence of R-spondin. At 2μg/ml, doxycycline did not affect normal organoid growth or expression of Axin2-LacZ in the crypt compartment (Supplementary figure 8).
As previously described, the growth of the crypt culture organoids was dependent on the presence of the Wnt signaling regulator R-spondin (Figure 6A).7 Intestinal organoids cultured in the absence of R-spondin failed to develop crypt domains and instead formed spherical structures at 3 days that subsequently degenerated with high levels of cell death from 4-5 days (Figure 6A). However, in the absence of R-spondin, treatment with doxycycline (2μg/ml) rescued the organoids from cell death (Figure 6A). In this context, doxycycline induced expression of high levels of ΔN89ß-catenin mRNA (29,000-fold; p=0.003, t-test, n=3) and activated Wnt target gene expression (Figure 6B and 6C). Interestingly, the doxycycline/ΔN89β-catenin-rescued organoids formed abnormal crypts that lacked the characteristic tear-shaped structures observed in normal tissues and control organoids. These doxycycline-rescued structures contained goblet cells, in contrast with Cre+APCfl/fl organoids (Supplementary figure 9). In addition, the doxycycline-stimulated structures showed low but uniform levels of Axin-2-lacZ expression as compared to the localized expression of Axin2-lacZ in the lower crypt region of uninduced structures treated with R-spondin (Figure 6A).
Figure 6.
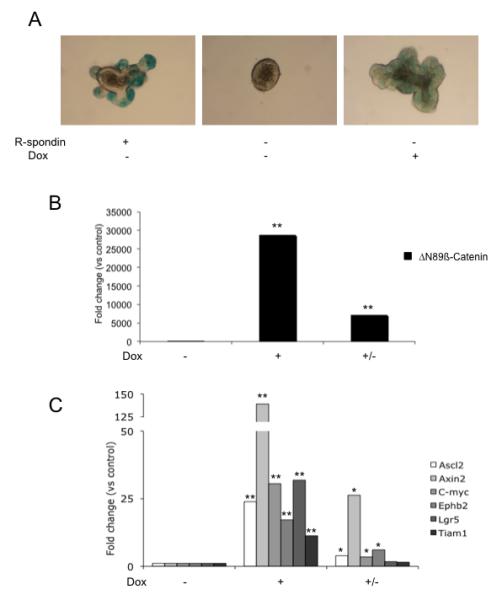
Doxycycline-dependent activation of Wnt signaling in Tet-OΔN89β-catenin organoids. (A) Tet-OΔN89β-catenin Axin2-LacZ organoids cultured for 72 hours with R-spondin, in the absence of R-spondin or with doxycycline and stained for β-galactosidase. Original magnification x20. (B) ΔN89β-catenin mRNA levels and (C) Wnt target gene mRNA levels in Tet-OΔN89β-catenin organoids without doxycycline treatment (−), after 72 hours doxycycline treatment (+) or after 48 hours doxycycline treatment followed by 24 hours without doxycycline (+/−). The level of the transcripts is expressed as fold of control. Significant difference compared to control (* p<0.05, ** p<0.01, n=3, paired t-test). Dox: doxycycline.
To recapitulate the in vivo effect of doxycycline removal, doxycycline-containing culture medium was removed from the matrigel-embedded structures for 24 hours after an initial 48 hours of doxycycline treatment. Under these conditions ΔN89ß-catenin mRNA levels were significantly reduced (p=0.02, t-test, n=3) leading to a strong reduction in Wnt target gene expression (Figure 6B and 6C).
