Abstract
Adipose tissue engineering has recently gained significant attention from materials scientists as a result of the exponential growth of soft tissue filler procedures being performed within the clinic. While several injectable materials are currently being marketed for filling subcutaneous voids, they often face limited longevity due to rapid resorption. Their inability to encourage natural adipose formation or ingrowth necessitates repeated injections for a prolonged effect, and thus classifies them as temporary fillers. As a result, a significant need for injectable materials that not only act as fillers, but also promote in vivo adipogenesis is beginning to be realized. This review will discuss the advantages and disadvantages of commercially available soft tissue fillers. It will then summarize the current state of research using injectable synthetic materials, biopolymers, and extracellular matrix-derived materials for adipose tissue engineering. Furthermore, the successful attributes observed across each of these materials will be outlined along with a discussion of the current difficulties and future directions for adipose tissue engineering.
Introduction
Traditional research regarding adipose tissue has focused on the obesity epidemic and growing prevalence of diabetes. However, more recently, researchers have begun to recognize the need of certain patients to actually gain or regenerate lost adipose tissue and, therefore, have started to apply the principles of tissue engineering to achieve this goal. In addition to the more common elective cosmetic procedures, there is a population of patients that would benefit from adipose regeneration due to congenital defects, trauma, or surgical resections. This patient population includes third-degree burn victims who have lost subcutaneous fat layers, children with hemifacial lipoatrophy or other congenital defects that cause underdeveloped features, and women undergoing lumpectomies following breast cancer treatment. These patients, among others suffering from congenital and post-traumatic defects, highlight the growing need for biomaterials that can not only replace lost or damaged adipose tissue, but also encourage its natural regeneration and continual integration with surrounding tissue throughout the lifetime of the patient.
Adipose tissue is most widely known for its role in energy metabolism through lipid storage and catabolism. However, it also has a prominent function in bodily aesthetics, symmetry, and cushioning, and even serves as a secretory organ releasing paracrine factors that communicate with the endocrine, immune, and cardiovascular systems [1]. Adipose tissue is primarily composed of lipid-filled adipocytes (Figure 1A), but also contains fibroblasts, neural and vascular cells, and more importantly, a population of multipotent progenitor cells. These adipose-derived adult stem cells, or ASCs, possess the ability to proceed down several mesodermal lineages,[2] including osteoblasts, chondrocytes, myoblasts, and preadipocytes (Figure 1B). ASCs have become the focus of many tissue engineering strategies as a result of their plasticity and potential autologous usage, as well as their relative ease of harvest. Furthermore, ASCs have been shown to reduce fibrous encapsulation of subcutaneously implanted materials [3]. As this review focuses on the materials used for adipose tissue engineering as opposed to the therapeutic potential of the cells themselves, readers are referred to a review by Gomillion et al for a more in-depth analysis of stem cell types being investigated for adipose regeneration [4].
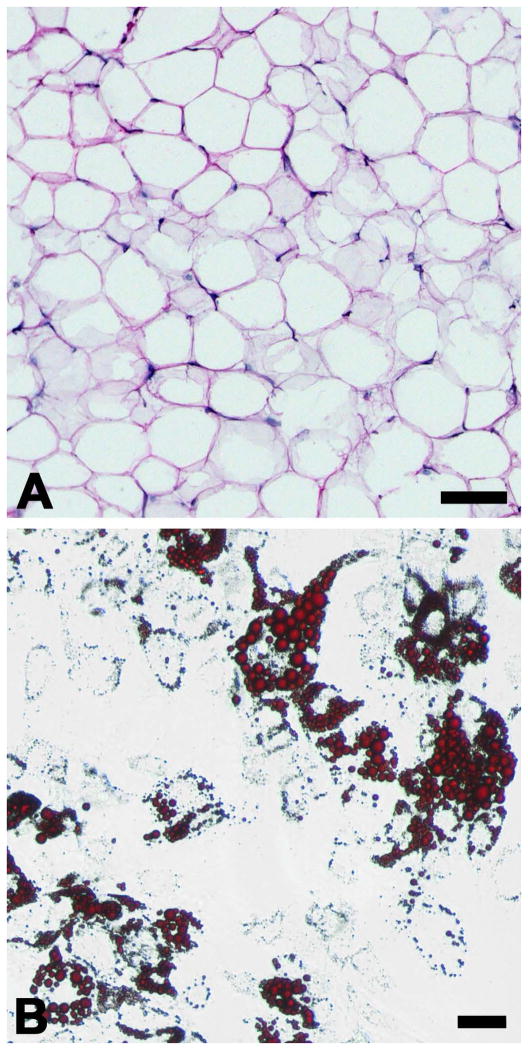
Figure 1. Adipose Tissue and Preadipocytes.
Histology of adipose tissue, visualized with H&E staining, reveals its characteristic architecture (A). The “voids” seen in this image are the location of lipid-filled vacuoles that have been washed away as a result of the staining reagents. Adipose-derived stem cells can be isolated from adipose tissue and differentiated in vitro into adipocytes that collect lipid filled vacuoles, stained here with Oil Red O (B). Scale bars = 100 μm.
Over the past decade, the development of various biomaterials for adipose tissue engineering has seen exponential growth. Synthetics and biopolymers alike have been investigated for their ability to interact with subcutaneous adipose tissue in animal models or promote adipogenesis of stem cells in vitro. While much of this work has been pursued using structured three-dimensional scaffolds, we will focus here solely on injectable materials for adipose engineering (those interested in a more predefined-shape approach are referred to a concise review by Flynn and Woodhouse in 2008 [5]). Injectable materials for tissue engineering offer the significant advantage of adapting to complex voids and thus allowing greater patient customization. This is especially beneficial for procedures attempting to replace excised malignant tissue. The initial fluid-like behavior of injectable materials allows immediate scalability for a range of defect shapes or sizes and facilitates the fine contouring of facial features. Furthermore, materials for adipose regeneration will generally be placed subcutaneously, just below the surface of the skin. Injectable materials allow for minimally-invasive delivery, which reduces surgical complications of implantation and prevents excessive scarring. This review will discuss injectable materials that are currently available to the clinician for soft tissue repair and then will summarize current research encompassing synthetics, biopolymers, and extracellular matrix materials for adipose tissue engineering.
