Abstract
The antidiabetic activity of Momordica charantia (L.), Cucurbitaceae, a widely-used treatment for diabetes in a number of traditional medicine systems, was investigated in vitro. Antidiabetic activity has been reported for certain saponins isolated from M. charantia. In this study insulin secretion was measured in MIN6 β-cells incubated with an ethanol extract, saponin-rich fraction, and five purified saponins and cucurbitane triterpenoids from M. charantia, 3β,7β,25-trihydroxycucurbita-5,23(E)-dien-19-al (1), momordicine I (2), momordicine II (3), 3-hydroxycucurbita-5,24-dien-19-al-7,23-di-O-β-glucopyranoside (4), and kuguaglycoside G (5). Treatments were compared to incubation with high glucose (27 mM) and the insulin secretagogue, glipizide (50 μM). At 125 μg/ml, an LC-ToF-MS characterized saponin-rich fraction stimulated insulin secretion significantly more than the DMSO vehicle, p=0.02. At concentrations 10 and 25 μg/ml, compounds 3 and 5 also significantly stimulated insulin secretion as compared to the vehicle, p≤0.007, and p= 0.002, respectively. This is the first report of a saponin-rich fraction, and isolated compounds from M. charantia, stimulating insulin secretion in an in vitro, static incubation assay.
Keywords: Momordica charantia, alternative medicine, hypoglycemia, insulin secretion, diabetes, bitter melon
Introduction
Diabetes is currently one of the most prevalent and serious diseases worldwide. According to the World Health Organization, diabetes causes nearly 5% of deaths worldwide, and is expected to rise by 50% in the next 10 years (World Health Organization, 2010). As of 2007, 7.8% of the United States population suffers from diabetes, and in that year alone, 1.6 million new cases were diagnosed (Centers for Disease Control and Prevention, 2010).
Type 2 diabetes, also known as non-insulin dependent diabetes mellitus (NIDDM), is responsible for 90% to 95% of diagnosed diabetes (Centers for Disease Control and Prevention, 2010). The progression of type 2 diabetes can be characterized both by insulin resistance, and loss of normal β-cell activity, such as hyperplasia of the pancreas and gradual decrease of insulin secretion (Talchai et al., 2009). As impaired β-cell function is central to the progression of type 2 diabetes, novel therapies that regulate insulin secretion and prevent β-cell damage and subsequent impaired function may be integral to the future of treatment (Talchai et al., 2009).
In addition to widely used modern medications, people throughout the world increasingly rely on complementary and alternative medicine, including plant-based traditional medicines, as a form of health care (Egede et al., 2002). In 2007, during the 12 months prior to an interview by the authors, 4 out of 10 adults used alternative and complementary medicine, 17.7% of this being natural products (Barnes et al., 2008). In another study, during an interview of Dominican patients in an emergency room setting, 24% were using alternative medicine for their emergency complaint (Allen et al., 2000). A study done in the United States in 2002 reported that 19% of subjects interviewed had used natural products in the form of herbal remedies, functional foods, and supplements in the prior 12 month period (Barnes et al., 2002). With this prevalence of herbal medicine, it is of paramount importance that these herbs are investigated.
Of traditional medicines used for diabetes treatment, Momordica charantia, L. (Cucurbitaceae) is reported to be the most common (Marles and Farnsworth, 1995). The hypoglycemic activity of various preparations of M. charantia is well substantiated with many recent mouse models of diet-induced obesity and type 2 diabetes (Miura et al., 2001; Miura et al., 2004; Nerurkar et al., 2008; Shih et al., 2008). Also, recent studies have begun to show that isolated triterpene glycosides known as saponins may be responsible, at least in part, for the antidiabetic activity seen in M. charantia. For example, the isolated cucurbitane triterpenoids 5β,19-epoxy-3β,25-dihydroxycucurbita-6,23(E)-diene, and 3β, 7β, 25-trihydroxycucurbita-5,23(E)-dien-19-al lowered blood sugar in diabetic mice (Harinantenaina et al., 2006), while a saponin-rich fraction isolated from M. charantia was also found to lower blood sugar and small intestine disaccharidase activity in rats (Oishi et al., 2007). In another study, momordicosides Q, R, S, and T increased GLUT4 translocation via the AMPK pathway in vitro, and momordicoside T improved glucose tolerance in mice fed a high fat diet during a glucose tolerance test (Tan et al., 2008).