Tet-OΔN89β-catenin-rtTA organoids, a useful in vitro system to screen for Wnt inhibitors
CCT036477 is a recently identified small-molecule inhibitor of Wnt-signaling that induced head and tail patterning defects in zebrafish embryos and inhibited the growth of colon cancer cell lines.16 Tet-OΔN89β-catenin-rtTA organoids were treated with 2μg/ml doxycycline for 72 hours, in the presence of 20uM CCT036477 for 4, 8, 12 and 24 hours. No effect of CCT036477 was noted on the levels of 3 control RNAs (Supplementary figure 10). The strongest inhibitory response was observed after 4 hours of CCT036477 exposure. The majority of the Wnt target genes (Ascl2, Ephb2, Lgr5 and Tiam1) were down-regulated by 60-90% (Figure 7). Ascl2, Ephb2 and Lgr5 levels after 4 hours of compound treatment were similar to that following 24 hours of doxycycline removal suggesting that CCT036477 treatment was as effective as lowering β-catenin levels by 75% (Figure 7). However, CCT036477 didn’t strongly affect Axin2 or C-myc expression. The reason that all Wnt targets were not similarly affected is unclear but may be related to the presence of multiple cell types that have different thresholds for Wnt responsiveness. After 8 and 12 hours of CCT036477 exposure, inhibitory effects decreased and were weak by 24 hours of exposure (Figure 7). The reason for this loss of response is unclear but may be related to the stability of the compound in culture or to a time-dependent oscillation of transcript levels since a similar time-dependence was observed in vivo following doxycycline-removal (Figure 5C).
Figure 7.
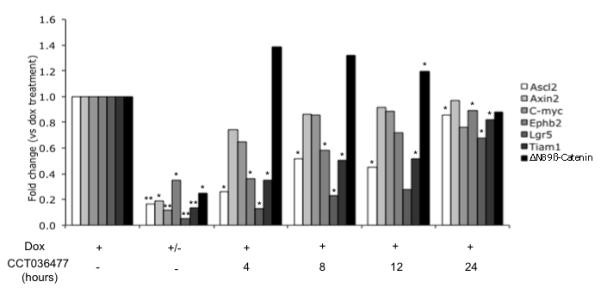
CCT036477 small molecule inhibitor down-regulates Wnt target gene expression in doxycycline-treated Tet-OΔN89β-catenin organoids. qRT-PCR analysis of Wnt target genes in Tet-OΔN89β-catenin organoids following 72 hours doxycycline treatment (+), 48 hours doxycycline treatment followed by 24 hours without doxycycline (+/−) or 72 hours doxycycline treatment with 20 μM CCT036477 for 4, 8, 12 or 24 hours. The level of the transcripts is expressed as fold of doxycycline-treated condition. Significant difference compared to control (* p<0.05, ** p<0.01, n=3, paired t-test). Dox: doxycycline.
A similar type of response was observed in culture with another molecule that has more stable properties. Interestingly, this small-molecule Wnt inhibitor recapitulated the in vitro responses in the in vivo system (unpublished data).
Discussion
The majority of human colorectal cancers are initiated by inappropriate activation of the Wnt pathway.8 Mechanisms that alter Wnt signaling involve the inactivation of the APC tumour suppressor or the stabilization of ß-catenin by mutation of residues within its N-terminus.17, 18 Several genetically encoded mouse models of Wnt pathway deregulation in the intestine have been established.11, 19, 20 Both forced expression of ΔN89β-catenin or conditional Apc deletion result in enlarged crypt compartments and increased levels of cell proliferation and apoptosis.11, 19, 20 In the present study, doxycycline-dependent expression of ΔN89β-catenin was associated with comparable alterations suggesting that the doxycycline-inducible model system recapitulated early abnormalities that lead to colorectal-cancer. The expression of ΔN89β-catenin did not change villus morphology or proliferation, suggesting that differentiated villus cells are unable to re-enter the cell cycle or to de-differentiate. Similar observations were made following ΔN89β-catenin expression in villus cells directed by the Fabp1 promoter.11
The aim of the in vivo study was to analyse the consequences of Wnt pathway inhibition in the mouse intestine since the removal of doxycycline should behave as an ‘ideal’ therapeutic modulator and should reverse oncogenic signaling, but not interfere with the restoration of normal crypt function. A combination of histological and molecular markers showed that ongoing ΔN89β-catenin expression in mice was necessary for the maintenance of the hyperplastic crypt phenotype and that loss of expression initiated a rapid cascade of responses that restored normal crypt morphology within 7 days (Figure 4). Of particular note, Wnt target gene expression rapidly dropped to a level 3 to 8 times lower than the control epithelium within 24 hours. A feature of Wnt signaling is the role of multiple Wnt transcriptional targets (Eg. Dkk1, Axin2, ß-TrCP, Nkd, TCF-1) in negative feedback inhibition of signaling through mechanisms including the blocking of Wnt receptor function and the stabilisation of the destruction complex.21, 22 Here, we speculate that feedback Wnt inhibitor proteins accumulate following ΔN89β-catenin expression and act to repress basal levels of Wnt signaling below levels in normal tissues after doxycycline removal. Interestingly, the pattern of Wnt target gene expression observed in Fig.5B shows characteristics of a time-dependent dampened wave that might result a feedback inhibition mechanism that showed a degree of overcompensation.
