Abstract
Objective To assess the risk of systemic adverse events associated with intravitreal injections of vascular endothelial growth factor inhibiting drugs.
Design Population based nested case-control study.
Setting Ontario, Canada.
Participants 91 378 older adults with a history of physician diagnosed retinal disease identified between 1 April 2006 and 31 March 2011. Cases were 1477 patients admitted to hospital for ischaemic stroke, 2229 admitted for an acute myocardial infarction, 1059 admitted or assessed in an emergency department for venous thromboembolism, and 2623 admitted for congestive heart failure. Event-free controls (at a ratio of 5:1) were matched to cases on the basis of year of birth, sex, history of the outcome in the previous 5 years, and diabetes.
Main exposure measure Exposure to vascular endothelial growth factor inhibiting drugs identified within 180 days before the index date.
Results After adjustment for potential confounders, participants who had ischaemic stroke, acute myocardial infarction, congestive heart failure, or venous thromboembolism were not more likely than control participants to have been exposed to either bevacizumab (adjusted odds ratios of 0.95 (95% confidence interval 0.68 to 1.34) for ischaemic stroke, 1.04 (0.77 to 1.39) for acute myocardial infarction, 0.81 (0.49 to 1.34) for venous thromboembolism, and 1.21 (0.91 to 1.62) for congestive heart failure) or ranibizumab (adjusted odds ratios 0.87 (0.68 to 1.10) for ischaemic stroke, 0.90 (0.72 to 1.11) for acute myocardial infarction, 0.88 (0.67 to 1.16) for venous thromboembolism, and 0.87 (0.70 to 1.07) for congestive heart failure). Similarly, a secondary analysis of exclusive users of bevacizumab or ranibizumab showed no differences in risk between the two drugs (adjusted odds ratios for bevacizumab relative to ranibizumab of 1.03 (0.67 to 1.60) for ischaemic stroke, 1.23 (0.85 to 1.77) for acute myocardial infarction, 0.92 (0.51 to 1.69) for venous thromboembolism, and 1.35 (0.93 to 1.95) for congestive heart failure). These findings were consistent for all but one outcome in subgroup analyses.
Conclusions Intravitreal injections of bevacizumab and ranibizumab were not associated with significant risks of ischaemic stroke, acute myocardial infarction, congestive heart failure, or venous thromboembolism.
Introduction
Age related macular degeneration is the leading cause of blindness in Western nations; the neovascular (“wet”) subtype is responsible for most cases of severe vision loss.1 2 3 4 Because vascular endothelial growth factor plays an important role in the growth of the pathological blood vessels that underlie neovascular age related macular degeneration, the development of vascular endothelial growth factor inhibitors has revolutionised the treatment of this disease.5 6 7 8 However, vascular endothelial growth factor functions in many physiological and pathological processes, including maintenance of normal blood vessels, wound healing responses, blood clotting processes, and stabilisation of atheromatous plaques.9 10 11 12 These wide ranging effects make the hypothesis that adverse vascular events may stem from inhibition of vascular endothelial growth factor biologically plausible. Furthermore, empirical clinical evidence suggests an association between inhibition of vascular endothelial growth factor and adverse vascular events. In particular, intravenous administration of the vascular endothelial growth factor inhibitor bevacizumab has been associated with increased risks of stroke, venous thromboembolism, and congestive heart failure.13 14 15 Whether this risk of systemic adverse events can be extrapolated to the small doses used in age related macular degeneration remains unclear.
Direct injection of vascular endothelial growth factor inhibitors into the eye decreases the concentration of drug reaching the systemic circulation.16 17 18 Clinical trials comparing intravitreal ranibizumab and bevacizumab with sham treated controls did not detect increased risks of vascular adverse events with either drug.6 7 19 In contrast, a small meta-analysis of early trials with ranibizumab found an increased risk of stroke in patients receiving ranibizumab injections.20 The Comparison of Age-related Macular Degeneration Treatments Trials, which directly compared intravitreal bevacizumab against intravitreal ranibizumab, found a higher risk of adverse events among participants receiving bevacizumab.8 21 However, this finding is difficult to interpret given that most of the observed adverse events were conditions not previously associated with inhibition of vascular endothelial growth factor, and that participants who received fewer doses of bevacizumab had a greater risk than did those who received more.
Clinical trials and meta-analyses have several important limitations, including a lack of power to detect adverse events and often poor generalisability.22 Hence, large post-marketing studies provide important information on safety that complements data from clinical trials.23 One previous population based study was inconclusive on the relative safety of ranibizumab and bevacizumab.24 Although the study’s primary analysis found that bevacizumab was associated with greater risk of stroke than was ranibizumab, the price gap between the drugs may have resulted in confounding due to differences in socioeconomic status between people receiving the two drugs. A secondary analysis from the same study, which controlled for the effect of socioeconomic status, found no difference in safety between the two drugs. Because of this discrepancy, questions regarding the risks of adverse events with intravitreal injection of vascular endothelial growth factor inhibiting drugs persist. To evaluate these risks, we did a population based, nested case-control study in a setting with universal healthcare insurance, which diminished the confounding effects of socioeconomic status.
Methods
Overview
We did a population based, nested case-control study to investigate the risk of serious adverse events associated with intravitreal injections of vascular endothelial growth factor inhibitors. We used linked healthcare databases to examine associations between four distinct outcomes (ischaemic stroke, acute myocardial infarction, venous thromboembolism, and congestive heart failure) and previous exposure to intravitreal injections of bevacizumab and ranibizumab.
Data sources
The province of Ontario provides universal healthcare insurance that covers all 13 million residents, and the resulting administrative data are population based. For this study, we linked records from seven health administrative databases. The Ontario Health Insurance Plan database contains information on inpatient and outpatient physician services and has excellent reliability for recording of medical procedures.25 The Ontario Drug Benefit database contains records of all outpatient prescriptions for formulary drugs dispensed to patients aged 65 years or older and has an error rate of less than 1%.25 26 The Canadian Institute for Health Information discharge abstract database provides detailed information on all hospital admissions in Ontario.27 The National Ambulatory Care Reporting System database contains information on all visits to hospital emergency departments.28 29 The registered persons database contains demographic information on all residents, and the Ontario diabetes database contains validated information on Ontarians with diabetes.30 The Institute for Clinical Evaluative Sciences physician database contains data on the specialty of all healthcare providers in Ontario. The databases used provide accurate data on the variables assessed in this study.25 26 27
Study population and base cohort identification
We used the Ontario Health Insurance Plan database to identify a base cohort consisting of patients diagnosed as having retinal disease (ICD-9 (international classification of diseases, 9th revision) code 362) within the previous two years who were aged 66 years and older between 1 April 2006 and 31 March 2011. We chose this period to include periods of significant use of both bevacizumab and ranibizumab in Ontario.31 To ensure that patients in the cohort could access intravitreal injections if clinically indicated, we limited our analysis to those who, during the study period, had seen an ophthalmologist who offered intravitreal injections.31
Study outcomes and case ascertainment
Cases were patients who were admitted to hospital with a primary diagnosis of ischaemic stroke (ICD-10 code I63 or I64), acute myocardial infarction (ICD-10 code I21), venous thromboembolism (ICD-10 code I26 or I80), or congestive heart failure (ICD-10 code I50). We defined the date of hospital admission or emergency department visit as the index date. Compared with direct chart review, the positive predictive values for the diagnoses of stroke and acute myocardial infarction in this database are both 87%.27 Similarly, the coding for congestive heart failure was highly predictive of the diagnosis made using clinical criteria in another validation study.32 Because some cases of venous thromboembolism are managed without hospital admission, we also included patients who visited an emergency room with a primary diagnosis of venous thromboembolism (ICD-10 code I26 or I80). For each outcome, if patients had multiple occurrences we included only the first in our analysis. Patients who experienced one outcome remained eligible to be controls for cases who experienced one of the other outcomes. Patients selected as controls remained eligible to have outcome events subsequently.