Although the insulin secretion activity of M. charantia has begun to be explored in vitro (Xiang et al., 2007), the exact mechanisms of the plant’s activity have yet to be fully elucidated. As this plant is used so widely throughout the world in traditional medicine for diabetes, knowledge of its bioactivity, including potential toxicity, and possible mechanisms of action are critical for informed public health decisions. It is also important to assess the potential efficacy of pure compounds, both acting together in a concentrated fraction of plant extract, and independently.
We hypothesized that the hypoglycemic activity of M. charantia is due, at least in part, to the stimulation of insulin secretion in pancreatic β-cells by cucurbitane triterpenoids. To test this hypothesis, we investigated the insulin secretion activity of M. charantia ethanol extract and an LC-ToF-MS characterized saponin-rich fraction; additionally, the five cucurbitane triterpenoids and saponin compounds 3β,7β,25-trihydroxycucurbita-5,23(E)-dien-19-al (1), momordicine I (2), momordicine II (3), 3-hydroxycucurbita-5,24-dien-19-al-7,23-di-O-β-glucopyranoside (4), and kuguaglycoside G (5) were also tested to ascertain the potential mechanism of the glucose-lowering activity of M. charantia in MIN6 β-cells, a mouse insulinoma β-cell line (Fig. 1). Of the clonal cell lines appropriate for testing potential insulin secretion, the glucose-stimulated insulin secretion previously observed with MIN6 cells was close to that of normal islets (Ishihara et al., 1993). Because the function of MIN6 cells closely resembles normal physiologic β-cell function, we chose this cell line to test our hypothesis in order to more precisely extrapolate a true pancreatic islet insulin secretion response. Extracts and compounds were tested in a static incubation assay, and insulin secretion and cell viability were measured.
Fig. 1.
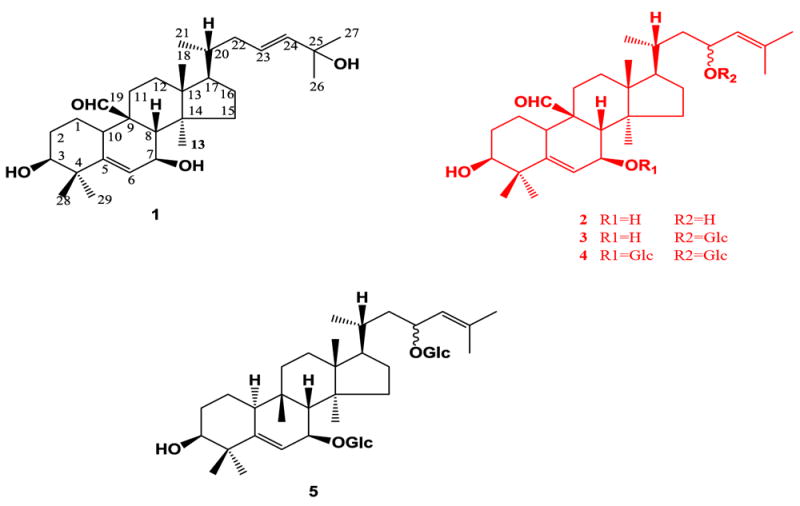
Compounds 3β,7β,25-trihydroxycucurbita-5,23(E)-dien-19-al (1), momordicine I (2), momordicine II (3), 3-hydroxycucurbita-5,24-dien-19-al-7,23-di-O-β-glucopyranoside (4), kuguaglycoside G (5).
Materials and methods
Plant material
Dried fruits, including seeds (1.94 kg), of M. charantia were identified by Naturex, Inc., and extracted in 75% ethanol, resulting in 578 g total solid extracted material. Spare extracted material was deposited in Naturex’s herbarium (South Hackensack, New Jersey). This resulting 75% ethanol-extracted material was tested in the cells and referred to as the ethanol extract, of which aliquots (24 g) were partitioned sequentially with methylene chloride and water-saturated butanol, repeatedly. The average yield of the dried butanol extract was 5.67% w/w of starting material. All dried butanol extracts were combined, and subsequently used for experiments.