Commercially Available Soft Tissue Fillers
Over the past decade, soft tissue filler procedures have increased nearly 200% with over one and a half million procedures in 2010 alone, as reported by the American Society of Plastic Surgeons [6]. Accordingly, the number of soft tissue fillers on the market has also magnified. The most prevalent products feature derivatives of hydroxylapatite, polylactic acid (PLA), collagen, or hyaluronic acid (HA). Other notable materials include polymethylmethacrylate (PMMA), polyacrylamide, and dextran [7]. PLA and hydroxylapatite both have established some of the longer persistence profiles, boasting subcutaneous durations out to 12 or even 18 months. However, they have also been frequently associated with granuloma formation in certain locations and foreign body reactions [8]. Hydroxylapatite also has the disadvantage of being radiopaque, which would be particularly undesirable in breast reconstruction following cancerous lumpectomy. Collagen-based injectables have recently experienced a precipitous decline in usage over the past few years, despite once dominating the soft tissue filler market. Their reduced usage can be partly attributed to their relatively rapid resorption within 6 months, but is also due to the rise of hyaluronic acid-based fillers. HA is a naturally occurring polysaccharide with an excellent immune profile and minimal allergy concerns [9, 10]. Currently, there are over 10 different derivatives of HA-based fillers that have received approval from the Food and Drug Administration. These fillers have a demonstrated longevity of up to 1 year and few adverse side effects [10]. However, despite these positive results, HA remains classified as a temporary soft tissue filler. As with all of these materials, repeated injections are required to maintain the desired outcome. Furthermore, these commercial products do not induce adipose tissue regeneration, serving only as temporary fillers, and thus highlight the need for biomaterials that will encourage sustained adipose formation in the injection region.
Challenging hyaluronic acid’s dominance among soft tissue fillers is a procedure known as lipotransfer, or autologous fat grafting. This technique takes a patient’s own adipose tissue, typically removed via liposuction, and re-injects it into a separate location on the same patient to fill subcutaneous voids. The use of autologous tissue obviates any concerns regarding immune rejection, and has even been seen to generate new adipose tissue in the site of injection [11]. Clinical evaluation of fat grafting has suggested it has a subcutaneous persistence over 1 year, with some of the success being attributed to both the presence of endogenous ASCs within the graft and biochemical similarities between the graft and host tissues [12, 13]. Concomitant injection of platelet-rich plasma (PRP), an autologous source of multiple growth factors derived from blood plasma, along with fat grafts resulted in a 70% retention of volume in a 1-year follow-up study [14]. Additionally, the absence of material costs and increased longevity associated with lipotransfer project it as a highly cost-effective option for filling subcutaneous voids [15]. However, this treatment is not without limitation. Mature adipocytes are poorly equipped to survive the trauma of liposuction and ischemic conditions present in the implant region. This results in potential necrotic portions of the fat graft and contributes to its relatively unpredictable performance. The injected lipoaspirate also contains a heterogeneous mixture of tissue pieces and free oils, which place constraints on fine contouring and behavior of the material during injection, thereby introducing variability to the survival of the implant. As a result, there remains a significant clinical need for engineered injectable materials that avoid immune complications and offer long-term maintenance of volume by encouraging new fat formation.
Synthetic Injectable Materials for Adipose Tissue Engineering
Synthetic polymers have become a popular choice for tissue engineers for a variety of applications as they offer stringent control over composition and mechanical properties of the implanted material. As a result, multiple synthetic polymers have seen use in adipose tissue engineering, ranging from polylactic acid (PLA) and polyethylene glycol (PEG) derivatives to polyethylene terephthalate (PET) and polytetrafluorethylene (PTFE) [16–19]. Several of these materials have already been approved by the Food and Drug Adminstration for various in vivo uses and are able to maintain the shape and volume of the scaffold in vivo, a highly desirable trait for adipose tissue engineering. However, synthetic materials lack any form of innate bioactivity to encourage adipogenesis on their own and many are not compatible with in situ gelation because of potentially toxic byproducts, which places constraints on injectable delivery. These drawbacks necessitate undesirable surgical implantation of the scaffold and often lead to fibrous encapsulation of the material and a foreign body response [20, 21]. As a result, degradable polymers, such as poly(lactic-co-glycolic acid) (PLGA), have gained considerable attention in the field because they could help reduce the immune response by allowing cellular infiltration and a positive resolution to the immune response. An interesting study by Brandl et al described the incorporation of a collagenase-sensitive substrate into the backbone of a PEG hydrogel, allowing it to be enzymatically degraded. When NIH 3T3-L1 preadipocytes were cultured on the degradable hydrogel, the authors noticed significantly higher levels of intracellular triglycerides and a more mature adipocyte phenotype compared to those cultured in non-degradable hydrogel controls [22]. These results highlight the potential importance of material degradation on adipose regeneration.
Researchers have also harnessed the tunable degradation of synthetic polymers for use in cell and drug delivery to adipose tissue. Choi et al were able to impart injectable functionality to PLGA by producing 250 μm diameter microspheres that could be delivered subcutaneously [23]. These particles were able to support proliferation and adipogenic differentiation of human ASCs. Furthermore, in vivo studies revealed that the PLGA microspheres were able to encourage significantly more adipose tissue formation after 2 months when combined with adipocyte-differentiated ASCs than when the cells were injected on their own [24]. In addition to being a cell delivery vehicle, PLGA particles have also been investigated for their ability to deliver growth factors and other bioactive molecules that encourage in vivo adipogenesis. Basic fibroblast growth factor (bFGF) has been shown to increase the proliferation and adipogenic potential of ASCs in vitro [25]. When PLGA particles were used to encapsulate and provide sustained release of FGF in vivo, studies saw improved cell survival and neovascularization in the injection region [26]. However, it should be noted that synthetic polyesters that degrade via hydrolysis, such as PLGA, have been shown to produce acidic byproducts during both in vitro and in vivo degradation. While these degradation products do not cause systemic toxicity, they can produce dramatic changes in pH of the local microenvironment if accumulated. Due to bulk hydrolysis of PLGA microspheres, several groups have reported an average pH of 3.5 within degrading microspheres, reaching as low as 1.5 in the center of larger particles (~ 40 μm diameter) [27, 28]. While diffusion of the by-products typically equilibrates the pH of the local environment over time, this drastic drop in pH could denature a protein payload or have adverse effects on cells or surrounding tissue. Despite these limitations, injectable synthetic polymers still hold promise for various functions in adipose tissue engineering, playing either a structural role by providing mechanical support to the implant or a supplemental role by delivering cells or bioactive molecules to the injection region.