Selection of controls
For each outcome, we selected up to five randomly selected controls for each case from the study population who had not experienced the adverse event. We assigned controls the same index date as their respective cases and matched them to cases according to year of birth, sex, history of the outcome in the five years preceding the index date, and diabetes.
Assessment of exposure to vascular endothelial growth factor inhibitors
We identified intravitreal injections of ranibizumab by using the Ontario Health Insurance Plan and Ontario Drug Benefit databases.31 Ranibizumab has been covered by the Ontario Drug Benefit Program since 25 March 2008. Before that date, use of ranibizumab in Ontario was minimal.31 Bevacizumab does not have approval from Health Canada for use in age related macular degeneration and is not covered by the Ontario Drug Benefit Program for this indication. However, bevacizumab received Health Canada approval for use in colorectal cancer in September 2005, and physicians can bill the provincial physician insurance plan for off-label intravitreal injections of this drug. Our previous work has documented the widespread use of off-label bevacizumab in the period before coverage of ranibizumab by the public drug insurance plan.31
We assigned exposure to bevacizumab injection indirectly. We identified all intravitreal injection procedures that were not associated with a prescription for ranibizumab by using the Ontario Drug Benefit and Ontario Health Insurance Plan databases and used several strategies to reduce misclassification of intravitreal injections of gas, antibiotics, or steroids. Firstly, because gas is injected as part of retinal detachment therapy, we excluded injections given to patients with a proximate diagnosis of retinal detachment, defined as having had a vitreous exchange procedure or a retinal reattachment procedure within 42 days before or seven days after the intravitreal injection. Secondly, because intravitreal steroid is used for retinal conditions that are also treated with laser, we excluded injections given to patients who had had retinal laser treatment in the previous 180 days. Additionally, we did a sensitivity analysis in which this time frame was shortened to 42 days. Finally, because antibiotics are injected for postoperative endophthalmitis, we excluded injections given to patients who had had retinal surgery, cataract surgery, corneal surgery, or glaucoma surgery in the 42 days before the injection.
Covariates
We adjusted for several potential confounders. We used the number of distinct drugs dispensed in the year before the index date as a measure of comorbidity.33 34 We estimated income from neighbourhood fifths of income based on census data. We defined diabetes status on the basis of the validated Ontario diabetes database.30 We identified patients with hypertension by using a validated algorithm.35 We also adjusted for exposure to several specific drugs within the year before the index date, grouped by mechanism of action to avoid over-fitting the statistical models. We also included specific comorbidities (including atrial fibrillation, cancer, and renal failure) in the models.
Statistical analysis
We used descriptive statistics to characterise cases and controls. We used a nested case-control analysis to evaluate the association between exposure to intravitreal vascular endothelial growth factor inhibitor and each outcome.36 37 This approach provides unbiased estimates of the rate ratios that would be obtained from traditional time to event analyses of the full cohort, with little or no loss of precision.38 39 40
We used conditional logistic regression to estimate the odds ratios and 95% confidence intervals for the association between adverse outcomes and exposure to vascular endothelial growth factor inhibitor injections, controlling for potential confounding variables. In the primary analysis, we assessed the risk associated with exposure to bevacizumab or ranibizumab within 180 days before the adverse event. In a secondary analysis, to facilitate direct comparison between the two vascular endothelial growth factor inhibitors, we assessed the risk associated with exclusive bevacizumab exposure (no ranibizumab exposure) by using exclusive ranibizumab exposure (no bevacizumab exposure) as the reference. Finally, we also did subgroup analyses on patients with and without diabetes.
For our study’s case-control ratio of one to five, independence in the probability of exposure among cases and controls, and a type 1 error probability of 5%, our a priori estimates of power were 89% for ischaemic stroke, 99% for acute myocardial infarction, 71% for venous thromboembolism, and 99% for congestive heart failure to detect a clinically important unadjusted odds ratio of 1.5. This was based on conservative estimates including a base cohort of approximately 50 000 patients over the age of 65 with retinal disease in each of the five study years, with 5% exposure rates, and expected annual percentages experiencing adverse events of 0.5 for ischaemic stroke, 1.0 for acute myocardial infarction, 0.3 for venous thromboembolism, and 1.0 for congestive heart failure.31 41 42 43 All analyses were done at the Institute for Clinical Evaluative Sciences using SAS, version 9.2.
Results
Baseline characteristics of cases and controls
We identified 91 378 older people with retinal disease diagnosed by a physician over the five year study period. Within this base cohort, we identified 7388 cases, including 1477 (1.6%) cases of ischaemic stroke, 2229 (2.4%) of acute myocardial infarction, 1059 (1.2%) of venous thromboembolism, and 2623 (2.9%) of congestive heart failure. These were matched to 35 962 control patients, with 95% of cases matched to five controls. Tables 1, 2, 3, and 4 show the characteristics of cases and controls for the four outcomes. The percentage of patients with diabetes ranged from approximately 31% (venous thromboembolism; table 3) to approximately 53% (congestive heart failure; table 4). Case patients were slightly more likely than controls to come from the lowest fifth of population income. Comorbidities, including a history of cancer, chronic renal insufficiency, and atrial fibrillation, were more common among patients who experienced adverse outcomes. Similarly, case patients were prescribed more drugs than were controls in the year before the index date.
Table 1.
