Carbon–nitrogen bonds are ubiquitous in medicinal agents and natural products.[1] Transition metal-catalyzed amination reactions, pioneered by Buchwald and Hartwig, are amongst the most powerful methods available for accessing these motifs.[1] Copper- and palladium-mediated aminations of aryl halides and triflates are now well-established,[1] and examples of mesylate[2] and tosylate[3] aminations have been reported. Most recent efforts have focused on the amination of classically “inert” phenolic derivatives (i.e., arylmethyl ethers[4] and aryl pivalate esters[5]), which could potentially be used in multistep synthesis.[6, 7] With the aim of assembling polysubstituted aryl amines, motifs commonly encountered in drug scaffolds, naturally occurring small molecules, pesticides, ligands for catalysis, and materials chemistry, we sought to uncover a versatile class of phenol-derived substrates that could undergo transition metal-catalyzed amination.
Although relatively unexplored, N,N-dialkylaryl O-sulfamates (e.g., 1, Scheme 1) are highly attractive electrophiles for cross-coupling reactions. They are easy to prepare,[8] stable to a variety of reaction conditions, and exhibit low reactivity toward Pd0.[9, 10] Moreover, the sulfamate moiety can be used to functionalize an arene at both the ortho or para positions,[9a, 10, 11] prior to carrying out a cross-coupling event. Despite that aryl O-sulfamates have been employed in carbon–carbon bond forming reactions,[9, 10] their use in carbon–nitrogen bond construction has remained undiscovered.[12] Herein, we report the first amination of aryl O-sulfamates (1→2) and the application of this methodology to a concise synthesis of the antibacterial drug linezolid (4).[13]
Scheme 1.
Amination of aryl sulfamates and linezolid (4).
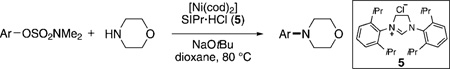
Initial studies were aimed at promoting the amination of dimethylsulfamate derivatives of phenol and 1-naphthol. Although catalytic systems based on nickel and PCy3 have been the cornerstone of several recent nickel-catalyzed cross-coupling reactions involving carbon–oxygen bonds,[14] including the Suzuki–Miyaura coupling of aryl O-sulfamates,[10] this metal/ligand combination was ineffective in our amination studies.[12] After conducting an extensive survey of reaction parameters (e.g., nickel catalysts, ligands, solvents, bases, temperature, etc.) it was observed that N-heterocyclic carbene (NHC) ligands uniquely facilitated the desired amination. Under optimal conditions, treatment of sulfamate 3 with morpholine in the presence of catalytic [Ni(cod)2] (cod= cyclooctadiene), SIPr·HCl (5), and NaOtBu, in dioxane at 80°C for 3 h afforded the aminated product in 95% yield (Table 1, entry 1).[15]
Table 1.
Cross-coupling of aryl sulfamates with morpholine.[a]
 | |||
|---|---|---|---|
| Entry | Ar-OSO2NMe2 | Product | Yield [%][b] |
| 1 | 95 | ||
| 2 | 76 | ||
| 3 |  |
 |
77 |
| 4 | 91 | ||
| 5[c] | 80 | ||
| 6[d] | 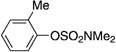 |
 |
80 |
| 7[e] | 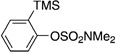 |
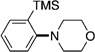 |
50 |
| 8[f] | 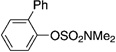 |
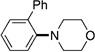 |
64 |
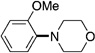
| 9[f] | 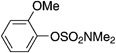 |
 |
74 |
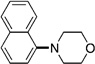
| 10 | 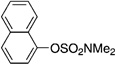 |
 |
96 |
| 11 |  |
86 | |
| 12[d] | 72 | ||
| 13 | 86 | ||
Conditions unless otherwise stated: [Ni(cod)2] (5 mol%), 5 (10 mol%), sulfamate substrate (1 equiv), morpholine (1.2 equiv), NaOtBu (1.4 equiv), dioxane (0.2 m), 80°C for 3 h.
Yields of isolated product.
[Ni(cod)2] (10 mol%), 5 (20 mol%), NaOtBu (1.5 equiv).
[Ni-(cod)2] (15 mol%), 5 (30 mol%), morpholine (1.8 equiv), NaOtBu (2.2 equiv).
[Ni(cod)2] (20 mol%), 5 (40 mol%), NaOtBu (1.7 equiv), 60°C.
[Ni(cod)2] (15 mol%), 5 (30 mol%), NaOtBu (1.6 equiv).
