Abstract
Background
The potential role of vitamin D and soy in prostate cancer (PCa) prevention/treatment has gained much attention in recent years. In this study, we evaluated the anticancer activity of calcitriol, the active form of vitamin D, dietary soy, and their combinations in a mouse model of PCa.
Methods
Athymic male nude mice bearing PC-3 human PCa xenografts received diets containing 10 kcal% or 20 kcal% soy, calcitriol injections, or a combination of dietary soy and calcitriol. Changes in tumor growth, serum levels of 1,25(OH)2D and calcium, and regulation of tumor gene expression were examined.
Results
The combination treatments resulted in substantially greater inhibition of tumor growth than either agent alone. Soy diets alone caused a modest elevation in serum 1,25(OH)2D, whereas the calcitriol-soy combinations led to substantially elevated serum 1,25(OH)2D, hypercalcemia, and in some cases lethal toxicity. The combinations enhanced calcitriol activity in regulating target gene expression, including greater up-regulation of anti-proliferative (p21, IGFBP-3) and pro-apoptotic (Bax) genes, increased inhibition of anti-apoptotic (Bcl-2) and cell cycle promoting (cyclin D1) genes, and suppression of prostaglandin (PG) synthesis and signaling (COX-2, 15-PGDH, PG receptors). Increases in serum calcium were accompanied by elevated expression of intestinal calcium absorption genes (TRPV6, calbindin-9k).
Conclusions
Soy increases the bioavailability of endogenous and administered calcitriol, thereby enhancing its anticancer effects and risk of hypercalcemia. Since both agents are easily available as dietary supplements, the increased potential for hypercalcemic toxicity becomes an important factor when considering the combined use of vitamin D and soy in PCa therapy.
Keywords: Vitamin D, calcitriol, soy, prostate cancer, CYP24A1, hypercalcemia
INTRODUCTION
Prostate cancer (PCa) is one of the most frequently diagnosed cancers among men in the United States and the second leading cause of cancer death in men after lung cancer [1]. While common treatment options include surgery, radiation, and androgen deprivation therapy, these methods are often insufficient for the treatment of advanced disease. Extensive research has indicated a potential role for vitamin D and soy in the prevention and treatment of PCa [2–8].
Calcitriol (1,25-dihydroxyvitamin D3) is the hormonally active form of vitamin D3 (cholecalciferol). It regulates gene transcription by binding to the vitamin D receptor (VDR) and plays a major role in maintaining calcium homeostasis in the body. Additionally, calcitriol has been shown to exhibit anti-proliferative, pro-differentiating, pro-apoptotic, and anti-inflammatory actions on a variety of cancer cells, including PCa cells [5,8–12]. An important component of the anti-inflammatory activity of calcitriol is the inhibition of the synthesis and signaling of prostaglandins (PGs), which play an important role in prostate carcinogenesis [5,13–17]. Despite these beneficial anticancer activities, clinical applications of calcitriol have been limited by the increased potential for developing hypercalcemia, hypercalciuria, and renal stones at therapeutically effective doses [4]. One approach to limit the toxicity is to use lower concentrations of calcitriol in combination with other agents that enhance its anticancer activity.
Epidemiological and experimental evidence points to the potential benefits of soy isoflavones, in particular genistein, in PCa treatment and prevention [18–20]. Some of the anticancer effects of genistein in PCa cells include the induction of apoptosis, interference with cell signaling, reduction of androgen receptor expression, and inhibition of the PG pathway [19–22]. Genistein in combination with calcitriol causes enhanced inhibition of the proliferation of LNCaP PCa cells and primary human prostatic epithelial cells due to the synergistic up-regulation of VDR expression and vitamin D signaling [23,24]. Phytoestrogens have been shown to affect the sensitivity of breast cancer cells to vitamin D through regulation of the VDR promoter [25]. Previous studies from our lab have demonstrated that the combination of calcitriol and genistein inhibits the proliferation of the calcitriol-resistant human PCa cell line DU145 [26]. These observations suggest that combining genistein or soy with low doses of calcitriol might be a beneficial chemopreventive or therapeutic strategy in PCa [8,27,28].
We have shown that in addition to exerting anti-proliferative activity, genistein also acts as a noncompetitive inhibitor of the enzymatic activity of 25-hydroxyvitamin D-24-hydroxylase (CYP24A1), the P450 enzyme that catalyzes the initial conversion of calcitriol to less active metabolites [26]. By inhibiting CYP24A1 activity, genistein co-treatment effectively increased the half-life of calcitriol, up-regulated VDR expression, and thereby increased growth arrest and apoptosis of DU145 cells that were resistant when calcitriol was used alone [26]. However, these findings raise the possibility that the increased bioavailability of calcitriol due to co-administered genistein or soy would also be associated with increased toxic effects including hypercalcemia. Since vitamin D and soy are frequently used as dietary supplements, patients being treated with calcitriol or vitamin D supplements may also be supplementing with dietary soy, and the potential toxicity of combining these readily available agents needs to be evaluated.