Baseline characteristics of cases of ischaemic stroke and matched controls*. Values are numbers (percentages) unless stated otherwise
| Characteristics | Cases (n=1477) | Controls (n=7349) | Standardised difference† |
|---|---|---|---|
| Demographics | |||
| Mean (95% CI) age at index date | 81.8 (81.4 to 82.2) | 81.8 (81.7 to 82.0) | 0 |
| Male sex | 591 (40.0) | 2946 (40.1) | 0 |
| Fifth of income: | |||
| Missing | ≤5 (0.1) | 31 (0.4) | 0.05 |
| 1 | 314 (21.3) | 1420 (19.3) | 0.05 |
| 2 | 341 (23.1) | 1530 (20.8) | 0.05 |
| 3 | 297 (20.1) | 1516 (20.6) | 0.01 |
| 4 | 276 (18.7) | 1418 (19.3) | 0.01 |
| 5 | 247 (16.7) | 1434 (19.5) | 0.01 |
| Rural residence | 238 (16.1) | 1027 (14.0) | 0.06 |
| Comorbidities | |||
| Mean (SD) No of unique drugs prescribed in previous year | 14.2 (8.1) | 12.2 (7.5) | 0.25 |
| Atrial fibrillation | 674 (45.6) | 1986 (27.0) | 0.41 |
| Coronary artery bypass grafting surgery | 37 (2.5) | 143 (1.9) | 0.04 |
| Cancer | 120 (8.1) | 414 (5.6) | 0.1 |
| Chronic renal insufficiency | 129 (8.7) | 283 (3.9) | 0.23 |
| Diabetes | 633 (42.9) | 3141 (42.7) | 0 |
| Drugs used in previous year | |||
| Angiotensin converting enzyme inhibitors | 712 (48.2) | 2911 (39.6) | 0.18 |
| Angiotensin receptor blockers | 386 (26.1) | 1731 (23.6) | 0.06 |
| Hydroxymethyl glutaryl coenzyme A reductase inhibitors (statins) | 825 (55.9) | 3645 (49.6) | 0.13 |
| Clopidogrel | 212 (14.4) | 541 (7.4) | 0.25 |
| Warfarin | 346 (23.4) | 962 (13.1) | 0.29 |
| Acetylsalicylic acid/dipyridamole | 118 (8.0) | 157 (2.1) | 0.34 |
| Hormone replacement therapy | 19 (1.3) | 71 (1.0) | 0.03 |
| Low molecular weight heparin | 24 (1.6) | 69 (0.9) | 0.07 |
| Diuretics | 781 (52.9) | 3383 (46.0) | 0.14 |
| Diuretics, potassium sparing | 116 (7.9) | 509 (6.9) | 0.04 |
| Calcium channel blocker | 652 (44.1) | 2825 (38.4) | 0.12 |
| History of outcome | |||
| Ischaemic stroke | 64 (4.3) | 284 (3.9) | 0.02 |
All cells with values of 5 or less are reported as ≤5 for privacy reasons.
*Up to 5 controls were matched to each case on age (±1 year), sex, diabetes, and history of outcome.
†Difference between cases and controls divided by pooled standard deviation of two groups; standardised differences <0.1 indicate good balance between groups.
Table 2.
Baseline characteristics of cases of acute myocardial infarction and matched controls*. Values are numbers (percentages) unless stated otherwise
| Characteristics | Cases (n=2229) | Controls (n=11 010) | Standardised difference† |
|---|---|---|---|
| Demographics | |||
| Mean (95% CI) age at index date | 80.6 (80.3 to 80.9) | 80.5 (80.4 to 80.7) | 0.01 |
| Male sex | 1129 (50.7) | 5578 (50.7) | 0 |
| Fifth of income: | |||
| Missing | 13 (0.6) | 49 (0.4) | 0.03 |
| 1 | 505 (22.7) | 2066 (18.8) | 0.1 |
| 2 | 445 (20.0) | 2282 (20.7) | 0.01 |
| 3 | 443 (19.9) | 2181 (19.8) | 0 |
| 4 | 442 (19.8) | 2241 (20.4) | 0.02 |
| 5 | 381 (17.1) | 2191 (19.9) | 0.07 |
| Rural residence | 401 (18.0) | 1584 (14.4) | 0.1 |
| Comorbidities | |||
| Mean (SD) No of unique drugs prescribed in previous year | 17.1 (8.5) | 12.1 (7.3) | 0.64 |
| Atrial fibrillation | 740 (33.2) | 2021 (18.4) | 0.37 |
| Coronary artery bypass grafting surgery | 61 (2.7) | 314 (2.9) | 0.01 |
| Cancer | 183 (8.2) | 613 (5.6) | 0.11 |
| Chronic renal insufficiency | 340 (15.3) | 418 (3.8) | 0.5 |
| Diabetes | 1098 (49.3) | 5374 (48.8) | 0.01 |
| Drugs used in previous year | |||
| Angiotensin converting enzyme inhibitors | 1284 (57.6) | 4571 (41.5) | 0.33 |
| Angiotensin receptor blockers | 616 (27.6) | 2584 (23.5) | 0.1 |
| Hydroxymethyl glutaryl coenzyme A reductase inhibitors (statins) | 1616 (72.5) | 5860 (53.2) | 0.39 |
| Clopidogrel | 832 (37.3) | 786 (7.1) | 0.98 |
| Warfarin | 397 (17.8) | 1403 (12.7) | 0.15 |
| Acetylsalicylic acid/dipyridamole | 52 (2.3) | 222 (2.0) | 0.02 |
| Hormone replacement therapy | 16 (0.7) | 82 (0.7) | 0 |
| Low molecular weight heparin | 28 (1.3) | 114 (1.0) | 0.02 |
| Diuretics | 1347 (60.4) | 5106 (46.4) | 0.28 |
| Diuretics, potassium sparing | 230 (10.3) | 784 (7.1) | 0.12 |
| Calcium channel blocker | 1090 (48.9) | 4104 (37.3) | 0.24 |
| History of outcome | |||
| Acute myocardial infarction | 181 (8.1) | 773 (7.0) | 0.04 |
All cells with values of 5 or less are reported as ≤5 for privacy reasons.
*Up to 5 controls were matched to each case on age (±1 year), sex, diabetes, and history of outcome.
†Difference between cases and controls divided by pooled standard deviation of two groups; standardised differences <0.1 indicate good balance between groups.
Table 3.
Baseline characteristics of cases of venous thromboembolism and matched controls*. Values are numbers (percentages) unless stated otherwise
| Characteristics | Cases (n=1059) | Controls (n=5259) | Standardised difference† |
|---|---|---|---|
| Demographics | |||
| Mean (95% CI) age at index date | 79.0 (78.5 to 79.4) | 79.0 (78.8 to 79.2) | 0 |
| Male sex | 402 (38.0) | 1987 (37.8) | 0 |
| Fifth of income: | |||
| Missing | ≤5 (0.4) | 23 (0.4) | 0 |
| 1 | 214 (20.2) | 991 (18.8) | 0.04 |
| 2 | 231 (21.8) | 1032 (19.6) | 0.05 |
| 3 | 213 (20.1) | 999 (19.0) | 0.03 |
| 4 | 203 (19.2) | 1122 (21.3) | 0.05 |
| 5 | 194 (18.3) | 1092 (20.8) | 0.06 |
| Rural residence | 173 (16.3) | 759 (14.4) | 0.05 |
| Comorbidities | |||
| Mean (SD) No of unique drugs prescribed in previous year | 15.0 (8.1) | 11.3 (7.2) | 0.49 |
| Atrial fibrillation | 272 (25.7) | 905 (17.2) | 0.22 |
| Coronary artery bypass grafting surgery | 20 (1.9) | 95 (1.8) | 0.01 |
| Cancer | 145 (13.7) | 278 (5.3) | 0.34 |
| Chronic renal insufficiency | 61 (5.8) | 135 (2.6) | 0.18 |
| Diabetes | 327 (30.9) | 1618 (30.8) | 0 |
| Drugs used in previous year | |||
| Angiotensin converting enzyme inhibitors | 408 (38.5) | 1992 (37.9) | 0.01 |
| Angiotensin receptor blockers | 248 (23.4) | 1178 (22.4) | 0.02 |
| Hydroxymethyl glutaryl coenzyme A reductase inhibitors (statins) | 499 (47.1) | 2524 (48.0) | 0.02 |
| Clopidogrel | 82 (7.7) | 301 (5.7) | 0.08 |
| Warfarin | 305 (28.8) | 582 (11.1) | 0.52 |
| Acetylsalicylic acid/dipyridamole | 20 (1.9) | 94 (1.8) | 0.01 |
| Hormone replacement therapy | 14 (1.3) | 48 (0.9) | 0.04 |
| Low molecular weight heparin | 128 (12.1) | 51 (1.0) | 0.69 |
| Diuretics | 551 (52.0) | 2242 (42.6) | 0.19 |
| Diuretics, potassium sparing | 100 (9.4) | 325 (6.2) | 0.13 |
| Calcium channel blocker | 399 (37.7) | 1768 (33.6) | 0.09 |
| History of outcome | |||
| Venous thromboembolism | 46 (4.3) | 194 (3.7) | 0.03 |
All cells with values of 5 or less are reported as ≤5 for privacy reasons.