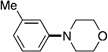
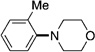
A variety of sulfamate substrates were examined in the nickel-catalyzed amination process (Table 1). Methyl substituents at the para and meta positions were tolerated (entries 2 and 3), in addition to the electron-withdrawing trifluoromethyl group and the electron-donating methoxy group (entries 4 and 5, respectively). Given the utility of the sulfamate in directed metalation chemistry,[9a, 10] we examined the amination of several ortho-substituted substrates bearing a methyl, trimethylsilyl (TMS), phenyl, or methoxy substituent. In all cases, amination proceeded smoothly (entries 6–9). Naphthyl-based substrates were found to be excellent amination substrates (entries 10 and 11). Furthermore, heterocycles, such as indole and pyridine, were tolerated in this methodology (entries 12 and 13).
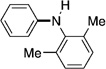
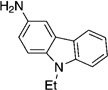
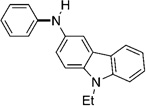
As shown in Table 2, the scope of aryl O-sulfamate amination is also broad with respect to the amine coupling partner. Both cyclic and acyclic secondary amines were found to be suitable substrates (entries 1–3). In addition, anilines could be employed (entries 4–6), including 2,6-dimethylaniline (entry 6). The methodology also allows for the coupling of amines with appended heterocycles, as demonstrated by the coupling of pyridine- and carbazole-containing substrates (entries 7 and 8).
Table 2.
Cross-coupling of aryl sulfamates with amines.[a]
Conditions unless otherwise stated: [Ni(cod)2] (5 mol%), 5 (10 mol%), sulfamate substrate (1 equiv), amine (1.2 equiv), NaOtBu (1.4 equiv), dioxane (0.2 m), 80°C for 3 h.
Yields of isolated product.
[Ni(cod)2] (10 mol%), 5 (20 mol%), NaOtBu (1.5 equiv).
[Ni-(cod)2] (15 mol%), 5 (30 mol%), amine (1.8 equiv), NaOtBu (2.2 equiv).
[Ni(cod)2] (15 mol%), 5 (30 mol%), amine (2.4 equiv), NaOtBu (2.2 equiv).
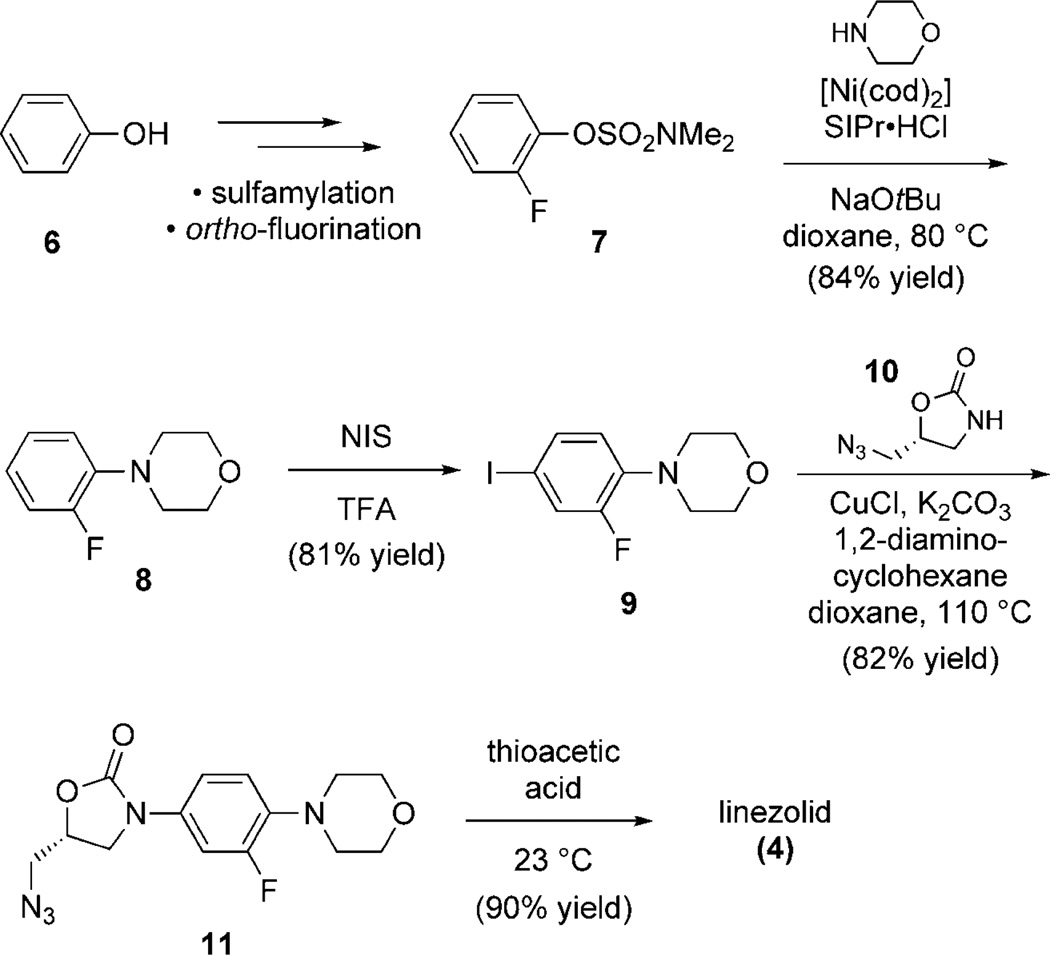
To further probe the scope and utility of the sulfamate amination methodology, a concise synthesis of the antibacterial drug linezolid (4) was performed (Scheme 2).[13] Beginning from phenol (6), fluorosulfamate 7 was readily prepared using our previously reported sequence.[10] This conversion proceeds by sulfamylation, followed by ortho-fluorination (two steps), and showcases the sulfamate’s directing ability. Nickel-catalyzed sulfamate amination proceeded smoothly to deliver intermediate 8, without interference from the fluoro substituent.[16] Subsequent iodination furnished trisubstituted arene 9, which in turn, underwent copper-catalyzed coupling with oxazolidinone 10 to afford arylated oxazolidinone 11 in good yield. In the final step, reductive acetylation of azide 11 furnished linezolid (4). Overall, our synthesis illustrates the merits of sulfamate-directed arene functionalization and coupling methodology in a complex setting.