Therefore, in the present study we evaluated the effects of combinations of calcitriol and soy in a mouse xenograft model of PCa. The data show that the combinations achieve significantly greater inhibition of tumor growth and enhanced anticancer activity compared to the individual agents. However, the combinations also increase the risk of hypercalcemia by prolonging serum calcitriol half-life and enhancing intestinal calcium absorption. These findings suggest that approaches aiming to reap the benefits of calcitriol and soy combinations in PCa treatment should proceed with caution.
MATERIALS AND METHODS
Materials
Calcitriol was kindly provided by Dr. Milan Uskokovic (BioXell). Tissue culture media, supplements, and fetal bovine serum (FBS) were obtained from Gibco BRL (Grand Island, NY), Lonza (Walkersville, MD), and Mediatech Inc. (Herndon, VA), respectively. Research animal diets, including soy-supplemented diets, were obtained from Research Diets, Inc. (New Brunswick, NJ). The basal diet contains 1000 I.U. of vitamin D per kg, a moderately high level of vitamin D [29].
Methods
Cell Culture
PC-3 human PCa cells were routinely cultured in MEM medium containing 10% FBS, 100 IU/ml penicillin, and 100 µg/ml streptomycin. Cells were maintained at 37°C and 5% CO2 in a humidified incubator. Cell cultures were screened for the absence of murine viruses before inoculation into nude mice. Cultures grown to ~75% confluence in T-150 flasks were washed and trypsinized to obtain cell pellets under sterile conditions. Cell pellets were resuspended (~10×107 cells/ml) in a small volume of sterile culture media and were mixed with an equal volume of Matrigel (BD Biosciences, Bedford, MA) prior to xenograft establishment in nude mice.
Xenograft establishment in nude mice
All animal procedures were performed in compliance with guidelines approved by the Stanford University Administrative Panels on Laboratory Animal Care (APLAC). Four- to six-week-old male athymic, nude (Foxnnu/nu) mice purchased from Harlan Laboratories (Indianapolis, IN) were housed in a designated pathogen-free area in the Research Animal Facility, Stanford University School of Medicine, and fed irradiated mouse chow and autoclaved reverse osmosis-treated water. Xenografts were established in the flanks of mice by subcutaneous injections of PC-3 cells (~5 million) suspended in a small volume (~100 µl) of the culture medium–Matrigel mixture. The tumors were allowed to grow for three weeks and attained a size of 100 mm3 when treatment was initiated.
Treatments
Animals with established xenografts were randomly assigned to a control group and five treatment groups. Treatment groups received either injections of 0.1 µg calcitriol in 50 µl of 0.1% ethanol in sterile saline, soy-supplemented diet with 10 kcal% derived from soy protein (10%-soy), soy-supplemented diet with 20 kcal% from soy protein (20%-soy), or a combination of calcitriol injections and soy-supplemented diets. All diets were accessible ad libitum. Calcitriol was administered every other day by intraperitoneal (i.p.) injections. The mice in the control group received i.p. injections of 0.1% ethanol in sterile saline every other day and the basal diet. All diets contained 1000 I.U./kg of vitamin D [29]. All treatments were for a period of 4 weeks. Animal body weights were recorded weekly during the course of treatment and tumor volumes were calculated as described previously [30]. At the end of 4 weeks, the mice were subjected to CO2 euthanasia and exsanguination by cardiac puncture 14 hours after the last injection of calcitriol. Sera were collected from the blood samples by centrifugation and stored at −80°C for later analysis. The tumors and intestinal mucosa were harvested and stored in Trizol reagent at −80° C until further use.
RNA isolation and real-time PCR
Tumor and intestinal mucosa samples were homogenized and total RNA was extracted using the Trizol reagent (Invitrogen, Life Technologies, Carlsbad, CA). Gene expression analysis by real-time RT-PCR was conducted as previously described [17]. Briefly, reverse transcription of the isolated RNA was performed with the SuperScript III First-Strand Synthesis kit (Invitrogen). Quantitative real-time PCR was conducted with DyNamo SYBR green qPCR (Finnzymes, New England Biolabs, Ipswich, MA) on the Opticon 2 DNA engine (Bio Rad, Hercules, CA) using gene-specific primers, and mRNA expression levels, normalized to TATA-box binding protein (TBP) and GAPDH, were determined by the 2−ΔΔC(t) method as described previously [17].
Serum calcium and 1,25-dihydroxyvitamin D measurements
Serum calcium levels were measured using the QuantiChrom calcium assay kit (BioAssay Systems, Hayward, CA) according to manufacturer instructions. Levels of 1,25-dihydroxyvitamin D (1,25(OH)2D), which includes both 1,25(OH)2D2 and 1,25(OH)2D3, were measured in pooled serum samples at Heartland Assays Inc. (Ames, IA). The assay involves a preliminary extraction and subsequent purification of vitamin D metabolites from serum using C18-OH cartridges. Following extraction, the sample is then assayed using a competitive RIA procedure [31]. The RIA method employs a polyclonal antibody that is specific for both 1,25(OH)2D2 and 1,25(OH)2D3. The inter-assay and intra-assay coefficients of variation for this assay are 12.6% and 9.8%, respectively.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism 5 (GraphPad Software, San Diego, CA). Data were evaluated by ANOVA and unpaired Student t tests with Welch correction.