*Up to 5 controls were matched to each case on age (±1 year), sex, diabetes, and history of outcome.
†Difference between cases and controls divided by pooled standard deviation of two groups; standardised differences <0.1 indicate good balance between groups.
Table 4.
Baseline characteristics of cases of congestive heart failure and matched controls*. Values are numbers (percentages) unless stated otherwise
| Characteristics | Cases (n=2623) | Controls (n=12 344) | Standardised difference† |
|---|---|---|---|
| Demographics | |||
| Mean (95% CI) age at index date | 82.0 (81.7 to 82.3) | 82.0 (81.9 to 82.2) | 0 |
| Male sex | 1196 (45.6) | 5636 (45.7) | 0 |
| Fifth of income: | |||
| Missing | 12 (0.5) | 42 (0.3) | 0.03 |
| 1 | 552 (21.0) | 2425 (19.6) | 0.04 |
| 2 | 569 (21.7) | 2566 (20.8) | 0.02 |
| 3 | 553 (21.1) | 2484 (20.1) | 0.02 |
| 4 | 479 (18.3) | 2446 (19.8) | 0.04 |
| 5 | 458 (17.5) | 2381 (19.3) | 0.05 |
| Rural residence | 419 (16.0) | 1812 (14.7) | 0.04 |
| Comorbidities | |||
| Mean (SD) No of unique drugs prescribed in previous year | 21.0 (8.7) | 12.8 (7.8) | 1.03 |
| Atrial fibrillation | 348 (13.3) | 1174 (9.5) | 0.12 |
| Coronary artery bypass grafting surgery | 121 (4.6) | 302 (2.4) | 0.13 |
| Cancer | 286 (10.9) | 690 (5.6) | 0.22 |
| Chronic renal insufficiency | 590 (22.5) | 569 (4.6) | 0.69 |
| Diabetes | 1413 (53.9) | 6561 (53.2) | 0.01 |
| Drugs used in previous year | |||
| Angiotensin converting enzyme inhibitors | 1547 (59.0) | 5346 (43.3) | 0.32 |
| Angiotensin receptor blockers | 824 (31.4) | 2957 (24.0) | 0.17 |
| Hydroxymethyl glutaryl coenzyme A reductase inhibitors (statins) | 1682 (64.1) | 6489 (52.6) | 0.23 |
| Clopidogrel | 464 (17.7) | 922 (7.5) | 0.36 |
| Warfarin | 1053 (40.1) | 1737 (14.1) | 0.69 |
| Acetylsalicylic acid/dipyridamole | 58 (2.2) | 264 (2.1) | 0 |
| Hormone replacement therapy | 27 (1.0) | 83 (0.7) | 0.04 |
| Low molecular weight heparin | 57 (2.2) | 121 (1.0) | 0.11 |
| Diuretics | 2345 (89.4) | 6070 (49.2) | 0.85 |
| Diuretics, potassium sparing | 592 (22.6) | 918 (7.4) | 0.51 |
| Calcium channel blocker | 1412 (53.8) | 4748 (38.5) | 0.31 |
| History of outcome | |||
| Congestive heart failure | 355 (13.5) | 1041 (8.4) | 0.18 |
All cells with values of 5 or less are reported as ≤5 for privacy reasons.
*Up to 5 controls were matched to each case on age (±1 year), sex, diabetes, and history of outcome.
†Difference between cases and controls divided by pooled standard deviation of two groups; standardised differences <0.1 indicate good balance between groups.
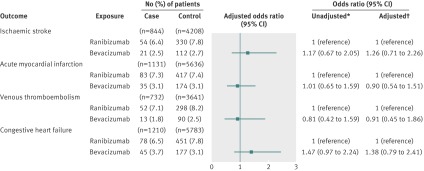
Association between ischaemic stroke and vascular endothelial growth factor inhibitors
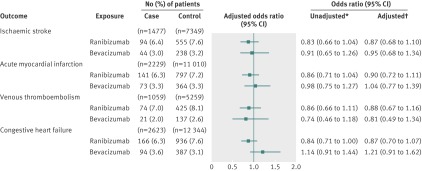
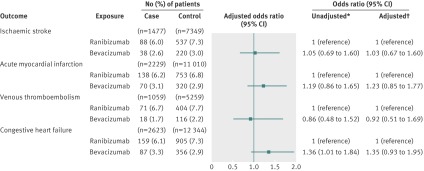
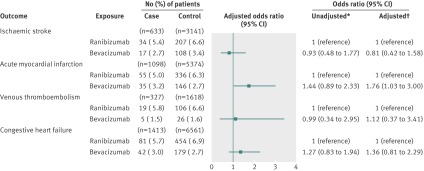
In our primary analysis of 1477 case patients and 7349 control patients, 94 (6.4%) case patients had received ranibizumab in the 180 days before their stroke (index date), and 555 (7.6%) control subjects had received ranibizumab in the 180 days before the index date. We found no statistically significant association between stroke and exposure to ranibizumab (adjusted odds ratio 0.87, 95% confidence interval 0.68 to 1.10) (fig 1). Forty-four (3.0%) case patients had received bevacizumab in the 180 days before their stroke, and 238 (3.2%) control patients had received bevacizumab in the 180 days before the index date. No significant association existed between stroke and exposure to bevacizumab (adjusted odds ratio 0.95, 0.68 to 1.34) (fig 1). In our secondary analysis, using exclusive ranibizumab exposure as the reference group, exclusive bevacizumab exposure was not significantly associated with stroke (adjusted odds ratio 1.03, 0.67 to 1.60) (fig 2).