Compound isolation
Compounds 1-5 were isolated from M. charantia as described previously (Ma et al., 2010).
β-Cell assay
MIN6 β-cells were grown at 37 °C with 5% CO2 in DMEM media (Invitrogen, California) with 15% fetal bovine serum (Invitrogen, California), 1% penicillin/streptomycin (Invitrogen, California), 1:200 gentamicin (Invitrogen, California), and 1% basel medium eagle (Invitrogen, California). Cells were trypsinized off the plate, split, and replated into 6-well plates and grown until confluent. The ethanol extract, saponin-rich fraction, and isolated compounds were re-suspended in 100% DMSO to make a stock solution of 125 mg/ml. The assay was performed similarly to a previously described method (Persaud et al., 1999). Cells were incubated with Kreb’s Ringer buffer (KRB) for 60 min on the plate. Cells were then rinsed with fresh KRB twice, and an aliquot of the second wash was saved as a baseline insulin measurement. Cells were then incubated for 60 min with KRB (negative control), 50 μM glipizide with 27 mM glucose in KRB (positive control), or 0.1% DMSO in KRB (vehicle control). The ethanol extract and saponin-rich fraction were tested at 25, 75, and 125 μg/ml concentrations, while compounds 1-5 were tested at 5, 10, and 25 μg/ml. Treatment solutions were collected at 60 min, and insulin concentrations were determined by ELISA (ALPCO, New Hampshire).
Cell viability
Immediately after incubation with treatment solutions, cells were trypsinized and resuspended in PBS buffer (Invitrogen, California). Cells (10 μl) were added to an equal amount of Trypan Blue (Invitrogen, California) and counted on a hemocytometer. Cells excluding Trypan Blue were assumed viable. Both live and dead cells were counted and are presented as an average of four counts for each treatment.
DNA extraction
For a total cell count, DNA was extracted by incubating cells overnight with 1% SDS/10 mM EDTA/10 mM Tris lysis buffer and 1% proteinase K. Phenol-chloroform 1:1 was added, and cells were centrifuged. Sodium acetate-isopropanol 1:3 was added to the supernatant, and material was incubated at -80 °C for 60 min. Precipitated DNA was then washed twice with 70% ethanol, and air-dried. DNA was reconstituted with 10:1 tris-EDTA buffer (pH 8.0), and the concentration was read with a spectrophotometer at 260 nm. The conversion factor of 5.8 μg of DNA = 1.0 × 106 cells was used.
LC-ToF-MS analysis
Samples were analyzed by LC-MS using a Waters LCT Premiere XE Time of Flight (ToF) mass spectrometer (Waters MS Technologies, Manchester, UK). Ionization was achieved using a multi-mode EScI source in electrospray (ESI) mode at the following conditions: +ESI capillary 3000 V, -ESI capillary 2800 V, both + and − ESI: cone: 20 V, aperture 1: 0 V, ion guide 1: 0 V, multichannel plate (MCP): 2600 V. Nitrogen was used for both cone and desolvation gases, with a cone gas flow of 20 l h-1, and desolvation gas flow of 600 l h-1 at 400 °C. The source temperature was 120 °C. Leucine-enkephaline was used as a reference mass and infused by a secondary reference probe. The reference mass was scanned once every five scans for each positive and negative data collection. Both positive and negative ESI data were collected using a scan time of 0.2 s, with an interscan time of 0.01 s, and a polarity switch time of 0.3 s. MS data were collected in centroid mode using MassLynx V4.1 Scn 727.
LC separation was conducted using a Waters Alliance 2695 HPLC coupled to a Waters 2998 PDA. Separation was achieved on a 150 × 2.0 mm 2.6 μm Kinetex C-18 column (Phenomenex, California) held at a constant temperature of 45 °C, using the following solvent gradient system: (A) 0.1% formic acid in water, (B) 0.1% formic acid in acetonitrile at a flow of 0.2 ml/min: 0-15 min B:10-70%, 15-20 min B:70-100%, 20-42 min B:100%.