RESULTS
Inhibition of Tumor Growth
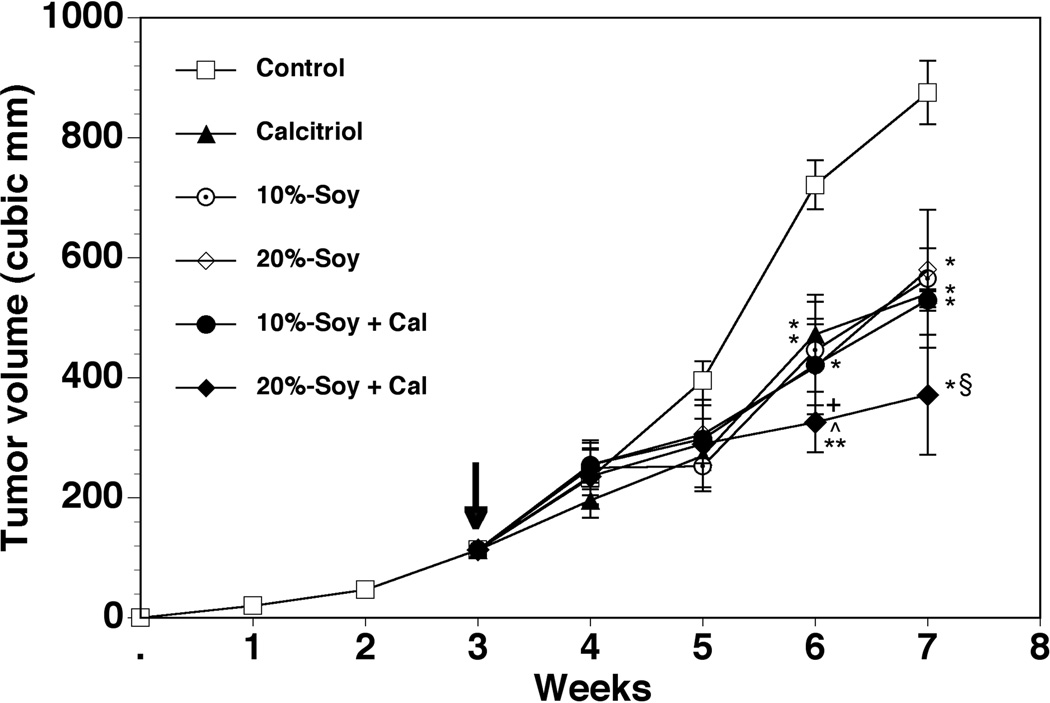
PCa xenografts established in the flanks of male nude mice grew to ~100 mm3 in size by three weeks following tumor cell inoculation, at which time treatments were initiated. The tumors in the control group continued to grow over the next 4 weeks, reaching a mean volume of ~870 mm3 by week 7 (Figure 1). In calcitriol-treated mice, decreases in tumor volumes were evident by about 2 weeks after treatment was initiated. Continued tumor growth was evident in the calcitriol-treated group, but at a slower rate than controls. By the end of four weeks, calcitriol treatment resulted in a nearly 40% reduction (p < 0.05) in the mean tumor volumes compared to control (539 mm3 vs. 875 mm3). The magnitude of tumor inhibition seen in the mice receiving soy-supplemented diets was comparable to that due to calcitriol. Reductions in the tumor volumes were similar in the mice treated with the 10%- (564 mm3) and 20%-soy diets (574 mm3). Only the 20%-soy diet in combination with calcitriol was able to achieve a further reduction in final tumor volume compared to calcitriol alone, achieving a mean final tumor volume of 371 mm3 (~60% inhibition when compared to control and a 40% greater inhibition compared to either drug alone). In contrast, the 10%-soy diet combined with calcitriol resulted in a final tumor volume of 529 mm3, similar to the effect achieved by either agent alone.
Figure 1. Effect of calcitriol, soy, and their combination on PC-3 xenograft growth.
Human PC-3 cells were established in the flanks of male nude mice and allowed to grow for three weeks to approximately 100 mm3 before initiation of treatment (arrow). Treatment groups received either i.p. injections of 0.1 µg calcitriol in 50 µl of 0.1% ethanol in sterile saline every other day; soy-supplemented diet with 10 kcal% soy protein (10%-soy); soy supplemented diet with 20 kcal% soy protein (20%-soy); or a combination of calcitriol injections and soy-supplemented diets. Animals in the control group received i.p. injections of 0.1% ethanol in sterile saline every other day. Tumor volumes were measured weekly over the course of the four-week treatment period. Values represent mean ± SE. Control group n = 14; calcitriol group n = 16; 10%-soy group n = 13; 20%-soy group n = 14; 10%-soy+cal group n = 14; 20%-soy+cal group n = 12. * p < 0.05, ** p < 0.01 compared to control. + p < 0.05 compared to calcitriol. ^ p < 0.05 compared to 20%-soy. § Does not include animals that died before end of treatment course.