Fig 1 Adverse events after intravitreal injections of vascular endothelial growth factor inhibitors. *Matched on age, sex, history of outcome, and diabetes. †Matched on age, sex, history of outcome, and diabetes; adjusted for: ischaemic stroke—angiotensin converting enzyme (ACE) inhibitors, acetylsalicylic acid/dipyridamole, cancer in previous 5 years, clopidogrel, hypertension, chronic renal insufficiency, warfarin; acute myocardial infarction—digoxin, ACE inhibitors, cancer in previous 5 years, clopidogrel, diuretics (regular), hypertension, No of unique prescriptions, chronic renal insufficiency, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin; venous thromboembolism—digoxin, glaucoma therapies, ACE inhibitors, cancer in previous 5 years, diuretics (potassium sparing), diuretics (regular), low molecular weight heparin, No of unique prescriptions, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin; congestive heart failure—digoxin, glaucoma therapies, ACE inhibitors, calcium channel blockers, cancer in previous 5 years, clopidogrel, diuretics (potassium sparing), diuretics (regular), hypertension, chronic renal insufficiency, warfarin
Fig 2 Risk of adverse events among exclusive users of bevacizumab or ranibizumab. *Matched on age, sex, history of outcome, and diabetes. †Matched on age, sex, history of outcome, and diabetes; adjusted for: ischaemic stroke—angiotensin converting enzyme (ACE) inhibitors, acetylsalicylic acid/dipyridamole, cancer in previous 5 years, clopidogrel, hypertension, chronic renal insufficiency, warfarin; acute myocardial infarction—digoxin, ACE inhibitors, cancer in previous 5 years, clopidogrel, diuretics (regular), hypertension, chronic renal insufficiency, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin; venous thromboembolism—digoxin, glaucoma therapies, ACE inhibitors, cancer in previous 5 years, diuretics (potassium sparing), diuretics (regular), low molecular weight heparin, No of unique prescriptions, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin; congestive heart failure—digoxin, glaucoma therapies, ACE inhibitors, calcium channel blockers, cancer in previous 5 years, clopidogrel, diuretics (potassium sparing), diuretics (regular), hypertension, income fifth, No of unique prescriptions
Association between acute myocardial infarction and vascular endothelial growth factor inhibitors
In our primary analysis of 2229 case patients and 11 010 control patients, 141 (6.3%) case patients had received ranibizumab in the 180 days before their acute myocardial infarction, and 797 (7.2%) control subjects had received ranibizumab in the 180 days before the index date. No significant association existed between acute myocardial infarction and exposure to ranibizumab (adjusted odds ratio 0.90, 0.72 to 1.11) (fig 1). Seventy-three (3.3%) case patients had received bevacizumab in the 180 days before their acute myocardial infarction, and 364 (3.3%) control patients had received bevacizumab in the 180 days before the index date. We found no significant association between acute myocardial infarction and exposure to bevacizumab (adjusted odds ratio 1.04, 0.77 to 1.39) (fig 1). In our secondary analysis, using exclusive ranibizumab exposure as the reference group, exclusive bevacizumab exposure was not significantly associated with acute myocardial infarction (adjusted odds ratio 1.23, 0.85 to 1.77) (fig 2).
Association between venous thromboembolism and vascular endothelial growth factor inhibitors
In our primary analysis of 1059 case patients and 5259 control patients, 74 (7.0%) case patients had received ranibizumab in the 180 days before their venous thromboembolism, and 425 (8.1%) controls had received ranibizumab in the 180 days before the index date. No significant association existed between venous thromboembolism and exposure to ranibizumab (adjusted odds ratio 0.88, 0.67 to 1.16) (fig 1). Twenty-one (2.0%) case patients had received bevacizumab in the 180 days before their venous thromboembolism, and 137 (2.6%) control patients had received bevacizumab in the 180 days before the index date. No significant association existed between venous thromboembolism and exposure to bevacizumab (adjusted odds ratio 0.81, 0.49 to 1.34) (fig 1). In our secondary analysis, using exclusive ranibizumab exposure as the reference group, exclusive bevacizumab exposure was not significantly associated with venous thromboembolism (adjusted odds ratio 0.92, 0.51 to 1.69) (fig 2).
Association between congestive heart failure and vascular endothelial growth factor inhibitors
In our primary analysis of 2623 case patients and 12 344 control patients, 166 (6.3%) case patients had received ranibizumab in the 180 days before being admitted for congestive heart failure, and 936 (7.6%) control patients had received ranibizumab in the 180 days before the index date. No significant association existed between congestive heart failure and exposure to ranibizumab (adjusted odds ratio 0.87, 0.70 to 1.07) (fig 1). Ninety-four (3.6%) case patients had received bevacizumab in the 180 days before being admitted for congestive heart failure, and 387 (3.1%) control patients had received bevacizumab in the 180 days before the index date. No significant association existed between congestive heart failure and exposure to bevacizumab (adjusted odds ratio 1.21, 0.91 to 1.62) (fig 1). In our secondary analysis, using exclusive ranibizumab exposure as the reference group, exclusive bevacizumab exposure was not significantly associated with congestive heart failure (adjusted odds ratio 1.35, 0.93 to 1.95) (fig 2).
Diabetes subgroup analyses
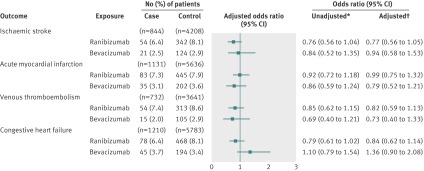
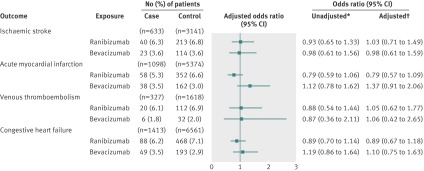
Our subgroup analyses of patients with and without diabetes showed no significant associations between either of the vascular endothelial growth factor inhibitors and any of the outcomes when compared with non-users (figure 3 and 4). Similarly, among both patients with and without diabetes, the secondary analysis of exclusive users showed no differences between bevacizumab and ranibizumab in the risk of ischaemic stroke, venous thromboembolism, or congestive heart failure (figures 5 and 6). In the subgroup with diabetes, we found a statistically significant association between exclusive bevacizumab exposure and acute myocardial infarction when exclusive ranibizumab exposure was used as the referent (adjusted odds ratio 1.76, 1.03 to 3.00) (fig 6).