Injectable Biopolymers for Adipose Tissue Engineering
Natural biopolymers have seen widespread use in adipose tissue engineering research and are currently dominating the clinical market for subcutaneous fillers. These materials offer the advantages of bioactivity and cell adhesion, accompanied by a limited immune response for most patients. Collagen was one of the first dermal fillers approved by the Food and Drug Administration and has been extensively researched in tissue engineering as a stiff sponge [29, 30]. However, in an injectable form, collagen has seen rapid resorption and limited vascular formation following subcutaneous injection [31, 32]. Other injectable biopolymers, such as alginate and fibrin, have produced only a slightly more favorable outcome. Marler et al described the development of an RGD-modified version of alginate that was able to maintain 58% of its original volume after 2 months in vivo and up to 88% when fibroblasts were added [33]. While no adipogenesis was reported in these studies, future versions of the RGD-modified alginate that also incorporated ASCs saw some lipid-filled adipocytes, vascular ingrowth, and limited fibrous encapsulation in the injection region [34]. Similar results have been seen with fibrin gels. Injections of fibrin alone were resorbed within 4 weeks in vivo but were able to maintain 50% of their original volume when delivering adipocyte-differentiated ASCs [35, 36]. Interestingly, when a polyglycolic acid (PGA) support structure was implanted subcutaneously to form a protective cage around the ASC-fibrin injection, the implant experienced relatively little volume loss and a significant increase in the number of new mouse- and human-origin adipocytes (Figure 2) [37]. While the subcutaneous environment is relatively static compared to the cyclic loads experienced by bone and cartilage tissues, the skin does exert a compressive force on any material placed subcutaneously. Perhaps these PGA cages bear the compressive load of the surrounding dermal tissue and thus protect the biopolymer hydrogel until it can establish adequate integration with the host tissue. These results suggest that maintaining the volume of an injectable scaffold in vivo plays a significant role in facilitating the formation of new adipose tissue. It is likely that this volume retention is highly dependent on the structural integrity of the injected material to withstand the compressive forces of the subcutaneous environment, however, the optimal strength for this interaction has yet to be identified.
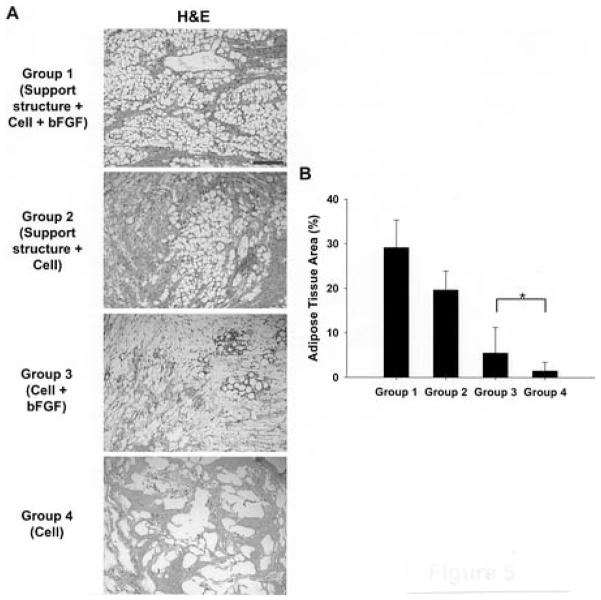
Figure 2. Fibrin Injections for Adipose Tissue Engineering.
Cho et al showed improved adipogenesis at 6 weeks when a PGA support structure was implanted subcutaneously to protect fibrin + preadipocyte injections from tissue constriction (Groups 1 & 2). Addition of bFGF to fibrin + preadipocyte injections further enhanced lipid accumulation (Groups 1 & 3). Group 4 also shows the relative inability of fibrin + preadipocyte injections to produce fatty tissue on their own. Scale bar = 200 μm (A). The relative amount of adipose tissue present was quantified using an Oil Red O stain to verify that the support structures significantly increased adipogenesis (B). Reprinted in part with permission from [37].
Overall, the inadequate structural strength and rapid resorption of the unmodified hydrogel versions of these biopolymers have resulted in their predominant use as delivery vehicles for cells and growth factors. Porous collagen microparticles, known as CultiSphers, have been shown to support the proliferation and adipogenic differentiation of ASCs, and has the potential to be delivered subcutaneously if suspended in a biocompatible liquid [38]. Other biological materials, such as microscale particulate forms of chitosan, have also been shown to support ASC attachment and proliferation and can deliver the cells via subcutaneous injection [39]. Alternatively, gelatin and alginate have been used to encapsulate bFGF for sustained delivery in vivo. Similar to the results seen with degradable PLGA, the sustained-release of bFGF or FGF-1 from gelatin and alginate beads increased neovascularization and volume retention [40, 41]. The prolonged diffusion of bFGF from fibrin gels, which is speculated to occur over the course of 7 days from a 0.5 mL injection, has also been shown to increase adipogenesis of simultaneously injected ASCs [37].
Hyaluronic acid (HA) is currently the most utilized biopolymer in adipose tissue engineering, seeing usage both in the clinic and research laboratories. While native HA has an abbreviated persistence profile comparable to the previously discussed biopolymers, it does have an available carboxylic acid side chain in its disaccharide backbone that makes it particularly amenable to chemical modification. This allows HA to be uniquely functionalized for a variety of applications by tailoring its degradation, strength, and interaction with other materials. Multiple implantable versions of HA have been created using PEG-diacrylate or benzyl esterification to crosslink the HA into a stiffer scaffold, but these have only produced limited adipogenesis and were not injectable, and therefore will not be discussed here [42–44]. Focusing on injectable versions, several derivatives of hyaluronic acid have been generated via amidation of the carboxylic acid to attach long, linear carbon chains and thereby increase the viscosity of HA gels. Unfortunately, these materials are relatively quickly broken down in vivo by endogenous hyaluronidases [45]. These studies suggested that chemical crosslinking is necessary for HA to function as a subcutaneous filler; a concept supported by the universal occurrence of crosslinking among commercial HA products. Tan et al recently introduced a “double-crosslinked” version of HA capable of in situ crosslinking and gelation. This study combined an amine-functionalized HA with an aldehyde-functionalized HA that spontaneously crosslink via a Schiff-base linkage, while concurrently using genipin, a biocompatible crosslinker derived from gardenia fruit, to create a second crosslinking action [46]. This allowed for broad tuning of the degradation and elastic modulus of the material, but has yet to be tested for adipogenesis. Alternatively, Chung et al used 1-ethyl-3-[3-dimethylaminopropyl]-carbodiimide hydrochloride (EDC) to covalently link HA to the surface of PLGA microspheres. When these particles were combined with primary ASCs, cell/microsphere aggregates developed that ranged in size up to 1 mm [47]. Despite some loss in volume, these aggregates did encourage the accumulation of some lipids upon subcutaneous injection and could be a possible cell delivery vehicle for adipose tissue engineering. Non-chemically modified versions of HA gels have also been created by blending HA with either collagen or thrombin and platelet-rich plasma (PRP). These blends showed good biocompatibility and cell attachment, but no adipose tissue formation was reported and both materials suffered from rather quick resorption [48, 49]. While each of these versions of HA has improved the longevity and cellular interaction of the injected material, there remains limited evidence to suggest HA alone is capable of being more than just a temporary filler material.