Scheme 2.
Synthesis of linezolid (4) using Ni-catalyzed amination. SIPr·HCl=1,3-bis-(2,6-diisopropylphenyl)imidazolinium chloride, [Ni-(cod)2]=bis(1,5-cycloactadiene)nickel(0), NIS=N-iodosuccinimide, TFA=trifluoroacetic acid.
In summary, we have discovered the first amination reactions of aryl O-sulfamates, which are attractive cross-coupling partners, particularly for use in multistep synthesis.
The amination is broad in scope with respect to both the sulfamate and amine coupling partners. The methodology presented herein provides an effective means for accessing polysubstituted aryl amines, as demonstrated by a concise synthesis of the antibacterial drug linezolid.
Supplementary Material
Footnotes
The authors are grateful to the NIH-NIGMS (R00 GM079922), Boehringer Ingelheim, DuPont, Eli Lilly, the University of California, Los Angeles, the Chemistry–Biology Interface training program (A.L.S., USPHS National Research Service Award GM08496), and the UCLA CSST Program (Y.Y.) for financial support. We thank the Garcia–Garibay laboratory (UCLA) for access to instrumentation, Dr. John Greaves (UC Irvine) for mass spectra, and Materia Inc. for chemicals.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201007325.
References
- 1. Wolfe JP, Wagaw S, Marcoux J-F, Buchwald SL. Acc. Chem. Res. 1998;31:805–818. Hartwig JF. Angew. Chem. 1998;110:2154–2177. Angew. Chem. Int. Ed. 1998;37:2046–2067. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L. c) “Practical Palladium Catalysts for C–N and C–O Bond Formation”: Muci AR, Buchwald SL. In: Topics in Current Chemistry. Miyaura N, editor. Vol. 219. Berlin: Springer; 2001. p. 131. Kienle M, Dubbaka SR, Brade K, Knochel P. Eur. J. Org. Chem. 2007:4166–4176.
- 2.a) Fors BP, Watson DA, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2008;130:13552–13554. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) So CM, Zhou Z, Lau CP, Kwong FY. Angew. Chem. 2008;120:6502–6506. [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:6402–6406. doi: 10.1002/anie.200802157. [DOI] [PubMed] [Google Scholar]
- 3.a) Hamann BC, Hartwig JF. J. Am. Chem. Soc. 1998;120:7369–7370. [Google Scholar]; b) Roy AH, Hartwig JF. J. Am. Chem. Soc. 2003;125:8704–8705. doi: 10.1021/ja035835z. [DOI] [PubMed] [Google Scholar]; c) Ogata T, Hartwig JF. J. Am. Chem. Soc. 2008;130:13848–13849. doi: 10.1021/ja805810p. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Gao C-Y, Yang L-M. J. Org. Chem. 2008;73:1624–1627. doi: 10.1021/jo7022558. [DOI] [PubMed] [Google Scholar]
- 4.The amination of aryl methyl ethers proceeds most efficiently using 2-methoxynaphthalene and cyclic secondary amines; see: Shimasaki T, Chatani N. Chem. Lett. 2009;38:710–711.