Fig 3 Adverse events after intravitreal injections of vascular endothelial growth factor inhibitors in patients without diabetes. *Matched on age, sex, history of outcome, and diabetes. †Matched on age, sex, history of outcome, and diabetes; adjusted for: ischaemic stroke—angiotensin converting enzyme (ACE) inhibitors, acetylsalicylic acid/dipyridamole, cancer in previous 5 years, clopidogrel, hypertension, chronic renal insufficiency, warfarin; acute myocardial infarction—digoxin, ACE inhibitors, cancer in previous 5 years, clopidogrel, diuretics (regular), hypertension, No of unique prescriptions, chronic renal insufficiency, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin; venous thromboembolism—digoxin, glaucoma therapies, ACE inhibitors, cancer in previous 5 years, diuretics (potassium sparing), diuretics (regular), low molecular weight heparin, No of unique prescriptions, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin; congestive heart failure—digoxin, glaucoma therapies, ACE inhibitors, calcium channel blockers, cancer in previous 5 years, clopidogrel, diuretics (potassium sparing), diuretics (regular), hypertension, chronic renal insufficiency, warfarin
Fig 4 Adverse events after intravitreal injections of vascular endothelial growth factor inhibitors in patients with diabetes. *Matched on: age, sex, history of outcome, and diabetes. †Matched on age, sex, history of outcome, and diabetes; adjusted for: ischaemic stroke—angiotensin converting enzyme (ACE) inhibitors, acetylsalicylic acid/dipyridamole, cancer in previous 5 years, clopidogrel, hypertension, chronic renal insufficiency, warfarin; acute myocardial infarction—digoxin, ACE inhibitors, cancer in previous 5 years, clopidogrel, diuretics (regular), hypertension, No of unique prescriptions, chronic renal insufficiency, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin; venous thromboembolism—digoxin, glaucoma therapies, ACE inhibitors, cancer in previous 5 years, diuretics (potassium sparing), diuretics (regular), low molecular weight heparin, No of unique prescriptions, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin; congestive heart failure—digoxin, glaucoma therapies, ACE inhibitors, calcium channel blockers, cancer in previous 5 years, clopidogrel, diuretics (potassium sparing), diuretics (regular), hypertension, chronic renal insufficiency, warfarin
Fig 5 Risk of adverse events in exclusive users of bevacizumab or ranibizumab among patients without diabetes. *Matched on age, sex, history of outcome, and diabetes. †Matched on age, sex, history of outcome and diabetes; adjusted for: ischaemic stroke—angiotensin converting enzyme (ACE) inhibitors, acetylsalicylic acid/dipyridamole, angiotensin receptor blockers, cancer in previous 5 years, clopidogrel, chronic renal insufficiency, warfarin; acute myocardial infarction—ACE inhibitors, clopidogrel, chronic renal insufficiency, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, No of unique prescriptions; venous thromboembolism—digoxin, glaucoma therapies, cancer in previous 5 years, diuretics (potassium sparing), oestrogen, low molecular weight heparin, No of unique prescriptions, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin; congestive heart failure—digoxin, glaucoma therapies, ACE inhibitors, calcium channel blockers, cancer in previous 5 years, clopidogrel, diuretics (potassium sparing), diuretics (regular), hypertension, income quintile, number of unique prescriptions, chronic renal insufficiency, warfarin, photodynamic therapy in previous 180 days, age
Fig 6 Risk of adverse events in exclusive users of bevacizumab and ranibizumab among patients with diabetes. *Matched on age, sex, history of outcome, and diabetes. †Matched on age, sex, history of outcome, and diabetes; adjusted for: ischaemic stroke—angiotensin converting enzyme (ACE) inhibitors, acetylsalicylic acid/dipyridamole, cancer in previous 5 years, clopidogrel, hypertension, chronic renal insufficiency, warfarin; acute myocardial infarction—ACE inhibitors, cancer in previous 5 years, clopidogrel, diuretics (regular), chronic renal insufficiency, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, warfarin, No of unique prescriptions; venous thromboembolism—cancer in previous 5 years, clopidogrel, low molecular weight heparin, No of unique prescriptions, warfarin; congestive heart failure—digoxin, glaucoma therapies, ACE inhibitors, calcium channel blockers, cancer in previous 5 years, clopidogrel, diuretics (potassium sparing), diuretics (regular), photodynamic therapy in previous 180 days, No of unique prescriptions, chronic renal insufficiency, warfarin
Discussion
The results of our population based study suggest that, overall, intravitreal injections of bevacizumab and ranibizumab are not associated with increased risks of ischaemic stroke, acute myocardial infarction, venous thromboembolism, or congestive heart failure. Furthermore, our analyses suggest that the risks of ischaemic stroke, venous thromboembolism, and congestive heart failure do not differ significantly between bevacizumab and ranibizumab injections. Although we found a statistically significant association between acute myocardial infarction and exclusive bevacizumab use when compared with exclusive ranibizumab exposure in the subgroup with diabetes, our primary analysis and other secondary analyses did not detect any excess risk with either bevacizumab or ranibizumab. As a result, the finding in the diabetes subgroup should be interpreted cautiously given that multiple comparisons were made, raising the risk of a type 1 error, and the fact that this observation was limited to a single analysis of one outcome in one subgroup.
Comparison with other studies
Data from the pivotal clinical trials of bevacizumab and ranibizumab for age related macular degeneration have been inconclusive on the question of safety. Trials comparing intravitreal ranibizumab and bevacizumab against sham injection controls did not detect increased risks of vascular adverse events with either drug.6 7 19 However, a small meta-analysis of early trials with ranibizumab suggested an increased risk of stroke in patients receiving ranibizumab injections.20 Additionally, direct comparison of intravitreal bevacizumab against intravitreal ranibizumab in the Comparison of Age-related Macular Degeneration Treatments Trials found a higher risk of adverse events among those receiving bevacizumab.8 21 However, most of the observed adverse events were conditions not previously associated with vascular endothelial growth factor inhibition.
Clinical trials and small meta-analyses have limitations including a lack of power to detect adverse events and poor generalisability to the types of patients who receive treatment in routine clinical practice.22 Hence, large scale post-marketing studies such as the one we present here provide important information on safety that complements data from clinical trials.23 Overall, our results are consistent with conclusions of previous large observational studies on this topic, providing some reassurance that the widespread use of intravitreal injections of vascular endothelial growth factor inhibitors has not resulted in any clinically important increase in risk for individual patients.24 44 Although the primary analysis of a study of US Medicare patients suggested the possibility that bevacizumab is associated with a greater risk of stroke than is ranibizumab, a secondary analysis, which controlled for the confounding effects of socioeconomic status and the price gap between ranibizumab and bevacizumab, did not confirm this difference in safety.24 45 46 Because of Ontario’s universal coverage of ranibizumab for people over the age of 65, we were able to reduce the potential confounding effects of socioeconomic status in our study. An unpublished study of Medicare patients has suggested an increased risk of death and haemorrhagic stroke with bevacizumab when compared with ranibizumab.47 Publication of these results as well as details regarding how the confounding effects of socioeconomic status were handled will add to our understanding.
Strengths and limitations of study
Strengths of our study include the large, real world study cohort and the population based approach, which maximised generalisability. The large population studied also provided for complete matching of most cases. We examined several important, biologically plausible outcomes and found largely consistent results. The use of validated databases and matching on important potential confounders are further strengths of our study. Our study also benefitted from the rapid transition from use of bevacizumab to ranibizumab in Ontario, which was induced by a shift in the coverage policy of the public drug plan.31 This allowed us to avoid the often unmeasured confounding factors underlying choices between drugs at the individual level, including socioeconomic status.
Several limitations to our study also warrant highlighting. Firstly, we did not capture adverse events that did not lead to hospital admission or an emergency department visit. This probably had a minimal effect on our findings, as the outcomes examined are generally severe enough to result in emergency department visits or hospital admissions. Secondly, we also did not capture severe events leading to death outside of hospital. Thirdly, because bevacizumab is used as an off-label treatment for age related macular degeneration, the indirect ascertainment of exposure could cause misclassification of exposure to this drug. However, we used a series of strategies to reduce the risk of exposure misclassification, and sensitivity analyses showed our results to be robust. Fourthly, to receive intravitreal injections, patients must be healthy enough to attend outpatient clinics. As a result, despite our adjustments for comorbidity, residual confounding could have made both bevacizumab and ranibizumab appear safer than they are in reality when compared with non-use (that is, no intravitreal injections). However, this would not be expected to affect comparisons of risk between the two drugs. Finally, although our estimates generally showed no increased or decreased risk with the use of intravitreal bevacizumab and ranibizumab, the observed confidence intervals include some potentially clinically relevant differences in risk, especially in the subgroup analyses.