More recently, synthetic materials have been combined with native biopolymers, such as collagen and hyaluronic acid, to create cell-adhesive, injectable synthetic polymers. These materials take advantage of the adjustable mechanical properties of a synthetic material while also incorporating natural cell binding domains of biopolymers. In 2007, Vashi et al published a study where they blended collagen with Pluronic F127, a non-ionic poloxamer surfactant. The results showed that the Pluronic F127 hydrogel did not inhibit adipogenesis of mesenchymal stem cells grown in adipogenic induction media [50]. Because the length of the co-polymer blocks that comprise Pluronics can be customized to impart a variety of mechanical properties to the material, the addition of cell-adhesive collagen generates a set of ASC-compatible hydrogels that do not interfere with adipogenesis; however, these materials have not yet been investigated for in vivo adipogenesis. A thermoresponsive version of hyaluronic acid was later developed by Tan et al by chemically linking it to poly(N-isopropylacrylamide) (PNIPAAm). This material has a lower critical solution temperature (LCST) of 30 °C and supported ASC growth for roughly one month. It also gelled upon subcutaneous injection, however, there was no reported cellular infiltration or neovascularization [51]. Further expanding on these biohybrids, Hillel et al recently published a report on an in situ crosslinked PEG-HA composite (Figure 3). The material blends PEG diacrylate with eosin Y and commercial formulations of HA, and then uses a light emitting diode (LED) to photocrosslink the components after subcutaneous injection [52]. These materials were evaluated in both rodent and human trials and demonstrated biocompatibility and excellent volume retention. However, some chronic inflammation was seen and a thin pseudocapsule had formed around the injections. No neovascularization was noted within the implants and they were also not assessed for adipose formation [52]. Together, these studies indicate a trend towards developing materials that attempt to harness each of the positive attributes of both synthetic and natural polymers to achieve a mechanically stable and biocompatible material for injectable adipose tissue engineering.
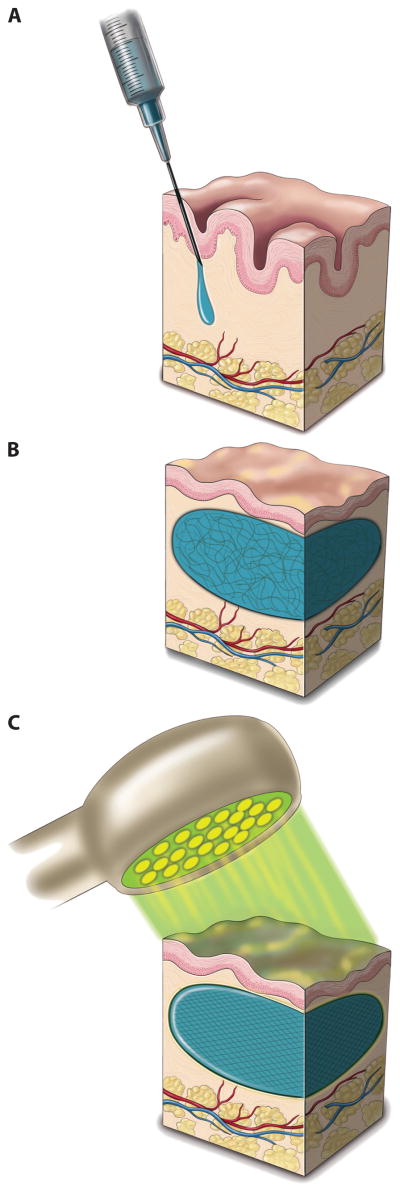
Figure 3. Injection of Photoactivated PEG-HA Hydrogel.
Hillel et al were able to create an in situ crosslinking injectable filler by combining hyaluronic acid (HA) with PEG-diacrylate and the photoinitiator, eosin Y. This mixture of components was injected transdermally (A) and could be massaged into a desired shape (B). The material was then crosslinked by shining an array of light emitting diodes (LEDs) emitting light at a wavelength of 520 nm (C). This light was shown to penetrate up to tissue depths of 4 mm, and a 2 minute exposure time was sufficient to activate the eosin Y and photocrosslink the composite implant. Reprinted with permission from [52].
Injectable Extracellular Matrix Based Materials
The relative success of autologous fat transfers in the clinic has altered the focus for materials development within adipose tissue engineering, shifting design criteria away from materials that only temporarily fill subcutaneous space, and toward those that potentially encourage the development and ingrowth of new adipose tissue. Despite the necrosis and resorption issues associated with fat grafting, new adipose tissue has been seen to develop in the injection region [11]. This implies that the extracellular composition of the fat graft may be contributing to the perceived benefit of the procedure, and emphasizes the need to explore injectable materials that more closely mimic the adipose microenvironment. Matrigel is a complex mixture of proteins and growth factors derived from the extracellular matrix of Engelbreth-Holm-Swarm mouse sarcoma cells, which have been considered a basement membrane tumor [53]. Some of the main components of Matrigel, such as laminin and type IV collagen, are also present in adipose tissue [54]. Several studies have demonstrated Matrigel to be highly adipogenic in vivo when injected alongside bFGF in mice (Figure 4) [55–57]. These experiments reported extensive neovascularization and adipose development that was specifically derived from host cells rather than exogenously implanted ones. Furthermore, controls consisting of bFGF alone or within a collagen gel did not promote adipogenesis [57]. While Matrigel is not a viable option for clinical usage due to its sarcoma cell origin, these results suggest that materials with complex combinations of extracellular matrix components could be critical to the regeneration of adipose tissue. Several groups have since used similar processing to produce Matrigel-like products from more clinically relevant sources. Abberton et al described the production of Myogel, a mixture of extracellular proteins derived from human, pig, or rat skeletal muscle explants. Myogel contains many basement membrane proteins and proteoglycans that are also found in adipose tissue. This material encouraged adipogenic differentiation of NIH 3T3-L1 preadipocytes and the development of mature adipocytes in an in vivo rat AV-loop model, significantly outperforming Matrigel [58]. Uriel et al saw similar results using rat subcutaneous adipose tissue derived proteins [59]. Although bFGF was not exogenously supplied in these experiments, it was found to be to be present in both materials at concentrations significantly higher than those seen in standard Matrigel. Other acellular materials, such as decellularized dermis or small intestinal submucosa, have not seen this adipogenic effect and function better as hASC delivery vehicles [26, 60]. From these studies, it is becoming apparent that a synergistic relationship exists between angiogenic growth factors and certain extracellular matrix components that promotes de novo development of adipose tissue.