An important question concerns the mechanism(s) involved in the rapid loss of crypt cells after Wnt pathway inhibition. In our study, about 70% of excess crypt cells were lost within 24 hours of doxycycline withdrawal, suggesting that even earlier experimental time points might be useful to understand the earliest processes. We noted raised levels of apoptosis in the intestine 1 and 2 days after doxycycline removal. Raised levels of apoptosis were recently observed following treatment of acute myeloid leukemia cells with small molecule inhibitors of Wnt signaling and were associated with decreased expression of Wnt target proteins, such as C-myc.23 Similarly, treatment of numerous cancer cell lines, including breast, lung, mesothelioma and sarcoma, with a monoclonal anti-Wnt-1 antibody induced apoptosis and down-regulation of Wnt pathway targets.24 These results suggest that the down-regulation of Wnt signaling enhances intestinal apoptosis during the reversal of the hyperplastic intestinal phenotype. However, it is important to note that the relationship between Wnt pathway activity and apoptosis is not always clear since Kuhnert et al. observed that Dkk-1 adenoviral expression in mice repressed intestinal epithelial proliferation and Wnt target gene expression, but did not modify apoptotic levels.6
Cell proliferation in the intestine was rapidly suppressed following doxycycline withdrawal in Tet-OΔN89β-catenin-rtTA mice. However, although the hyperplastic phenotype was rapidly reversible, it is unknown whether polyp development or colorectal cancers generated by multiple cooperating mutations would be similarly dependent on continued Wnt signaling. Recently, using colorectal cancer cells containing a doxycycline-inducible ß-catenin shRNAs system, Scholer-Dahirel et al. demonstrated that sustained Wnt pathway activation was required for the maintenance of APC-mutated colorectal tumors in immuno-compromised mice.25 Similarly, Gunther et al. used a doxycycline-inducible approach to conditionally express the Wnt1 oncogene in the mammary gland.26 They noted that Wnt1-initiated mammary tumors completely regressed following doxycycline withdrawal. Coste et al. demonstrated that an oncogenic β-catenin allele that was expressed sporadically in vivo did not result in intestinal tumourigenesis and speculated that β-catenin’s oncogenic effect may require the presence of a field of mutant cells termed βfield cancerizationβ.27 Studies of mutant ß-catenin in melanoma by contrast suggest that a ‘just right’ level of ß-catenin expression (neither too high or low) may be necessary for tumourigenesis.28 Future studies will be needed to determine whether intestinal tumors derived in the Tet-OΔN89β-catenin-rtTA background will be similarly dependent on the maintenance of mutant ß-catenin expression.