Conclusions and policy implications
Because of the enormous costs involved, policies on the use of intravitreal vascular endothelial growth factor inhibitors have broad implications for healthcare systems.48 For instance, despite considerable use of bevacizumab, ranibizumab sales in the United States surpassed $1.6bn (£1.0bn; €1.3bn) in 2011, making ranibizumab the single greatest drug expenditure covered under Medicare part B.49 50 Bevacizumab costs approximately $50-70 per injection, and ranibizumab is priced at about $2000 per injection,8 and public leaders in the United States have recently requested a national coverage determination supporting the use of bevacizumab for age related macular degeneration.51 Similarly, the UK Department of Health, through the National Institute for Health and Clinical Excellence, has begun working towards formally evaluating bevacizumab for age related macular degeneration, and corresponding evaluations are anticipated in many other countries.52 53
Our results suggest that, overall, intravitreal injections of bevacizumab and ranibizumab are not associated with significant risks of ischaemic stroke, acute myocardial infarction, venous thromboembolism, or congestive heart failure. In conjunction with data from efficacy trials including the Comparison of Age-related Macular Degeneration Treatments Trials and the Inhibit Vascular Endothelial Growth Factor in Age-related Choroidal Neovascularisation studies, our safety data support the use of bevacizumab in the treatment of age related macular degeneration.8 54 However, several important questions require clarification. In particular, further studies focusing on the risk of acute myocardial infarction in patients with diabetes treated with intravitreal vascular endothelial growth factor inhibitors are warranted. Additionally, the potential for bevacizumab’s low price to discourage investment in innovative treatments for age related macular degeneration, and the need to design safe and accountable processes to support distribution of bevacizumab for intravitreal use, remain important.55 56 57 Furthermore, the entrance of additional vascular endothelial growth factor inhibitors onto the market will necessitate further comparative studies of effectiveness and safety. Clearly, with billions of dollars of healthcare at stake, stakeholders on all sides—from patients and physicians to governments, third party payers, and industry—must continue to evaluate all available data to ensure that accessibility, affordability, and quality of care are maximised.
What is already known on this topic
Intravenous administration of vascular endothelial growth factor inhibitors for cancer has been associated with several adverse vascular events
Clinical trials have been inconclusive regarding the risks associated with the smaller doses of vascular endothelial growth factor inhibitors used to treat age related macular degeneration
What this study adds
This population based, nested case-control study was carried out in a setting with universal healthcare insurance to minimise the confounding effects of socioeconomic status
Overall, intravitreal injections of neither bevacizumab nor ranibizumab were associated with increased risks of ischaemic stroke, acute myocardial infarction, venous thromboembolism, or congestive heart failure
We thank Erica Campbell for assistance with project management, creation of tables, and manuscript editing. She is funded through research grants from the Canadian Institutes of Health Research and the Ontario Ministry of Health and Long-Term Care. We also thank Brogan Inc, Ottawa, Canada, for use of their drug product and therapeutic class database.
Contributors: All authors were involved in the study concept and design and in the analysis and interpretation of data. RJC drafted the manuscript and approved the final version to be published. SSG, SEB, JMP, MW, and CMB critically revised the manuscript for important intellectual content. RJC, MW, SEB, SSG, and CMB did the statistical analysis. RJC and SSG obtained funding. RJC, SEB, and SSG supervised the study. RJC is the guarantor.
Funding: This study was funded by the Canadian Institutes of Health Research (CIHR) and the Ontario Ministry of Health and Long-Term Care’s (MOHLTC) drug innovation fund. RJC is supported by a clinician scientist award from the Southeastern Ontario Academic Medical Organization. SSG and SEB are supported by CIHR new investigator awards from the Institute of Aging. CMB is supported by a CIHR and Canadian Patient Safety Institute chair in patient safety and continuity of care. The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit for publication. The authors are affiliated with the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario MOHLTC. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: RJC is supported by a Clinician Scientist Award from the Southeastern Ontario Academic Medical Organization, which receives funding from the Ontario Ministry of Health and Long-Term Care (MOHLTC) and holds research grants from the Canadian Institutes of Health Research (CIHR) and the MOHLTC for related work; SSG and SEB are supported by CIHR new investigator awards from the Institute of Aging; CMB is supported by a CIHR and Canadian Patient Safety Institute chair in patient safety and continuity of care; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; SSG is a member of the Ontario MOHLTC Committee to Evaluate Drugs, which provides advice to the Ontario MOHLTC on drug related matters (such as drugs to list on provincial formulary), and RJC is an adviser to the Ontario MOHLTC Committee to Evaluate Drugs (in these roles, neither has reviewed bevacizumab or ranibizumab); no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study protocol was approved by the Research Ethics Board at Queen’s University, Kingston, Ontario, Canada (file No 6004374).
Data sharing: No additional data available.
Cite this as: BMJ 2012;345:e4203
References
- 1.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol 2004;137:486-95. [DOI] [PubMed] [Google Scholar]
- 2.Cruess A, Zlateva G, Xu X, Rochon S. Burden of illness of neovascular age-related macular degeneration in Canada. Can J Ophthalmol 2007;42:836-43. [DOI] [PubMed] [Google Scholar]
- 3.Javitt JC, Zhou Z, Maguire MG, Fine SL, Willke RJ. Incidence of exudative age-related macular degeneration among elderly Americans. Ophthalmology 2003;110:1534-9. [DOI] [PubMed] [Google Scholar]
- 4.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med 2008;358:2606-17. [DOI] [PubMed] [Google Scholar]
- 5.Ip MS, Scott IU, Brown GC, Brown MM, Ho AC, Huang SS, et al. Anti-vascular endothelial growth factor pharmacotherapy for age-related macular degeneration—a report by the American Academy of Ophthalmology. Ophthalmology 2008;115:1837-46. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432-44. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-31. [DOI] [PubMed] [Google Scholar]
- 8.CATT Research Group, Martin D, Maguire M, Ying G, Grunwald J, Fine S, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamba T, McDonald D. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 2007;96:1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuñón J, Ruiz-Moreno JM, Martín-Ventura JL, Blanco-Colio LM, Lorenzo O, Egido J. Cardiovascular risk and antiangiogenic therapy for age-related macular degeneration. Surv Ophthalmol 2009;54:339-48. [DOI] [PubMed] [Google Scholar]
- 11.Snider KL, Maitland ML. Cardiovascular toxicities: clues to optimal administration of vascular endothelial growth factor signaling pathway inhibitors. Targeted Oncology 2009;4:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csaky K, Do DV. Safety implications of vascular endothelial growth factor blockade for subjects receiving intravitreal anti-vascular endothelial growth factor therapies. Am J Ophthalmol 2009;148:647-56. [DOI] [PubMed] [Google Scholar]
- 13.Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 2007;99:1232-9. [DOI] [PubMed] [Google Scholar]
- 14.Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol 2011;29:632-8. [DOI] [PubMed] [Google Scholar]
- 15.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008;300:2277. [DOI] [PubMed] [Google Scholar]
- 16.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci 2005;46:726-33. [DOI] [PubMed] [Google Scholar]
- 17.Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol 1999;27:536-44. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26:859-70. [DOI] [PubMed] [Google Scholar]
- 19.Tufail A, Patel PJ, Egan C, Hykin P, da Cruz L, Gregor Z, et al. Bevacizumab for neovascular age related macular degeneration (ABC trial): multicentre randomised double masked study. BMJ 2010;340:c2459. [DOI] [PubMed] [Google Scholar]
- 20.Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology 2009;116:362. [DOI] [PubMed] [Google Scholar]
- 21.Martin DF, Maguire MG, Fine SL, Ying G, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012; published online 1 May. [DOI] [PubMed]
- 22.Liew G, Mitchell P, Gillies MC, Wong TY, Rosenfeld PJ, Brown DM, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2007;356:747. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, Psaty BM. Benefits and risks of drug treatments. JAMA 2008;300:2417-9. [DOI] [PubMed] [Google Scholar]
- 24.Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol 2010;128:1273-9. [DOI] [PubMed] [Google Scholar]
- 25.A summary of studies on the quality of health care administration databases in Canada. In: Goel V, Williams JI, Anderson GM, Blackstien-Hirsch P, Fooks C, Naylor D, eds. Patterns of health care in Ontario: the ICES practice atlas. 2nd ed. Canadian Medical Association, 1996:339-45 (available at www.ices.on.ca/file/Practice2-appendix.pdf).