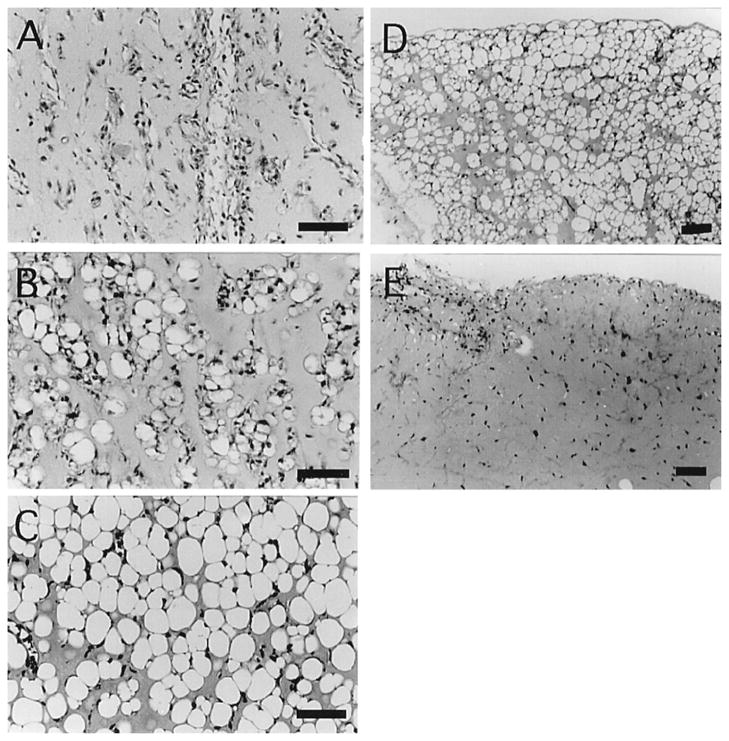
Figure 4. bFGF Required for Matrigel-induced Adipogenesis in vivo.
Subcutaneous injections of Matrigel and 1 μg/mL bFGF were performed in mice and H&E stained sections were analyzed over a 10-week period by Kawaguchi et al [57]. After 1 week, neovascularization could be seen in the injection region (A), and by 2 weeks fibroblast-like cells had invaded and differentiated into adipocytes (B). At 5 weeks, significant adipocyte formation and maturation was seen in the injection region (C), which persisted through the entire 10-week duration of the study (D). However, when bFGF was not included in the Matrigel injections, limited adipogenesis was seen (E). Copyright 1998, National Academy of Sciences, USA.
Expanding on the results of these Matrigel-like products, recent attempts have been made to produce extracellular matrix materials directly from components of human lipoaspirate. Choi et al reported the development of a powder form of human lipoaspirate by homogenizing the adipose tissue and lyophilizing the remaining material beneath the oily supernatant. This processing produced a fine powder of adipose extracellular components that supported ASC proliferation and could be delivered in vivo as a suspension. Subcutaneous injections of this material led to neovascularization in the injection region and, when ASCs were also supplied, the formation of new fatty tissue and adipose-specific gene expression [61]. Turner and Flynn have also produced a particulate form of human adipose tissue that could be used for injectable cell delivery [62]. Alternatively, Young et al published a method for directly decellularizing and delipidizing human lipoaspirate [63]. This decellularized matrix material retained many of the extracellular matrix proteins and proteoglycans characteristic of native adipose tissue. The adipose matrix could be further processed into a thermoresponsive liquid formulation that would self-assemble into a stable hydrogel upon warming to 37 °C, allowing for subcutaneous delivery through a small gauge needle (Figure 5). While this material has yet to be examined for in vivo adipogenesis, decellularized skeletal muscle and cardiac tissues that were processed in a similar manner have been shown to promote the maturation of tissue-specific progenitor cells [64]. Collectively, all of these results suggest that materials that mimic the complex composition of extracellular components specific to adipose tissue, combined with the angiogenic activity of certain growth factors, may provide an advantageous platform for the development of inherently adipogenic tissue engineering strategies.
Figure 5. Lipoaspirate-derived Hydrogels.
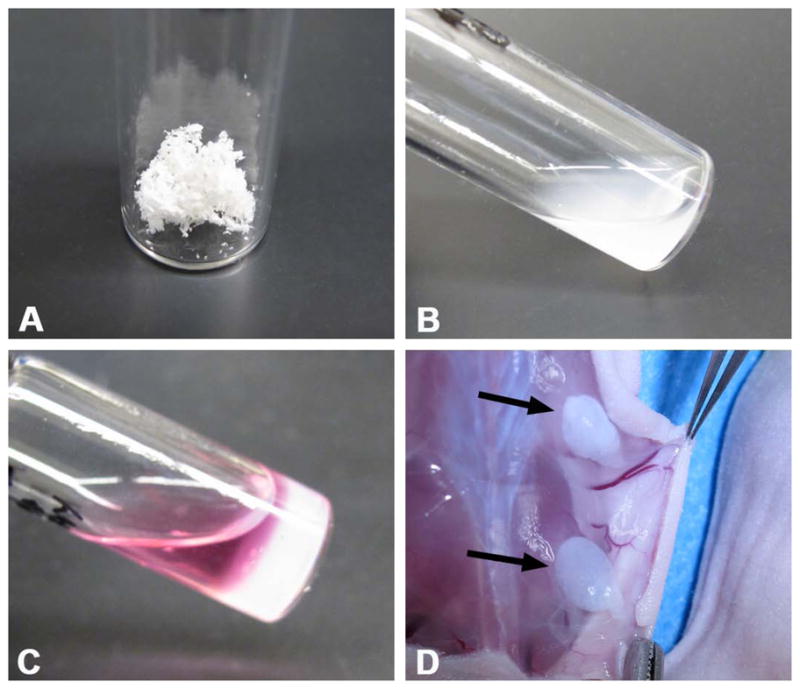
Human lipoaspirate was decellularized and delipidized to isolate adipose extracellular matrix components (A). This powder was subsequently reduced to liquid form via enzymatic digestion (B). This formulation would then self-assemble into a soft hydrogel in vitro when brought to physiologic temperature (C). Subcutaneous injections of the liquid matrix into nude mice would also self-assemble within 15 minutes (D). Arrows indicate subcutaneous hydrogels of injected adipose matrix.