Effects of calcitriol-soy combinations on body weight and serum levels of calcium and 1,25-dihydroxyvitamin D
Serum samples were obtained ~14 hours after the final calcitriol injection. No significant changes were observed in final body weight in the animals treated with calcitriol or the soy diets as single agents (Table I). However, serum calcium levels in animals treated with calcitriol registered a modest but statistically significant increase (10.0 mg/dl, p < 0.05). Signs of toxicity were most apparent in mice on the calcitriol-20%-soy combination treatment. Five of the sixteen mice in this group died within two weeks of treatment initiation. The body weights of the surviving mice receiving this combination treatment were significantly lower than the control mice at the end of 4 weeks, indicating toxicity (Table I). Serum calcium levels in the surviving mice treated with this combination showed a substantial and significant elevation demonstrating hypercalcemia (12.1 mg/dl, p < 0.01). Elevations in serum calcium levels were also seen in the mice treated with the combination of calcitriol and 10%-soy, though to a lesser but still substantial degree (11.3 mg/dl, p < 0.01), and one mouse died in this group as well.
TABLE I.
Body weight, serum calcium, and serum 1,25(OH)2D measurements
| GROUPS | BODY WEIGHT (g) (n=14) |
SERUM CALCIUM (mg/dl) (n=9) |
SERUM 1,25(OH)2D (pg/ml) (n=5) |
|---|---|---|---|
| Control | 27.1±0.7 | 8.9±0.5 | 56.6±2 |
| Calcitriol (0.1µg) | 25.8±0.9 | 10.0±0.2* | 100.4±10* |
| 10%-Soy | 28.1±0.7 | 9.1±0.6 | 133.8±16** |
| 20%-Soy | 28.6±0.7 | 9.1±0.3 | 122.3±16* |
| 10%-Soy + Cal | 25.1±0.6 | 11.3±0.4** | 170.2±19** + |
| 20%-Soy + Cal | 24.6±0.5* | 12.1±0.8** | 266.7±20*** ++ ^^ § |
p < 0.05 when compared to control.
p < 0.01 when compared to control.
p < 0.001 when compared to control.
p < 0.05 when compared to calcitriol.
p < 0.01 when compared to calcitriol.
p < 0.01 when compared to 20%-soy.
Does not include animals that died before end of treatment course.
Serum 1,25(OH)2D levels in the various experimental groups were also assayed (Table I). While serum 1,25(OH)2D levels of animals receiving calcitriol injections were elevated as expected, 1,25(OH)2D levels of the animals on the soy diets without calcitriol treatment were significantly increased as well, suggesting that the soy diets elevated endogenously synthesized 1,25(OH)2D levels. Consistent with the significantly elevated serum 1,25(OH)2D levels, both combination treatment groups showed significantly (p < 0.001) increased serum calcium levels. A dose-dependent effect was observed with the 20%-soy and calcitriol combination, resulting in much higher serum 1,25(OH)2D and calcium levels than the 10%-soy and calcitriol combination. However, although elevated, no difference in serum 1,25(OH)2D levels between 10%-soy alone and 20%-soy alone was observed at this time point. It should be emphasized that all blood samples were drawn 14 hours after the final calcitriol injection; the data therefore represent nadir values for serum 1,25(OH)2D and calcium [32].
Changes in mRNA expression in PC-3 xenografts
We evaluated the ability of soy to modulate the regulatory effects of calcitriol on the expression of several target genes in the tumor. Because both the enhanced anticancer activity and increased toxicity were observed with the calcitriol-20%-soy combination, changes in gene expression when 20%-soy was administered alone or with calcitriol are presented here. Unless otherwise noted, treatments with the 10%-soy diet elicited similar responses as those seen with the 20%-soy diet, albeit to a somewhat lesser degree.
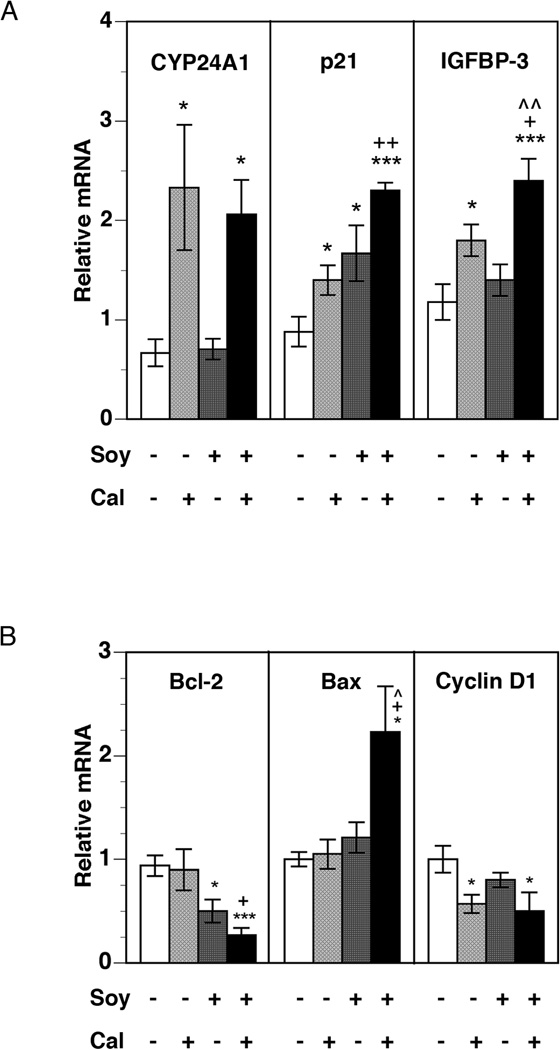
To understand the effects of soy supplementation on calcitriol-mediated transcriptional activity in the tumor, we first examined the gene expression of CYP24A1, whose transcription in target tissues is induced by calcitriol [8]. As expected, calcitriol increased CYP24A1 mRNA in the tumor 2.3-fold (p < 0.05) compared to untreated mice (Figure 2A). Soy supplementation alone did not appear to increase levels of CYP24A1 gene expression. The calcitriol-20% soy combination also significantly increased CYP24A1 mRNA 2.1-fold (p < 0.05), though this does not represent enhancement above the level achieved by calcitriol alone.