The aim of the in vitro study was to develop a crypt culture system that allowed a kinetic analysis of early changes during oncogenic reversal. Tetracycline-driven ΔN89β-catenin expression replaced the requirement for the Wnt pathway activator R-spondin in the growth of intestinal organoids, presumably by inducing expression of Wnt target genes required for cell survival. However, ΔN89β-catenin over-expression did not fully recapitulate the normal crypt morphology induced by R-spondin. Several explanations may account for the different phenotypes. Firstly, doxycycline-induced ΔN89β-catenin expression was probably expressed throughout the cultured crypts while R-spondin has been shown to enhance endogenous Wnt signals that may be localised to paneth cells at the base of the crypt.29, 30 This contention is supported by the observation that Axin-2-lacZ was localized in R-spondin treated structures but was broadly expressed when replaced by doxycycline-induced ß-catenin expression. Secondly, ΔN89β-catenin over-expression may insufficient to a high enough level of Wnt target gene expression to drive a normal development program. The developmental and oncogenic outcomes of deregulated Wnt signaling vary from tissue to tissue and can be highly dependent on the absolute level of TCF-dependent transcriptional activity.31 Lastly, expression of ΔN89β-catenin may be unable to rescue branches of non-canonical Wnt signaling that are responsible for morphological aspects of crypt development.32, 33
Using the in vitro system a small molecule Wnt pathway inhibitor was shown to down-regulate ΔN89β-catenin-driven Wnt target gene expression. The early time-point of maximal Wnt-target transcriptional response (4 hours) emphasises the importance of early analysis of drug action since the later responses may be masked by compound clearance/degradation. The early timescale of responses in vitro corresponded well with the rapid responses observed in vivo where maximal responses were observed within 24 hours of doxycycline removal. Importantly, at later times in vivo, responses to compound may be obscured by a rebound in target transcripts levels that accompanies the restoration of normal levels of signaling. In conclusion, we have shown that Wnt pathway up-regulation induced a proliferative response in the intestine, and that the reversal of Wnt signaling rapidly restored the initial structure of the intestine. These observations provide evidence that down-regulation of the Wnt pathway has potent anti-hyperplastic activities in vivo. Targeting oncogenic mutations involving the Wnt pathway, using a specific therapy, may therefore reverse an abnormal phenotype in vivo. As observed in this Tet-O mouse model, the effect of a βgold standardβ treatment targeting the Wnt pathway should induce an almost immediate response, with the first effects on target gene expression being observed within 24 hours. Similarly, the in vitro system demonstrates that transcriptional response associated with the inhibition of Wnt signaling should be studied at early timepoints. These combinations of the in vitro and in vivo models provide a complementary pair of approaches for the discovery of inhibitors of Wnt signaling and intestinal tumourigenesis. For the first time these constitute a reversible system that can be used to characterize the early-stage compound efficacy before time-consuming medicinal chemistry is deployed to optimize hit compound pharmacodynamics.
Materials and Methods
Generation of tet-O-ß-catenin mutant mice
The pBS31 flp-in vector was engineered to contain the following four cassettes. The pgk promoter followed by an ATG and an FRT site. A splice acceptor double polyA cassette derived from the ROSA26 target vector. The CMV minimal promoter containing tetracycline response operator sequences from pTETOP and an SV40 polyA signal.13, 34, 35 This aid rapid cloning of longer cDNAs, pBS31pgkATGFRTTet-O was converted into a gateway destination vector at the EcoR1 site. Human N-terminal myc-tagged ΔN89β-catenin cDNA was introduced into the vector by gateway cloning. Constructs were introduced into KH2 embryonic stem (ES) cells containing M2-rtTA under control of the endogenous ROSA26 promoter as described previously.13 Mice were generated by diploid blastocyst injection into 3.5dpc blastocysts from C57/Bl6-J females. Approximately 30 injected blastocysts were transferred into pseudopregnant CD1 recipient females (Transgenic Facility, Wales Gene Park). Chimeras were crossed with C57/BL6-J mice. The full method is described in supplementary method 1.
Crypt isolation and culture
Organoid culture was conducted as previously described.7, 36 Crypts were released from small intestine tissue fragments by incubation for 30 min at 4°C in 2mM EDTA-PBS solution. Isolated crypts were mixed with 50 μl of Matrigel (BD Biosciences), seeded in 24-well plates and 500 μl of crypt culture medium (DMEM/F12 containing 50 ng/ml EGF (Sigma), 100 ng/ml Noggin (Peprotech) and 600 ng/ml R-spondin 1 (R&D)) was added. Organoids were maintained in a 37 °C humidified atmosphere under 5% CO2.
Doxycycline induction and small molecule inhibitor treatment
Induction of ΔN89β-catenin expression in ES cells was achieved by culturing cells with 2μg/ml doxycycline (Sigma) for 2 days. For in vivo induction, eight-week-old mice were fed 2mg/ml doxycycline and 10mg/ml sucrose in their drinking water for 10 days. Doxycycline was then withdrawn for periods up to 14 days (n=4-6 for each group). Mice negative for Tet-OΔN89 β-catenin and/or rtTA were used as controls. For in vitro experiments, organoids were seeded in 24-well plates and 500μl of R-spondin 1-free crypt culture medium containing 2μg/ml doxycycline was added. After 2 days, culture medium was replaced by fresh medium containing 2μg/ml doxycycline (for 24 hours), both 2μg/ml doxycycline (for 24 hours) and 20μM CCT036477 (for 4, 8, 12 and 24 hours) or no doxycycline (for 24 hours) (n=3).