Statistics
The JMP software, version 8.0, was used to determine statistical significance between measured values. Significant differences were determined using least square means contrast within a one or two-way ANOVA.
Results
We tested a saponin-rich fraction of the total ethanol extract from M. charantia to investigate the mechanism of action behind the plant’s hypoglycemic activity. This fraction stimulated insulin secretion above that of the DMSO vehicle at the 125 μg/ml concentration (Fig. 2, p=0.02). The activity of the saponin-rich fraction at 125 μg/ml was 4.9-fold greater than that of the DMSO vehicle, while the positive control’s activity was 20.2-fold greater than the DMSO vehicle (Fig. 2).
Fig. 2.
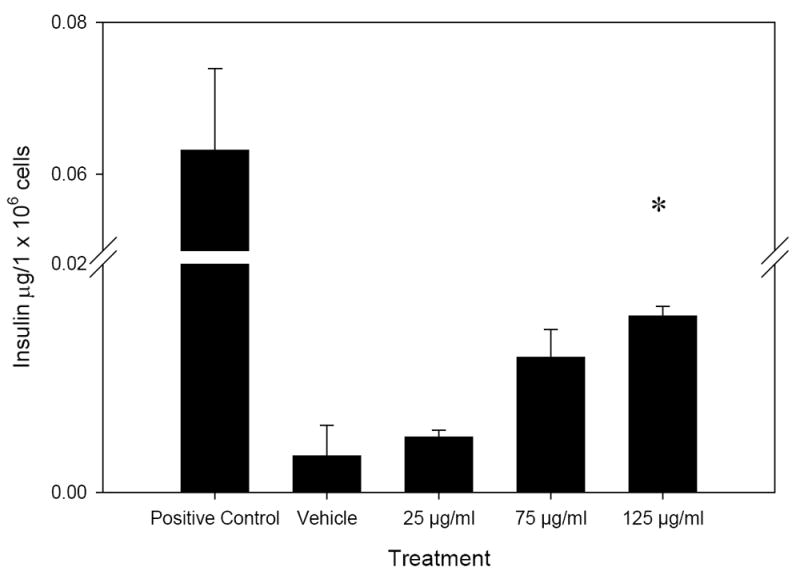
Insulin secretion activity of the saponin-rich fraction, after 60 min incubation with MIN6 β-cells. The (*) denotes significantly different activity as compared to DMSO vehicle, n=3. Error bars represent standard error.
To ascertain that the potentially active compounds present in the saponin-rich fraction of M. charantia were saponins, the fraction was characterized using LC-ToF-MS. These compounds are characterized by their dammarane-type triterpene skeleton, found in cucurbitanes (Hostettmann and Marston, 1995). The cucurbitane saponins found in M. charantia are glycosylated at carbons 7 and 23, and sometimes contain an aldehyde group attached at carbon 9 (Fig. 1) (Miro, 1995). Using the extracted ion chromatogram which corresponds to a common saponin basal skeleton with m/z 437.3290, we were able to characterize most of the ionizable components in the extract as saponins (Fig. 3).
Fig. 3.
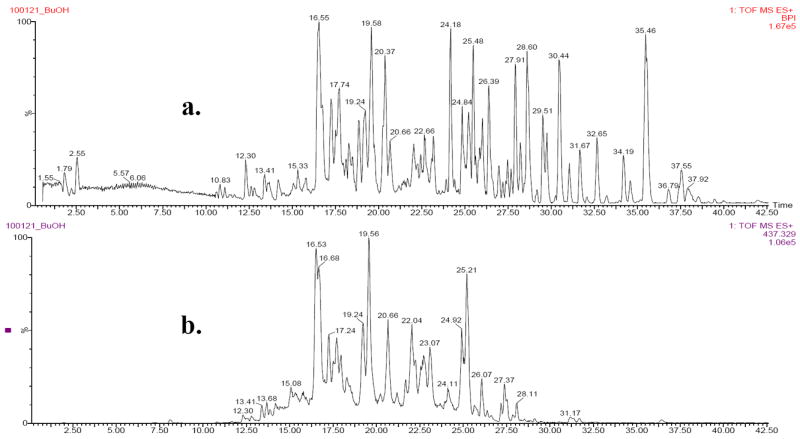
Base peak intensity chromatogram (a), derived from a total ion chromatogram, of saponin-rich fraction of M. charantia. Extracted ion chromatogram (b) for a common cucurbitane skeleton, m/z = 437.3290.