Figure 2. Changes in tumor mRNA expression of classical calcitriol target genes.
PC-3 xenografts were harvested from mice at the end of four weeks of treatment. Total RNA was isolated from harvested tumor tissue and mRNA levels of the calcitriol inactivating enzyme CYP24A1 (A), anti-proliferative genes p21 and IGFBP-3 (A), apoptosis-related genes Bcl-2 and Bax (B), and cell cycle promoter cyclin D1 (B) were analyzed by qRT-PCR, as described in Materials and Methods. Relative mRNA expression for each gene in tumors from control mice was set at 1. Values represent mean ± SE of 6–11 measurements in each group. * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to control. + p < 0.05, ++ p< 0.01 compared to calcitriol. ^ p< 0.05, ^^ p < 0.01 compared to 20%-soy.
Next, we examined the expression of genes that are known to control proliferation (cyclin D1, p21 and IGFBP-3), apoptosis (Bcl-2 and Bax), and inflammation (COX-2, 15-PGDH, and EP receptors) in PCa. Significant increases in the mRNA levels of p21, an inhibitor of cyclin-dependent kinase (CDK) activity in promoting cell division, were seen in all treatment groups (Figure 2A). Consistent with the greatest degree of tumor retardation seen in this cohort, the 20%-soy and calcitriol combination increased p21 expression maximally (~2.5-fold over control mice, p < 0.001), which was significantly higher than calcitriol alone, indicating the potentiation of calcitriol effects by soy. Increases in the mRNA levels of IGFBP-3, a direct transcriptional target of calcitriol with anti-proliferative and pro-apoptotic effects [33], were also observed (Figure 2A). Calcitriol increased IGFBP-3 mRNA expression 1.8-fold over control, whereas soy did not produce a significant change. However, administration of soy in combination with calcitriol led to a further increase in IGFBP-3 mRNA level (~2.5-fold over control), indicating an enhanced effect. Soy co-administration greatly enhanced the down-regulation of the expression of the anti-apoptotic gene Bcl-2 and the up-regulation of the pro-apoptotic gene Bax by calcitriol (Figure 2B). Expression of mRNA for cyclin D1, an activator of CDK, was reduced approximately two-fold by calcitriol but was not enhanced by soy co-treatment (Figure 2B).
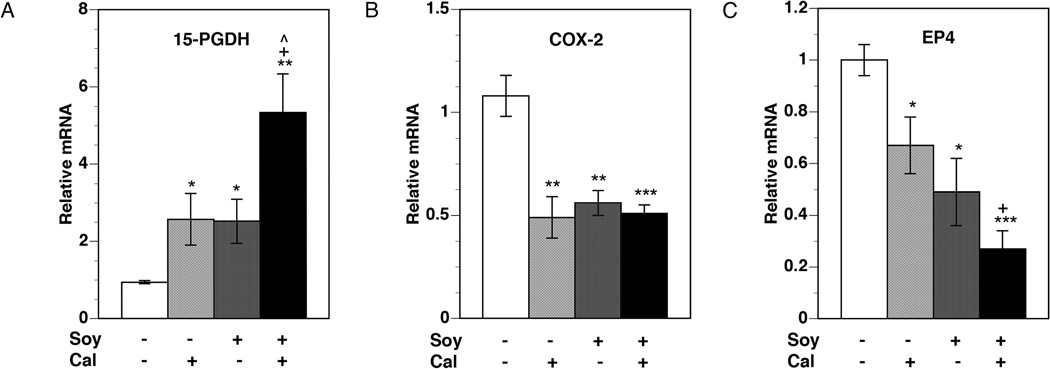
Significant up-regulation of the expression of the PG-degrading enzyme 15-PGDH was observed in all treatment groups (Figure 3A). Calcitriol and soy when used individually increased 15-PGDH mRNA levels approximately 2.5-fold over control. Combining the two agents significantly increased 15-PGDH mRNA levels over the increase seen with either agent alone, resulting in more than a five-fold increase over control. As expected, the expression of COX-2, the enzyme catalyzing PG synthesis, was significantly reduced by 50% by calcitriol (Figure 3B). Treatment with soy also decreased COX-2 expression levels to a similar degree. The combination of the two agents, however, produced no further decreases in COX-2 mRNA levels. Changes in the mRNA levels of three PG receptors were also analyzed. EP4 mRNA levels decreased significantly with both calcitriol and soy treatments, with the levels falling to 67% and 49% of control levels, respectively (Figure 3C). The combination was significantly more effective than calcitriol alone, reducing EP4 mRNA levels to 27% of control. Expression of the EP2 and FP receptors did not change significantly with the treatments (data not shown).