β-Galactosidase staining
Tet-OΔN89β-catenin-rtTA mice were crossed with Axin2-LacZ mice, a direct reporter of canonical Wnt pathway activity.37 β-Galactosidase staining was conducted as previously described.20
Immunoblot analysis
Proteins were extracted by scraping lysed ES cells from a confluent 10cm2 plate or by crushing liquid nitrogen-frozen intestinal tissues using a mortar and pestle. Equal amounts of cellular protein (80μg) were separated on a 4-12% Bis Tris Gel (Invitrogen) and transferred onto nitrocellulose membranes. Immunoblotting utilised antibodies against the myc epitope tag (1:1000, Sigma) and GAPDH as a protein loading control (1:5000, Chemicon).
Immunohistochemistry and Immunofluorescence
Tissues were fixed in 10% formalin, paraffin embedded and cut into 5μm sections for immunostaining. Briefly, slides were boiled for 20 min in antigen retrieval solution (Dako) followed by blocking in 0.3% hydrogen peroxide for 5 min, avidin/biotin solutions for 30 min (Vector) and goat serum for 45 min (Vector). Slides where then incubated overnight with rabbit primary antibodies directed against Ki67 (1/500, Millipore), lysozyme (1/400, Dako), mucin2 (1/500, Santa Cruz), myc epitope tag (1/1500, Sigma), ß-catenin (1/100, Cell Signaling) or active caspase 3 (1/750, R&D Systems). After 1-hour incubation with rabbit biotinylated secondary antibody, detection was carried out using a Vectastain ABC kit (Vector) for 30 min and visualized with DAB substrate chromogen for 10 min (Sigma).
For immunofluorescence, slides were treated as described above and were incubated overnight with anti-ß-catenin antibody (1/1000, BD Transduction Laboratories). Slides were then exposed to Alexa Fluor® 488 goat anti-mouse IgG (1/1000, Invitrogen) for 20 min and DAPI stained (1/5000). Fluorescent images were taken on an IX-71 inverted microscope (Olympus) via an ORCA-ER camera utilising SimplePCI software (Hamamatsu Corporation). Illumination intensity, exposure, offset and gain settings were maintained between samples.
Scoring apoptosis and mitosis
Mitotic cells on H&E-stained sections were scored as previously described.38 Fifty half-crypts were counted for each mouse (n=4-5 per group). In addition, the number of cells per villus was counted (50 half-villus, n=4-5 per group). Alkaline phosphatase staining was performed as described by the manufacturer (Fast Red Substrate System, Dako).
qRT-PCR
Three to five mice of each genotype were harvested after 10-day doxycycline exposure and at 1, 2, 7, and 14 days following doxycycline removal. RNA was extracted from a 1cm portion of small intestine taken approximately 7 cm from the stomach and frozen in liquid N2. It is worth noting that intestinal RNA contains both crypt, villus and stromal/muscle tissues that dilute stem cell markers such as Lgr-5. The tissue was homogenized and RNA extracted using the RNeasy mini kit (Qiagen). DNA contamination was removed using the TurboDnase kit (Ambion). For in vitro experiments, matrigel was mechanically dissociated and organoids were pelleted by centrifugation at 1000 rpm for 3 min. RNA was extracted using the RNeasy micro kit (Qiagen).
For both in vivo and in vitro experiments, 1μg of total RNA was reverse transcribed using random hexamers (Promega) and Superscript III reverse transcriptase (RT; Invitrogen). The qRT-PCR was performed using FastSybr Green Master Mix kit (Applied Biosystems). Duplicate samples were analysed on a StepOnePlus (Applied Biosystems) qRT-PCR machine using the fast protocol. ß-Actin, ß-2 microglobulin and keratin 20 primers were used to equalize the data. Threshold cycle (CT) values and the 2-ΔΔCT method were used to calculate expression changes. The supplementary table 1 contains the list of primers used in this study.