Five pure compounds isolated from M. charantia fruit were tested in the β-cell insulin secretion assay. Although in a previous study, glycoside compound momordicoside U was found to be moderately active in the β-cell assay at both 10 and 25 μg/ml concentrations, (Ma et al., 2010) its aglycone, compound 1 (Fig. 1), did not stimulate insulin secretion.
Compound 3 (Fig. 4) significantly stimulated insulin secretion at 0.1 μg/1 × 106 cells for both concentrations 10 and 25 μg/ml, 7.3 and 7.1 times more than the vehicle (Fig. 3A, p=0.006, 0.007, respectively), while the positive control’s activity was 14.3 times above the vehicle (Fig. 3A). However, its aglycone, compound 2, was not active.
Fig. 4.
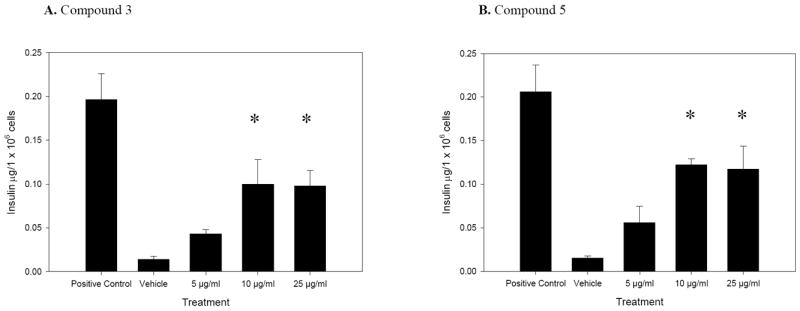
A and B. Insulin secretion activity of compounds 3 and 5, after 60 min incubation with MIN6 β-cells. Positive control is 27 mM glucose together with 50 μM glipizide, and vehicle is DMSO. The (*) denotes significantly different activity as compared to vehicle, n=3. Error bars represent standard error.
Compound 5 (Fig. 4) stimulated insulin secretion at 0.1 μg/1 × 106 cells at both 10 and 25 μg/ml concentrations, 8.1 and 7.8 times the vehicle (Fig. 3B, p=0.002); the positive control stimulated insulin secretion 13.7 times the vehicle (Fig. 3B). The aldehyde of compound 5, compound 4 (Fig. 1), showed no activity.
To ensure that the insulin secretion was due to treatment-induced insulin secretion and not cell death, cells were stained with Trypan Blue and counted for viability. The viability of cells exposed to compound 2 at 25 μg/ml was significantly less as compared to the vehicle (Table 1C, p=0.002). As there was no significantly lower cell viability in any of the other treatment groups for either the ethanol extract or saponin-rich fraction, or pure compounds tested when compared to the DMSO control (Table 1), insulin secretion due to cell death was ruled out.
Table 1.
A-F. β-Cell viability was measured by staining cells with Trypan Blue immediately after static incubation, and is an average of counting four quadrants on a hemocytometer. Viability was assessed as percentage (%) live cells and is ± standard error, n=3. Error bars represent standard error.