Figure 3. Changes in tumor mRNA expression of PG pathway genes.
PC-3 xenografts were harvested from mice at the end of four weeks of treatment. Total RNA was isolated from harvested tumor tissue and mRNA levels of 15-PGDH (A), COX-2 (B), and PG receptor EP4 (C) were analyzed by qRT-PCR, as described in Materials and Methods. Relative mRNA expression for each gene in tumors from control mice was set at 1. Values represent mean ± SE of 6–11 measurements in each group. * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to control. + p < 0.05 compared to calcitriol. ^ p< 0.05 compared to 20%-soy.
Changes in mRNA Expression in the Intestinal Mucosa
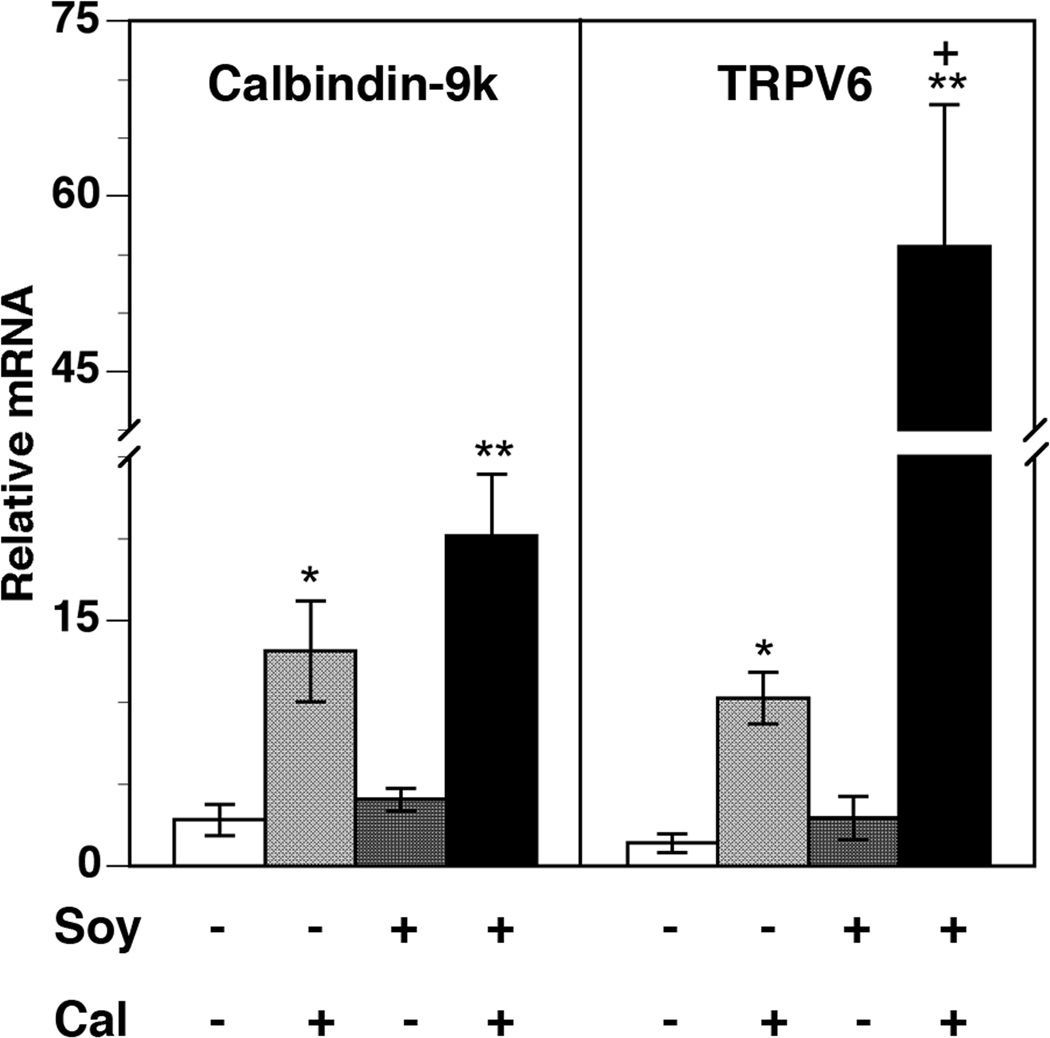
To further examine the mechanism of lethal hypercalcemia produced by the 20%-soy and calcitriol combination, the expression of genes involved in calcium transport, namely TRPV6 and calbindin-9k, was measured in the intestinal epithelia of the tumor-bearing mice (Figure 4). A significant induction of TRPV6 and calbindin mRNA expression was seen in the calcitriol-treated group. When used alone, the soy diets did not achieve any significant change in TRPV6 or calbindin-9k mRNA levels compared to control. In contrast, a striking enhancement of the increase in the expression of both calcium absorption genes was observed in the soy-calcitriol combination treatment groups. When calcitriol was combined with the 20%-soy diet, TRPV6 mRNA increased 55-fold, representing a substantial enhancement over the 10-fold increase seen with calcitriol alone. Calbindin-9k mRNA levels also increased more with the combination treatment than with calcitriol alone, resulting in a 20-fold increase in the group receiving the combination.