Conclusion
As seen in this review, a wide variety of injectable biomaterials have been investigated for use in adipose tissue engineering. For synthetic polymers, the greatest success has been seen using degradable materials, especially for transient delivery of cells or growth factors. Biopolymers, being naturally biodegradable, have also been positively used as delivery vehicles and temporary filler materials, but lack the structural integrity to generate a lasting effect. Promising results have been seen by combining materials from both of these categories to generate injectable scaffolds with both advantageous cell adhesiveness and tailorable degradation profiles. However, the pinnacle of tissue engineering is to generate healthy, functional tissue in place of damaged or absent tissue. So far, injectable materials derived from complex, basement membrane-like sources offer the greatest potential for encouraging de novo adipogenesis. Based on the observations reported in these experiments, a set of specific criteria is beginning to be formulated that outlines the qualities needed for a successful injectable material for adipose tissue engineering. Depending on the desired outcome of the injectable material, certain qualities may receive heightened priority; however, for traditional tissue engineering, encouragement of adipogenesis remains paramount. This likely will necessitate a complex composition of basement membrane proteins that resemble native adipose tissue. Several methods have recently emerged that will facilitate this process, but the exact proteins of interest have yet to be identified. Recapitulating this tissue-specific molecular content is not the only important interaction located at the cell-matrix interface. Alongside surface composition, the relative hydrophobicity of a surface can also influence cellular attachment, spreading, migration, and even differentiation [65]. Degradation of the injected material also plays a crucial role in the final resolution. It is clear that scaffold breakdown at too rapid of a pace is undesirable for adipose tissue engineering. However, permanent implants often lead to chronic inflammation. This highlights one of the greatest challenges that remain in the field: tailoring degradation to allow cellular infiltration and prevent a negative immune response, while also maintaining a structured and supportive environment for the newly developing tissue. Closely associated with degradation are the mechanical properties of the material. While increased stiffness will lengthen degradation times, it has also been shown that substrates that match the native tissue stiffness can increase maturation of progenitor cells [66]. Preliminary studies have estimated adipose tissue to have an elastic modulus around 1–2 kPa and toughness of 4.1 kJm−2 [67, 68]. However, few materials for adipose tissue engineering, whether injectable or implantable, attempt to recapitulate this characteristic. Additionally, neovascularization, as with most tissue engineering strategies, has also improved the function of injectable biomaterials for adipose tissue engineering. As seen above, the inclusion of bFGF alongside several different materials has resulted not only in increased vessel formation in the injection region, but also increased adipogenesis. These outcomes suggest that for an injectable material to facilitate adipose regeneration, it should capitalize on these attributes: a nominal immune response in vivo, an in situ gelation mechanism, an appropriate degradation rate, a composition resembling native adipose ECM, and a positive angiogenic action in vivo. As a result, future success in the field of injectable adipose engineering will likely feature hybrid materials that utilize the strengths of several materials, each uniquely contributing to the specific requirements of adipose tissue development.
Acknowledgments
Funding was provided in part by the NIH Director’s New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant number 1-DP2-OD004309. D.A.Y. would also like to thank the National Science Foundation Graduate Research Fellowship Program.
References
- 1.Wang P, Mariman E, Keijer J, Bouwman F, Noben J-P, Robben J, Renes J. Profiling of the secreted proteins during 3t3-l1 adipocyte differentiation leads to the identification of novel adipokines. Cell Mol Life Sci. 2004;61:2405–17. doi: 10.1007/s00018-004-4256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuk P, Zhu M, Mizuno H, Huang J, Futrell J, Katz A, Benhaim P, Lorenz H, Hedrick M. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Engineering. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3.Prichard HL, Reichert W, Klitzman B. Ifats collection: Adipose-derived stromal cells improve the foreign body response. Stem Cells. 2008;26:2691–5. doi: 10.1634/stemcells.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomillion CT, Burg KJL. Stem cells and adipose tissue engineering. Biomaterials. 2006;27:6052–63. doi: 10.1016/j.biomaterials.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Flynn L, Woodhouse K. Adipose tissue engineering with cells in engineered matrices. Organogenesis. 2008;4:228. doi: 10.4161/org.4.4.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American society of plastic surgeons report of the 2010 plastic surgery statistics. Arlington Heights, Illinois: ASPS National Clearinghouse of Plastic Surgery Procedural Statistics; 2011. pp. 1–25. [Google Scholar]
- 7.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic plastic surgery. 2003;27:354–66. doi: 10.1007/s00266-003-3022-1. discussion 67. [DOI] [PubMed] [Google Scholar]
- 8.Cohen G, Treherne A. Treatment of facial lipoatrophy via autologous fat transfer. J Drugs Dermatol. 2009;8:486–9. [PubMed] [Google Scholar]
- 9.Verpaele A, Strand A. Restylane subq, a non-animal stabilized hyaluronic acid gel for soft tissue augmentation of the mid- and lower face. Aesthet Surg J. 2006;26:S10–7. doi: 10.1016/j.asj.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Allemann IB, Baumann L. Hyaluronic acid gel (juvederm) preparations in the treatment of facial wrinkles and folds. Clinical Interventions in Aging. 2008;3:629–34. doi: 10.2147/cia.s3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serra-Renom JM, Muñoz-Olmo JL, Serra-Mestre JM. Fat grafting in postmastectomy breast reconstruction with expanders and prostheses in patients who have received radiotherapy: Formation of new subcutaneous tissue. Plastic and reconstructive surgery. 2010;125:12–8. doi: 10.1097/PRS.0b013e3181c49458. [DOI] [PubMed] [Google Scholar]