- 26.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 2003;10:67-71. [PubMed] [Google Scholar]
- 27.Juurlink DN, Preyra C, Croxford R, Chong A, Austin P, Tu J, et al. Canadian Institute for Health Information discharge abstract database: a validation study. ICES investigative report. Institute for Clinical Evaluative Sciences, 2006 (available at www.ices.on.ca/file/CIHI_DAD_Reabstractors_study.pdf).
- 28.Gruneir A, Bell CM, Bronskill SE, Schull M, Anderson GM, Rochon PA. Frequency and pattern of emergency department visits by long-term care residents—a population-based study. J Am Geriatr Soc 2010;58:510-7. [DOI] [PubMed] [Google Scholar]
- 29.Gill SS, Anderson GM, Fischer HD, Bell CM, Li P, Normand SLT, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med 2009;169:867. [DOI] [PubMed] [Google Scholar]
- 30.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512-6. [DOI] [PubMed] [Google Scholar]
- 31.Campbell RJ, Bronskill SE, Bell CM, Paterson JM, Whitehead M, Gill SS. Rapid expansion of intravitreal drug injection procedures, 2000 to 2008: a population-based analysis. Arch Ophthalmol 2010;128:359-62. [DOI] [PubMed] [Google Scholar]
- 32.Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care 2005;43:182-8. [DOI] [PubMed] [Google Scholar]
- 33.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001;154:854-64. [DOI] [PubMed] [Google Scholar]
- 34.Bell CM, Hatch WV, Fischer HD, Cernat G, Paterson JM, Gruneir A, et al. Association between tamsulosin and serious ophthalmic adverse events in older men following cataract surgery. JAMA 2009;301:1991. [DOI] [PubMed] [Google Scholar]
- 35.Tu K, Campbell NRC, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Medicine 2007;1:e18. [PMC free article] [PubMed] [Google Scholar]
- 36.Breslow N, Day N. Fitting models to continuous data. 82nd ed. IARC Scientific Publications, 1987:178-229.
- 37.Suissa S. Novel approaches to pharmacoepidemiology study design and statistical analysis. John Wiley and Sons, 2005:811-29.
- 38.Breslow N, Lubin J, Marek P, Langholz B. Multiplicative models and cohort analysis. J Am Stat Assoc 1983;78:1-12. [Google Scholar]
- 39.Essebag V, Platt RW, Abrahamowicz M, Pilote L. Comparison of nested case-control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol 2005;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liddell F, McDonald J, Thomas D. Methods of cohort analysis: appraisal by application to asbestos mining. J R Stat Soc Ser A 1977;140:469-91. [Google Scholar]
- 41.Institute for Clinical Evaluative Sciences. Ontario stroke evaluation report 2011: improving system efficiency by implementing stroke best practices. Supplementary materials. www.ices.on.ca/webpage.cfm?site_id=1&org_id=68&morg_id=0&gsec_id=0&item_id=6813&type=report.
- 42.White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I-4,I-8. [DOI] [PubMed] [Google Scholar]
- 43.Public Health Agency of Canada. Tracking heart disease and stroke in Canada. 2009. www.phac-aspc.gc.ca/publicat/2009/cvd-avc/index-eng.php.
- 44.French DD, Margo CE. Age-related macular degeneration, anti-vascular endothelial growth factor agents, and short-term mortality: a postmarketing medication safety and surveillance study. Retina 2011;31:1036-42. [DOI] [PubMed] [Google Scholar]
- 45.Rosenfeld PJ. Bevacizumab versus ranibizumab for AMD. N Engl J Med 2011;364:1966-7. [DOI] [PubMed] [Google Scholar]
- 46.Al-Qureshi S, Shaikh S. Intravitreous bevacizumab adult safety data: the evidence so far. Clin Experiment Ophthalmol 2012;40:3-5. [DOI] [PubMed] [Google Scholar]
- 47.Gower EW, Cassard S, Chu L, Varma R, Klein R. Adverse event rates following intravitreal injection of Avastin or Lucentis for treating age-related macular degeneration [abstract 6644]. ARVO Visionary Genomics, Fort Lauderdale, Florida, May 2011 (available at www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=3a667d20-f42d-421e-a859-e1b680de80ed&cKey=4e534aee-b678-4b9d-91dc-20a9d6ae0c56&mKey=%7b6F224A2D-AF6A-4533-8BBB-6A8D7B26EDB3%7d).
- 48.Campbell RJ, Dhalla IA, Gill SS, Bell CM. Implications of “not me” drugs for health systems: lessons from age related macular degeneration. BMJ 2012;344:e2941. [DOI] [PubMed] [Google Scholar]
- 49.Annual report 2011. F. Hoffmann-La Roche, 2012:66 (available at www.roche.com/investors/annual_reports.htm).
- 50.Medicare Payment Advisory Commission. A data book: healthcare spending and the Medicare program. MedPAC, 2010 (available at www.medpac.gov/documents/Jun10DataBookEntireReport.pdf).
- 51.Mitka M. Macular degeneration coverage. JAMA 2011;306:1073. [Google Scholar]
- 52.National Institute for Health and Clinical Excellence. NICE’s report on the feasibility of appraising the use of bevacizumab (Avastin) to treat eye conditions. NICE, 2010 (available at: www.nice.org.uk/newsroom/pressreleases/BevacizumabAvastinToTreatEyeConditions.jsp).
- 53.National Institute for Health and Clinical Excellence. Bevacizumab (Avastin) for eye conditions: Report of findings from a workshop held at NICE on 13 July 2010. www.nice.org.uk/media/AC2/3C/BevacizumabEyeConditionsPrescopingReport.pdf.
- 54.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012; published online 10 May. [DOI] [PubMed]
- 55.Ferner R, Hughes DA, Aronson J. NICE and new: appraising innovation. BMJ 2010;340:245-7. [DOI] [PubMed] [Google Scholar]
- 56.Pollack A. Doctors grow wary of Avastin for eye treatment. New York Times 2011. Oct 4. http://prescriptions.blogs.nytimes.com/2011/10/04/doctors-grow-wary-of-avastin-for-eye-treatment/.
- 57.Berkrot B, Pierson R. VA halts Avastin use for eye disease. Reuters (US edition) 2011 Sep 21. www.reuters.com/article/2011/09/21/us-roche-va-idUSTRE78K6F520110921.