Figure 4. Changes in intestinal mucosa mRNA expression of calcium absorption genes.
Intestinal mucosa was collected from mice at the end of four weeks of treatment. Total RNA was isolated from the intestinal mucosa and mRNA levels of calbindin-9k and the TRPV6 calcium channel were analyzed by qRT-PCR. Relative mRNA expression for each gene in mucosa from control mice was set at 1. Values represent mean ± SE of 5–6 measurements in each group. * p < 0.05, ** p < 0.01 compared to control. + p < 0.05 compared to calcitriol.
DISCUSSION
Pre-clinical research has demonstrated the benefit of calcitriol in the treatment of PCa, especially in combination with other cytotoxic agents [2,4,6,34], whereas large clinical trials thus far have been disappointing [35]. In this study we evaluated a potential treatment strategy, namely the combination of calcitriol and dietary soy. Evidence suggests that both these agents may have a beneficial role in the prevention and treatment of PCa [3,8–10,18–20,28,36,37]. Furthermore, earlier studies have demonstrated a synergistic enhancement of inhibition of prostate cell proliferation when these two agents are combined [24,26]. These observations provide the rationale for investigating the anticancer potential of calcitriol-soy combination in vivo using an animal model, wherein the potential for the development of hypercalcemic toxicity could also be evaluated.
Our data showed that calcitriol administration in combination with dietary soy protein resulted in more substantial inhibition of tumor growth than either agent alone, with the greatest inhibition being seen when the 20%-soy diet was combined with calcitriol. Consistent with enhanced inhibition of tumor growth was the enhancement by soy of calcitriol regulation of target gene expression, including greater up-regulation of anti-proliferative and pro-apoptotic genes and a more substantial decrease in the expression of anti-apoptotic and cell cycle regulatory genes. Enhanced inhibition of PG synthesis and signaling by calcitriol and soy was also observed in the tumor tissue, consistent with observations we have previously made in PCa cell cultures [16,17,27]. However, some calcitriol target genes (CYP24A1, COX-2, cyclin D1) examined in our study did not demonstrate an enhanced response to the combination treatment. This could be because increases in circulating calcitriol levels following soy co-administration could have had a threshold effect on the expression of some genes, which are also direct targets of soy [22,23], or because the timing of our measurement of gene transcription missed the maximum changes in levels of expression of some genes.
As demonstrated by these findings in vivo, previous studies have established the ability of soy components such as genistein to potentiate calcitriol actions on gene transcription and prostate cell proliferation [19,21,24,26]. While the soy component genistein has independent actions on many of these genes, some of them, such as p21 and IGFBP-3, are also targeted by calcitriol [19,22,23]. Further vitamin D-independent actions of soy could also contribute to its anticancer activity in PCa cells [38,39]. However, we believe that the major mechanism underlying the synergistic activity of the calcitriol-soy combination is the ability of the soy component genistein to modulate the metabolism of vitamin D.
Metabolism of vitamin D in target tissues is mediated by two key enzymes: 1α-hydroxylase (CYP27B1), which catalyzes the synthesis of calcitriol from 25(OH)D, and 24-hydroxylase (CYP24A1), which catalyzes the initial step in the degradation of 25(OH)D and calcitriol to inactive metabolites. One potential mechanism by which soy may influence the metabolism of calcitriol is by increasing its synthesis from dietary precursors. The standard AIN76 animal research diet contains 1000 I.U./kg of diet of vitamin D [29], as does the basal diet used in our study. Previous studies have shown increased expression of CYP27B1 mRNA in the colon of mice that received the AIN76 diet with genistein supplementation, but not in mice receiving AIN76 alone [40]. In our study, we found that serum 1,25(OH)2D levels in mice treated with 10%- or 20%-soy were elevated in a dose-dependent manner to levels comparable to those in mice receiving only calcitriol injections, with maximal serum 1,25(OH)2D levels achieved with the calcitriol-soy combinations. Thus, in order for circulating levels of 1,25(OH)2D to increase in the absence of exogenous calcitriol administration, soy is likely enhancing the endogenous conversion of vitamin D from the diet to active calcitriol and/or inhibiting its degradation.