- 12.Meier J, Glasgold R, Glasgold M. Autologous fat grafting: Long-term evidence of its efficacy in midfacial rejuvenation. Archives of Facial Plastic Surgery. 2009;11:24–8. doi: 10.1001/archfacial.2008.518. [DOI] [PubMed] [Google Scholar]
- 13.Toledo LS, Mauad R. Fat injection: A 20-year revision. Clin Plast Surg. 2006;33:47–53. vi. doi: 10.1016/j.cps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Cervelli V, Gentile P, Scioli MG, Grimaldi M, Casciani CU, Spagnoli LG, Orlandi A. Application of platelet-rich plasma in plastic surgery: Clinical and in vitro evaluation. Tissue engineering Part C, Methods. 2009;15:625–34. doi: 10.1089/ten.TEC.2008.0518. [DOI] [PubMed] [Google Scholar]
- 15.Kanchwala SK, Holloway L, Bucky LP. Reliable soft tissue augmentation: A clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Annals of plastic surgery. 2005;55:30–5. doi: 10.1097/01.sap.0000168292.69753.73. discussion 5. [DOI] [PubMed] [Google Scholar]
- 16.Shanti RM, Janjanin S, Li W-J, Nesti LJ, Mueller MB, Tzeng MB, Tuan RS. In vitro adipose tissue engineering using an electrospun nanofibrous scaffold. Annals of plastic surgery. 2008;61:566–71. doi: 10.1097/SAP.0b013e31816d9579. [DOI] [PubMed] [Google Scholar]
- 17.Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: Implications in soft tissue augmentation and reconstruction. Tissue Engineering. 2005;11:556–66. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 18.Kral JG, Crandall DL. Development of a human adipocyte synthetic polymer scaffold. Plastic and Reconstructive Surgery. 1999;104:1732–8. doi: 10.1097/00006534-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Kang X, Xie Y, Kniss DA. Adipose tissue model using three-dimensional cultivation of preadipocytes seeded onto fibrous polymer scaffolds. Tissue Engineering. 2005;11:458–68. doi: 10.1089/ten.2005.11.458. [DOI] [PubMed] [Google Scholar]
- 20.Cronin KJ, Messina A, Knight KR, Cooper-White JJ, Stevens GW, Penington AJ, Morrison WA. New murine model of spontaneous autologous tissue engineering, combining an arteriovenous pedicle with matrix materials. Plastic and reconstructive surgery. 2004;113:260–9. doi: 10.1097/01.PRS.0000095942.71618.9D. [DOI] [PubMed] [Google Scholar]
- 21.Patrick C, Jr, Chauvin P, Hobley J, Reece G. Preadipocyte seeded plga scaffolds for adipose tissue engineering. Tissue Engineering. 1999;5:139–51. doi: 10.1089/ten.1999.5.139. [DOI] [PubMed] [Google Scholar]
- 22.Brandl FP, Seitz AK, Tessmar JKV, Blunk T, Göpferich AM. Enzymatically degradable poly(ethylene glycol) based hydrogels for adipose tissue engineering. Biomaterials. 2010;31:3957–66. doi: 10.1016/j.biomaterials.2010.01.128. [DOI] [PubMed] [Google Scholar]
- 23.Choi YS, Park S-N, Suh H. Adipose tissue engineering using mesenchymal stem cells attached to injectable plga spheres. Biomaterials. 2005;26:5855–63. doi: 10.1016/j.biomaterials.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Choi YS, Cha SM, Lee YY, Kwon SW, Park CJ, Kim M. Adipogenic differentiation of adipose tissue derived adult stem cells in nude mouse. Biochemical and biophysical research communications. 2006;345:631–7. doi: 10.1016/j.bbrc.2006.04.128. [DOI] [PubMed] [Google Scholar]
- 25.Neubauer M, Hacker M, Bauer-Kreisel P, Weiser B, Fischbach C, Schulz MB, Goepferich A, Blunk T. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Engineering. 2005;11:1840–51. doi: 10.1089/ten.2005.11.1840. [DOI] [PubMed] [Google Scholar]
- 26.Marra KG, Defail AJ, Clavijo-Alvarez JA, Badylak SF, Taieb A, Schipper B, Bennett J, Rubin JP. Fgf-2 enhances vascularization for adipose tissue engineering. Plastic and reconstructive surgery. 2008;121:1153–64. doi: 10.1097/01.prs.0000305517.93747.72. [DOI] [PubMed] [Google Scholar]
- 27.Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (plga) microspheres. Pharm Res. 2000;17:100–6. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 28.Mader K, Gallez B, Liu KJ, Swartz HM. Non-invasive in vivo characterization of release processes in biodegradable polymers by low-frequency electron paramagnetic resonance spectroscopy. Biomaterials. 1996;17:457–61. doi: 10.1016/0142-9612(96)89664-5. [DOI] [PubMed] [Google Scholar]
- 29.von Heimburg D, Zachariah S, Kühling H, Heschel I, Schoof H, Hafemann B, Pallua N. Human preadipocytes seeded on freeze-dried collagen scaffolds investigated in vitro and in vivo. Biomaterials. 2001;22:429–38. doi: 10.1016/s0142-9612(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 30.Stosich MS, Mao JJ. Adipose tissue engineering from human adult stem cells: Clinical implications in plastic and reconstructive surgery. Plastic and Reconstructive Surgery. 2007;119:71–83. doi: 10.1097/01.prs.0000244840.80661.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orbay H, Takami Y, Hyakusoku H, Mizuno H. Acellular dermal matrix seeded with adipose-derived stem cells as a subcutaneous implant. Aesthetic plastic surgery. 2011 doi: 10.1007/s00266-011-9683-2. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Gentleman E, Nauman EA, Livesay GA, Dee KC. Collagen composite biomaterials resist contraction while allowing development of adipocytic soft tissue in vitro. Tissue Engineering. 2006;12:1639–49. doi: 10.1089/ten.2006.12.1639. [DOI] [PubMed] [Google Scholar]
- 33.Marler JJ, Guha A, Rowley J, Koka R, Mooney D, Upton J, Vacanti JP. Soft-tissue augmentation with injectable alginate and syngeneic fibroblasts. Plastic and reconstructive surgery. 2000;105:2049–58. doi: 10.1097/00006534-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Halberstadt C, et al. A hydrogel material for plastic and reconstructive applications injected into the subcutaneous space of a sheep. Tissue Engineering. 2002;8:309–19. doi: 10.1089/107632702753725067. [DOI] [PubMed] [Google Scholar]
- 35.Cho S-W, Kim I, Kim S-H, Rhie JW, Choi CY, Kim B-S. Enhancement of adipose tissue formation by implantation of adipogenic-differentiated preadipocytes. Biochemical and biophysical research communications. 2006;345:588–94. doi: 10.1016/j.bbrc.2006.04.089. [DOI] [PubMed] [Google Scholar]
- 36.Torio-Padron N, Baerlecken N, Momeni A, Stark GB, Borges J. Engineering of adipose tissue by injection of human preadipocytes in fibrin. Aesthetic plastic surgery. 2007;31:285–93. doi: 10.1007/s00266-006-0221-6. [DOI] [PubMed] [Google Scholar]
- 37.Cho S-W, Song KW, Rhie JW, Park MH, Choi CY, Kim B-S. Engineered adipose tissue formation enhanced by basic fibroblast growth factor and a mechanically stable environment. Cell transplantation. 2007;16:421–34. doi: 10.3727/000000007783464795. [DOI] [PubMed] [Google Scholar]
- 38.Rubin JP, Bennett JM, Doctor JS, Tebbets BM, Marra KG. Collagenous microbeads as a scaffold for tissue engineering with adipose-derived stem cells. Plastic and reconstructive surgery. 2007;120:414–24. doi: 10.1097/01.prs.0000267699.99369.a8. [DOI] [PubMed] [Google Scholar]