| A: |
| Treatment Live Cells (%) |
| Ethanol extract 25 μg/ml 77 ± 5 |
| Ethanol extract 75 μg/ml 76 ± 4 |
| Ethanol extract 125 μg/ml 73 ± 5 |
| Saponin-rich fraction 25 μg/ml 94 ± 5 |
| Saponin-rich fraction 75 μg/ml 93 ± 2 |
| Saponin-rich fraction 125 μg/ml 93 ± 3 |
| B: |
| Treatment Live Cells (%) |
| Compound 1 (0.005 mg/ml) 97 ± 1 |
| Compound 1 (0.010 mg/ml) 97 ± 1 |
| Compound 1 (0.025 mg/ml) 93 ± 3 |
| C: |
| Treatment Live Cells (%) |
| Compound 2 (0.005 mg/ml) 96 ± 1 |
| Compound 2 (0.010 mg/ml) 95 ± 1 |
| Compound 2 (0.025 mg/ml) 89 ± 3* |
| D: |
| Treatment Live Cells (%) |
| Compound 3 (0.005 mg/ml) 98 ± 0 |
| Compound 3 (0.010 mg/ml) 98 ± 0 |
| Compound 3 (0.025 mg/ml) 99 ± 0 |
| E: |
| Treatment Live Cells (%) |
| Compound 4 (0.005 mg/ml) 98 ± 0 |
| Compound 4 (0.010 mg/ml) 99 ± 0 |
| Compound 4 (0.025 mg/ml) 98 ± 0 |
| F: |
| Treatment Live Cells (%) |
| Compound 5 (0.005 mg/ml) 97 ± 0 |
| Compound 5 (0.010 mg/ml) 95 ± 1 |
Discussion
Saponins are well known bioactive phytochemicals, and have been investigated for a multitude of activities, including antimicrobial, cytotoxic, anti-inflammatory, and immunostimulatory (Hostettmann and Marston, 1995; Francis et al., 2002). Although saponins are known to have hypoglycemic activity (Harinantenaina et al., 2006; Oishi et al., 2007; Tan et al., 2008), the cellular and molecular mechanisms of action are only beginning to be explored, and may be varied.
The saponin-rich fraction from M. charantia, along with compounds 3 and 5, stimulated insulin secretion in MIN6 pancreatic β-cells in a concentration-dependent manner. The compounds were compared to a strong positive control; thus, it is possible to compare the strength of activity relative to both positive and negative controls. As the saponin-rich fraction and compounds 3 and 5 stimulated insulin secretion significantly above the vehicle, but less than a highly active positive control, we conclude that the saponin-rich fraction and isolated compounds’ activity is robust. The saponin-rich fraction was also found to contain saponins as detected by LC-ToF-MS, suggesting that these compounds may be responsible for part or all of the hypoglycemic activity of M. charantia. These results are consistent with previous reports of hypoglycemic activity of saponins (Francis et al., 2002; Harinantenaina et al., 2006; Oishi et al., 2007; Tan et al., 2008).
The ranges in bioactivity of compounds 1-5 may be due to their differences in structure. The previously tested compound, momordicoside U (Ma et al., 2010), along with the active compound 3, are monodesmoside cucurbitanes as opposed to their aglycones, inactive compounds 2 and 4, respectively (Fig. 1). Although compound 4 is a bidesmoside and is inactive, it also contains an aldehyde group whereas the bidesmoside compound 5 does not, and is the only compound tested to contain both of these moieties (Fig. 1). Cytotoxicity was observed only when testing the 25 μg/ml concentration of compound 2, and may help explain its lack of bioactivity in this insulin secretion assay, in addition to the structural differences mentioned above. Some saponins are known to be cytoxic at certain concentrations, and this is consistent with our findings for compound 2 (Oleszek et al., 1999; Francis et al., 2002).
These detailed structural differences, especially glycosylation, most likely account for the bioactivity and lack thereof observed. It has been previously reported that both the type of sugar and other moieties along with the specific triterpene skeleton factor greatly in a saponin’s bioactivity (Gauthier et al., 2009). For example, when investigating lupane-type saponins for cytotoxic activity, it was reported that the different sugar moieties reportedly explained the variation in activity of both monodesmosides and bidesmosides (Gauthier et al., 2009). The authors also mention that the basal skeleton of saponins can impact the mechanism of action of bioactivity, in addition to general activity (Gauthier et al., 2009). In other studies, saponin functional groups, such as sugars, also influenced the compounds’ effects on cell membranes (Francis et al., 2002). Two saponins differing in only one glucose group, and neither having an acyl group, showed significantly different insulin absorption activity (Francis et al., 2002). In another study, the presence or absence of sugar moities affected several saponins’ anticancer activity (Wang et al., 2007).
As compounds 1-5 all shared the same basal skeleton, we observed that the sugar moities, and possibly the presence of an aldehyde group, may have been responsible for their bioactivity or lack thereof, in agreement with the studies described above.