- 5.Shimasaki T, Tobisu M, Chatani N. Angew. Chem. 2010;122:2991–2994. doi: 10.1002/anie.200907287. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:2929–2932. doi: 10.1002/anie.200907287. [DOI] [PubMed] [Google Scholar]
- 6.A single example of an aryl carbamate undergoing amination has been described in the literature using N,N-diethylphenylcarbamate (see Ref. [5]).
- 7.For pertinent reviews, see: Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita A-M, Garg NK, Percec V. Chem. Rev. 2010 doi: 10.1021/cr100259t. Yu D-G, Li B-J, Shi Z-J. Acc. Chem. Res. 2010 doi: 10.1021/ar100082d.
- 8.Aryl N,N-dimethylsulfamates are readily prepared from aryl alcohols and commercially available N, N-dimethylsulfamoyl chloride (approximate cost $0 80 per gram from Aldrich Chemical Co., Inc.).
- 9.For Kumada couplings, see: Macklin TK, Snieckus V. Org. Lett. 2005;7:2519–2522. doi: 10.1021/ol050393c. Wehn PM, Du Bois J. Org. Lett. 2005;7:4685–4688. doi: 10.1021/ol051896l.
- 10.For the use of aryl sulfamates in directed metalation, electrophilic aromatic substitution, multistep synthesis, and Suzuki–Miyaura couplings, see: Quasdorf KW, Riener M, Petrova KV, Garg NK. J. Am. Chem. Soc. 2009;131:17748–17749. doi: 10.1021/ja906477r.
- 11.Other phenol-based amination partners cannot be used effectively for directed metalation/functionalization. Specifically, aryl sulfonates and pivalates are not suitable substrates, whereas aryl methyl ethers are poor metalation substrates. For reviews see: Snieckus V. Chem. Rev. 1990;90:879–933. Hartung CG, Snieckus V. In: Modern Arene Chemistry. Astruc D, editor. New York: Wiley-VCH; 2002. pp. 330–367. Macklin T, Snieckus V. In: Handbook of C-H Transformations. Dyker G, editor. New York: Wiley-VCH; 2005. pp. 106–119.
- 12.Although an initial oxidative addition step would be common to both C–C and C–N bond-forming reactions of aryl sulfamate substrates, the remaining steps of the presumed catalytic cycles are quite different.
- 13.Linezolid, marketed by Pfizer Inc. under the name Zyvox, is used to treat a variety of infections. In recent years, worldwide sales of Zyvox have been approximately 11 billion dollars per year; see: http://www.pfizer.com/investors/financial_reports/annual_reports/key_medicines.jsp.
- 14.For phenolic ester couplings, see: Quasdorf KW, Tian X, Garg NK. J. Am. Chem. Soc. 2008;130:14422–14423. doi: 10.1021/ja806244b. Guan B-T, Wang Y, Li B-J, Yu D-G, Shi Z-J. J. Am. Chem. Soc. 2008;130:14468–14470. doi: 10.1021/ja8056503. Li B-J, Li Y-Z, Lu X-Y, Liu J, Guan B-T, Shi Z-J. Angew. Chem. Int. Ed. 2008;47:10124–10127. doi: 10.1002/anie.200803814. Angew. Chem. 2008;120:10278–10281. for methyl ether couplings, see: d) Tobisu M, Shimasaki T, Chatani N. Angew. Chem. 2008;120:4944–4947. doi: 10.1002/anie.200801447. Angew. Chem. Int. Ed. 2008;47:4866–4869. doi: 10.1002/anie.200801447. Dankwardt JW. Angew. Chem. 2004;116:2482–2486. Angew. Chem. Int. Ed. 2004;43:2428–2432. doi: 10.1002/anie.200453765. for carbamate couplings, see: f) Antoft-Finch A, Blackburn T, Snieckus V. J. Am. Chem. Soc. 2009;131:17750–17752. doi: 10.1021/ja907700e. Xi L, Li B-J, Wu Z-H, Lu X-Y, Guan B-T, Wang B-Q, Zhao K-Q, Shi Z-J. Org. Lett. 2010;12:884–887. doi: 10.1021/ol9029534.
- 15.Although other N-heterocyclic carbene ligands could be used to achieve this exact coupling, commercially available SIPr·HCl (5, CAS# 258278-25-0) proved most generally useful, particularly for the amination of ortho-substituted substrates.
- 16.For nickel-catalyzed Kumada and Suzuki–Miyaura couplings of aryl fluorides, see: Yoshikai N, Mashima H, Nakamura E. J. Am. Chem. Soc. 2005;127:17978–17979. doi: 10.1021/ja056327n. Dankwardt JW. J. Organomet. Chem. 2005;690:932–938. Schaub T, Backes M, Radius U. J. Am. Chem. Soc. 2006;128:15964–15965. doi: 10.1021/ja064068b.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.