Supplementary Material
Acknowledgements
This work was supported by Cancer Research UK and the Breast Cancer Campaign. The authors thank C. Beard and R. Jaenisch (The Whitehead Institute for Biomedical Research, Massachusetts) for the ES cell lines and for technical advice, K. Ewan, A. Offergeld and S. Braun (Cardiff School of Biosciences) for their assistance in tissue staining and cell counting, P. Watson (Cardiff School of Biosciences) for his assistance in fluorescence microscopy, O. Sansom and K. Myant for their assistance in organoid culture, B. Allen and O. Asby for technical assistance in blastocyst injections and chimera crosses and D. Scarborough for help with histology.
Grant support: Breast Cancer Campaign, Cancer Research UK
Footnotes
Conflict of interest The authors declare no conflict of interest
Involvement with manuscript: Study concept and design: TCD, ARC, TJ; Funding and study supervision: TCD, TJ; Acquisition and analysis of data: TJ, RJE, KLM, LP, GJF, BA; Drafting manuscript: TJ, TCD; Critical Revision: ARC, RJE, LP, GJF.
References
- 1.Van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 2.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, et al. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 6.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 8.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 9.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 10.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong MH, Rubinfeld B, Gordon JI. Effects of forced expression of an NH2-terminal truncated beta-Catenin on mouse intestinal epithelial homeostasis. J Cell Biol. 1998;141:765–777. doi: 10.1083/jcb.141.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romagnolo B, Berrebi D, Saadi-Keddoucci S, Porteu A, Pichard AL, Peuchmaur M, et al. Intestinal dysplasia and adenoma in transgenic mice after overexpression of an activated beta-catenin. Cancer Res. 1999;59:3875–3879. [PubMed] [Google Scholar]
- 13.Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44:23–8. doi: 10.1002/gene.20180. [DOI] [PubMed] [Google Scholar]
- 14.Bocker R, Estler CJ, Maywald M, Weber D. Comparison of distribution of doxycycline in mice after oral and intravenous application measured by a high-performance liquid chromatographic method. Arzneimittelforschung. 1981;31:2116–2117. [PubMed] [Google Scholar]
- 15.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 16.Ewan K, Pajak B, Stubbs M, Todd H, Barbeau O, Quevedo C, et al. A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription. Cancer Res. 2010;70:5963–5973. doi: 10.1158/0008-5472.CAN-10-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 18.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 19.Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 20.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 22.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 23.Minke KS, Staib P, Puetter A, Gehrke I, Gandhirajan RK, Schlosser A, et al. Small molecule inhibitors of WNT signaling effectively induce apoptosis in acute myeloid leukemia cells. Eur J Haematol. 2009;82:165–175. doi: 10.1111/j.1600-0609.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 24.He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, et al. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6:7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R, et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2011;108:17135–17140. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunther EJ, Moody SE, Belka GK, Hahn KT, Innocent N, Dugan KD, et al. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev. 2003;17:488–501. doi: 10.1101/gad.1051603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coste I, Freund JN, Spaderna S, Brabletz T, Renno T. Precancerous lesions upon sporadic activation of beta-catenin in mice. Gastroenterology. 2007;132:1299–1308. doi: 10.1053/j.gastro.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Lucero OM, Dawson DW, Moon RT, Chien AJ. A re-evaluation of the “oncogenic” nature of Wnt/beta-catenin signaling in melanoma and other cancers. Curr Oncol Rep. 2010;12:314–318. doi: 10.1007/s11912-010-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchert M, Athineos D, Abud HE, Burke ZD, Faux MC, Samuel MS, et al. Genetic dissection of differential signaling threshold requirements for the Wnt/beta-catenin pathway in vivo. PLoS Genet. 2010;6:e1000816. doi: 10.1371/journal.pgen.1000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y. Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther. 2009;8:2103–2109. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- 33.van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- 34.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 35.Wutz A, Jaenisch R. A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol Cell. 2000;5:695–705. doi: 10.1016/s1097-2765(00)80248-8. [DOI] [PubMed] [Google Scholar]
- 36.Shorning BY, Jarde T, McCarthy A, Ashworth A, de Leng WW, Offerhaus GJ, et al. Intestinal renin-angiotensin system is stimulated after deletion of Lkb1. Gut. 2012;61:202–213. doi: 10.1136/gutjnl-2011-300046. [DOI] [PubMed] [Google Scholar]
- 37.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merritt AJ, Allen TD, Potten CS, Hickman JA. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene. 1997;14:2759–2766. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