Also, the pattern of insulin secretion in response to the cells’ incubation with the saponin-rich fraction is different than the response seen with compounds 3 and 5 (Fig. 2). The response from the saponin-rich fraction is concentration-dependent from 25 to 125 μg/ml, whereas the response to compounds 3 and 5 levels off between 10 and 25 μg/ml (Figs. 2 and 4). As certain bioactive saponins have previously been reported as being agonists, this phenomena may suggest that compounds 3 and 5 are binding to specific receptors, resulting in the observed bioactivity (Yang et al., 2008).
Momordica charantia is one of the most popular medicinal plants used worldwide for diabetes treatment (Marles and Farnsworth, 1995), and is also a widely used food (Basch et al., 2003). With the prevalence of this plant used both for diabetes treatment and food, it is crucial that health practitioners and researchers know and investigate the possible mechanisms of action of its hypoglycemic activity. The more that is known about the efficacy and mechanism of action of M. charantia, the better its use in health care settings throughout the world. All of this points to the potential to provide more informed care, and to avoid any potential ill effects such as herb-drug interactions, among other risks associated with herbal medicine.
Although studies suggest the consistent, but limited bioavailability of saponins (Xu et al., 2003; Podolak et al., 2010), we are currently investigating the saponin-rich fraction in an animal model of type 2 diabetes. In summary, our data support our hypothesis that M. charantia may work to lower blood glucose via promoting insulin secretion, and we theorize that this mechanism may contribute substantially to the plant’s overall hypoglycemic effect.
Acknowledgments
The authors wish to acknowledge Drs. Michael Balick, Anthony Ferrante, Fredi Kronenberg, Jeanne I. Rader, Ina Vandebroek, and Nao Wakae.
Funding
This work was supported by PSC-CUNY and NIH-NCCAM F31-AT004548 “Antidiabetic Constituents from the Dominican Medicinal Plant Momordica charantia” (A.C.K.). The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of NIH-NCCAM.
Footnotes
Declarations of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen R, Cushman LF, Morris S, Feldman J, Wade C, McMahon D, Moses M, Kronenberg F. Use of complementary and alternative medicine among Dominican emergency department patients. Am J Emerg Med. 2000;18:51–54. doi: 10.1016/s0735-6757(00)90048-2. [DOI] [PubMed] [Google Scholar]
- Barnes PM, Bloom B, Nahin RL. N C f C a A Medicine. Washington, D.C.: National Institutes of Health; 2008. Complementary and alternative medicine use among adults and children: United States, 2007. [PubMed] [Google Scholar]
- Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Semin Int Med. 2002;2:54–71. [PubMed] [Google Scholar]
- Basch E, Gabardi S, Ulbricht C. Bitter melon (Momordica charantia): a review of efficacy and safety. Am J Health Syst Pharm. 2003;60:356–359. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Controland Prevention. National Diabetes Fact Sheet, 2007. 2010 Retrieved April, 2010 from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf.