- 39.Natesan S, Baer DG, Walters TJ, Babu M, Christy RJ. Adipose-derived stem cell delivery into collagen gels using chitosan microspheres. Tissue Engineering Part A. 2010;16:1369–84. doi: 10.1089/ten.tea.2009.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–21. doi: 10.1016/s0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 41.Moya ML, Cheng M-H, Huang J-J, Francis-Sedlak ME, Kao S-W, Opara EC, Brey EM. The effect of fgf-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. 2010;31:2816–26. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stillaert FB, Di Bartolo C, Hunt JA, Rhodes NP, Tognana E, Monstrey S, Blondeel PN. Human clinical experience with adipose precursor cells seeded on hyaluronic acid-based spongy scaffolds. Biomaterials. 2008;29:3953–9. doi: 10.1016/j.biomaterials.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Flynn L, Prestwich G, Semple J, Woodhouse K. Proliferation and differentiation of adipose-derived stem cells on naturally derived scaffolds. Biomaterials. 2008;29:1862–71. doi: 10.1016/j.biomaterials.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 44.von Heimburg D, Zachariah S, Low A, Pallua N. Influence of different biodegradable carriers on the in vivo behavior of human adipose precursor cells. Plastic and Reconstructive Surgery. 2001;108:411–20. doi: 10.1097/00006534-200108000-00020. discussion 21–2. [DOI] [PubMed] [Google Scholar]
- 45.Hemmrich K, Van de Sijpe K, Rhodes NP, Hunt JA, Di Bartolo C, Pallua N, Blondeel P, von Heimburg D. Autologous in vivo adipose tissue engineering in hyaluronan-based gels--a pilot study. J Surg Res. 2008;144:82–8. doi: 10.1016/j.jss.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Tan H, Li H, Rubin JP, Marra KG. Controlled gelation and degradation rates of injectable hyaluronic acid-based hydrogels through a double crosslinking strategy. J Tissue Eng Regen Med. 2011 doi: 10.1002/term.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung HJ, Jung JS, Park TG. Fabrication of adipose-derived mesenchymal stem cell aggregates using biodegradable porous microspheres for injectable adipose tissue regeneration. Journal of biomaterials science Polymer edition. 2011;22(16):107–22. doi: 10.1163/092050609X12580983495681. [DOI] [PubMed] [Google Scholar]
- 48.Kreger ST, Voytik-Harbin SL. Hyaluronan concentration within a 3d collagen matrix modulates matrix viscoelasticity, but not fibroblast response. Matrix Biol. 2009;28:336–46. doi: 10.1016/j.matbio.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okabe K, Yamada Y, Ito K, Kohgo T, Yoshimi R, Ueda M. Injectable soft-tissue augmentation by tissue engineering and regenerative medicine with human mesenchymal stromal cells, platelet-rich plasma and hyaluronic acid scaffolds. Cytotherapy. 2009;11:307–16. doi: 10.1080/14653240902824773. [DOI] [PubMed] [Google Scholar]
- 50.Vashi AV, Keramidaris E, Abberton KM, Morrison WA, Wilson JL, O'Connor AJ, Cooper-White JJ, Thompson EW. Adipose differentiation of bone marrow-derived mesenchymal stem cells using pluronic f-127 hydrogel in vitro. Biomaterials. 2008;29:573–9. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Tan H, Ramirez C, Miljkovic N, Li H, Rubin J, Marra K. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009;30:6844–53. doi: 10.1016/j.biomaterials.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hillel AT, et al. Photoactivated composite biomaterial for soft tissue restoration in rodents and in humans. Science Translational Medicine. 2011;3:93ra67. doi: 10.1126/scitranslmed.3002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145:204–20. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type iv procollagen, laminin, and heparan sulfate proteoglycan from the ehs sarcoma. Biochemistry. 1982;21:6188–93. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 55.Toriyama K, Kawaguchi N, Kitoh J, Tajima R, Inou K, Kitagawa Y, Torii S. Endogenous adipocyte precursor cells for regenerative soft-tissue engineering. Tissue Engineering. 2002;8:157–65. doi: 10.1089/107632702753503144. [DOI] [PubMed] [Google Scholar]
- 56.Stillaert F, Findlay M, Palmer J, Idrizi R, Cheang S, Messina A, Abberton K, Morrison W, Thompson EW. Host rather than graft origin of matrigel-induced adipose tissue in the murine tissue-engineering chamber. Tissue Engineering. 2007;13:2291–300. doi: 10.1089/ten.2006.0382. [DOI] [PubMed] [Google Scholar]
- 57.Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA. 1998;95:1062–6. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abberton KM, Bortolotto SK, Woods AA, Findlay M, Morrison WA, Thompson EW, Messina A. Myogel, a novel, basement membrane-rich, extracellular matrix derived from skeletal muscle, is highly adipogenic in vivo and in vitro. Cells Tissues Organs. 2008;188:347–58. doi: 10.1159/000121575. [DOI] [PubMed] [Google Scholar]
- 59.Uriel S, Huang J-J, Moya ML, Francis ME, Wang R, Chang S-Y, Cheng M-H, Brey EM. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials. 2008;29:3712–9. doi: 10.1016/j.biomaterials.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 60.Yoo G, Lim JS. Tissue engineering of injectable soft tissue filler: Using adipose stem cells and micronized acellular dermal matrix. J Korean Med Sci. 2009;24:104–9. doi: 10.3346/jkms.2009.24.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi JS, Yang H-J, Kim BS, Kim JD, Kim JY, Yoo B, Park K, Lee HY, Cho YW. Human extracellular matrix (ecm) powders for injectable cell delivery and adipose tissue engineering. J Control Release. 2009;139:2–7. doi: 10.1016/j.jconrel.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 62.Turner AEB, Flynn LE. Design and characterization of tissue-specific extracellular matrix-derived microcarriers. Tissue engineering Part C. Methods. 2011 doi: 10.1089/ten.TEC.2011.0246. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 63.Young DA, Ibrahim DO, Hu D, Christman KL. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomaterialia. 2011;7:1040–9. doi: 10.1016/j.actbio.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ayala R, et al. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials. 2011;32:3700–11. doi: 10.1016/j.biomaterials.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Comley K, Fleck NA. A micromechanical model for the young's modulus of adipose tissue. International Journal of Solids and Structures. 2010;47:2982–90. [Google Scholar]
- 68.Comley K, Fleck NA. The toughness of adipose tissue: Measurements and physical basis. Journal of Biomechanics. 2010;43:1823–6. doi: 10.1016/j.jbiomech.2010.02.029. [DOI] [PubMed] [Google Scholar]