- Egede L, Ye X, Zheng D, Silverstein MD. The prevalence and pattern of complementary and alternative medicine use in individuals with diabetes. Diabetes Care. 2002;25:324–329. doi: 10.2337/diacare.25.2.324. [DOI] [PubMed] [Google Scholar]
- Francis G, Kerem Z, Makker HPS, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Gauthier C, Legault J, Girard-Lalancette K, Mshvildadze V, Pichette A. Haemolytic activity, cytotoxicity and membrane cell permeabilization of semi-synthetic and natural lupane- and oleanane- type saponins. Bioorg Med Chem. 2009;17:2002–2008. doi: 10.1016/j.bmc.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Harinantenaina L, Tanaka M, Takaoka S, Oda M, Mogami O, Uchida M, Asakawa Y. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem Pharm Bull. 2006;54:1017–1021. doi: 10.1248/cpb.54.1017. [DOI] [PubMed] [Google Scholar]
- Hostettmann K, Marston A. Chemistry and Pharmacology of Natural Products: Saponins. Cambridge University Press; Cambridge: 1995. pp. 233–283. [Google Scholar]
- Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki J-I, Oka Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia. 1993;36:1139–1145. doi: 10.1007/BF00401058. [DOI] [PubMed] [Google Scholar]
- Ma J, Whittaker P, Keller AC, Mazzola EP, Pawar RS, White KD, Callahan JH, Kennelly EJ, Krynitsky AJ, Rader JI. Cucurbitane-type triterpenoids from Momordica charantia. Planta Med. 2010 doi: 10.1055/s-0030-1249807. [DOI] [PubMed] [Google Scholar]
- Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomed. 1995;2:137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- Miro M. Cucurbitacins and their pharmacological effects. Phyto Res. 1995;9:159–168. [Google Scholar]
- Miura T, Itoh C, Iwamoto N, Kato M, Kawai M, Park SR, Suzuki I. Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J Nutri Sci Vitaminol. 2001;47:340–344. doi: 10.3177/jnsv.47.340. [DOI] [PubMed] [Google Scholar]
- Miura T, Itoh Y, Iwamoto N, Kato M, Ishida T. Suppressive activity of the fruit of Momordica charantia with exercise on blood glucose in type 2 diabetic mice. Biol Pharm Bull. 2004;27:248–250. doi: 10.1248/bpb.27.248. [DOI] [PubMed] [Google Scholar]
- Nerurkar PV, Lee YK, Motosue M, Adeli K, Nerurkar VR. Momordica charantia (bitter melon) reduces plasma apolipoprotein B-100 and increases hepatic insulin receptor substrate and phosphoinositide-3 kinase interactions. Br J Nutr. 2008;100:751–759. doi: 10.1017/S0007114508937430. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Sakamoto T, Udagawa H, Taniguchi H, Kobayashi-Hattori K, Ozawa Y, Takita T. Inhibition of increases in blood glucose and serum neutral fat by Momordica charantia saponin fraction. Biosci Biotechnol Biochem. 2007;71:735–740. doi: 10.1271/bbb.60570. [DOI] [PubMed] [Google Scholar]
- Oleszek W, Junkuszew M, Stochmal A. Determination and toxicity of saponins from Amaranthus cruentus seeds. Journal of Agriculture and Food Chemistry. 1999;47:3685–3687. doi: 10.1021/jf990182k. [DOI] [PubMed] [Google Scholar]
- Persaud SJ, Al-Majed H, Raman A, Jones PM. Gymnema sylvestre stimulates insulin release in vitro by increased membrane permeability. J Endocrinol. 1999;163:207–212. doi: 10.1677/joe.0.1630207. [DOI] [PubMed] [Google Scholar]
- Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev. 2010;9:425–474. doi: 10.1007/s11101-010-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C-C, Lin C-H, Lin W-L. Effects of Momordica charantia on insulin resistance and visceral obesity in mice on high-fat diet. Diabetes Res Clin Pract. 2008;81:134–143. doi: 10.1016/j.diabres.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Talchai C, Lin HV, Kitamura T, Accili D. Genetic and biochemical pathways of β-cell failure in type 2 diabetes. Diab Obes Met. 2009;11:38–45. doi: 10.1111/j.1463-1326.2009.01115.x. [DOI] [PubMed] [Google Scholar]
- Tan M-J, Ye J-M, Turner N, Hohnen-Behrens C, Ke C-Q, Tang C-P, Chen T, Weiss H-C, Gesing E-R, Rowland A, James DEYY. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem Biol. 2008;15:263–273. doi: 10.1016/j.chembiol.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Diabetes Programme. 2010 Retrieved April, 2010 from http://www.who.int/diabetes/en/
- Xiang L, Huang X, Chen L, Rao P, Ke L. The reparative effects of Momordica charantia Linn. extract on HIT-T15 pancreatic β-cells. Asia Pac J Clin Nutr. 2007;16:249–252. [PubMed] [Google Scholar]
- Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Yang C-S, Ko S-R, Cho B-G, Shin D-M, Yuk J-M, Li S, Kim J-M, Evans RM, Jung J-S, Song D-K, Jo E-K. The ginsenoside metabolite compound K, a novel agonist of glucocorticoid receptor, induces tolerance to endotoxin-induced lethal shock. J Cell Mol Mech. 2008;12:1739–1753. doi: 10.1111/j.1582-4934.2007.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