A second mechanism which we favor to underlie both the elevated 1,25(OH)2D serum levels and the synergistic effect of the soy-calcitriol combination on tumor growth is the ability of genistein to inhibit the enzymatic activity of CYP24A1 [24,26], the main P450 enzyme in the calcitriol degradation pathway. Although genistein has also been shown to inhibit the transcription of CYP24A1 and CYP27B1 in DU-145 PCa cells [41,42], in this study we observed that CYP24A1 gene expression was not reduced below control levels in tumors from mice receiving soy-supplemented diets. Additionally, soy did not appear to attenuate CYP24A1 gene expression in the tumors when combined with calcitriol, allowing for comparable increases in CYP24A1 mRNA levels between the calcitriol-only and the combination treatment groups. In spite of this upregulation of CYP24A1 gene expression, serum 1,25(OH)2D levels and calcitriol anti-tumor effects were both effectively enhanced by the combination treatment. Therefore, the ability of soy to prolong calcitriol serum half-life and enhance its activity must be primarily mediated through inhibition of the enzymatic activity of the 24-hydroxylase protein as we previously demonstrated [26] and not an effect on its mRNA expression. By inhibiting the degradation of calcitriol by CYP24A1, soy supplementation enabled higher levels of circulating calcitriol to exert a greater degree of anticancer activity, as our findings demonstrate. The role of CYP24A1 in antagonizing calcitriol activity against PCa has previously been explored using the P450 enzyme inhibitors liarozole and ketoconazole, whose combination with calcitriol resulted in greater tumor growth inhibition than achieved when either agent was used alone [43,44]. Accordingly, CYP24A1, now recognized as a candidate oncogene [45,46], has become the target of many potential therapeutic agents for PCa, including CYP24A1-resistant calcitriol analogs [47,48], tetralone derivatives [49], and other CYP24A1-inhibitory compounds.
Since CYP24A1 inhibition by soy increases calcitriol bioavailability [26], soy is expected to increase both calcitriol-mediated beneficial activity against PCa as well as its positive regulation of calcium absorption. Studies in CYP24A1-knock-out mice have demonstrated that the inability to degrade the active hormone greatly increases calcitriol activity, leading to decreased bone mineralization and high serum calcium levels [50]. Similarly, loss of function mutations in CYP24A1 have been shown to cause severe infantile hypercalcemia following vitamin D supplementation because of the inability to inactivate calcitriol [51]. The same mechanism would account for the findings in our study, where calcitriol-mediated up-regulation of the TRPV6 calcium channel and calbindin-9k [52–54] in intestinal epithelia was substantially enhanced by soy supplementation. The subsequent increase in calcium absorption led to hypercalcemia, which likely explains the increased mortality in the group treated with the calcitriol-20%-soy combination. In contrast, only modest elevations in serum calcium levels were seen in mice receiving calcitriol alone. Although we did not detect a significant difference in serum calcium levels between the two combination treatment groups, it is reasonable to assume that the deceased animals in the 20%-soy and calcitriol combination group had much higher serum calcium levels than the surviving mice in the immediate hours after calcitriol injections. This would suggest that actual peak values were higher than those determined when blood samples were drawn from surviving mice 14 hours after calcitriol injections, which may well represent nadir values [32]. The elevations in serum 1,25(OH)2D levels and the degree of toxicity from soy-calcitriol co-administration also appeared to be dose-dependent. The 10%-soy diet clearly caused hypercalcemic toxicity, with one animal dying in this group. The higher 20%-soy concentration probably provided a greater degree of CYP24A1 inhibition and higher 1,25(OH)2D levels, leading not only to more anticancer potency but also more severe hypercalcemia that proved fatal to several mice in this group. Thus, these results highlight the possible risk of increased hypercalcemia while using the calcitriol-soy combination in PCa therapy.
CONCLUSIONS
In conclusion, our study demonstrates the anticancer activities of calcitriol and soy in vivo in a mouse xenograft model of PCa. When combined, they exhibit enhanced anticancer activity but also increase the risk of toxicity from hypercalcemia. These findings, while further supporting the role of calcitriol and other dietary components in the treatment and prevention of PCa, also suggest the need for vigilance about the potential for toxicity if a combination of soy supplements and calcitriol administration or dietary vitamin D supplements are ingested. Dietary supplements of vitamin D were adequate to cause severe infantile hypercalcemia in children with CYP24A1 loss of function mutations because of their inability to inactivate 25(OH)D or 1,25(OH)2D [51]. In our study, the modest levels of vitamin D in the basal diet also led to elevated serum calcium and 1,25(OH)2D levels in the presence of soy supplements, likely due to soy inhibition of CYP24A1 activity. Since dietary vitamin D and soy are easily available agents, the potential risk of hypercalcemia becomes an important factor when considering the combined use of vitamin D and soy as a therapeutic approach in PCa
ACKNOWLEDGEMENTS
We thank Milan Uskokovic (BioXell, Nutley, NJ) for the generous gift of calcitriol and Ron Horst (Heartland Assays Inc., Ames, IA) for assay of serum 1,25(OH)2D levels.
Grant Support: The research was supported by NCI Grant CA130991 to DF and NCI Grant CA130991 American Recovery and Reinvestment Act administrative supplement to JYW.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Beer TM, Myrthue A. Calcitriol in the treatment of prostate cancer. Anticancer Res. 2006;26(4A):2647–2651. [PubMed] [Google Scholar]
- 3.Chen TC, Holick MF. Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol Metab. 2003;14(9):423–430. doi: 10.1016/j.tem.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Gross C, Stamey T, Hancock S, Feldman D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol) J Urol. 1998;159(6):2035–2039. doi: 10.1016/S0022-5347(01)63236-1. discussion 2039–2040. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan AV, Feldman D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer. 2010;17(1):R19–R38. doi: 10.1677/ERC-09-0139. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG. Vitamin D and intervention trials in prostate cancer: from theory to therapy. Ann Epidemiol. 2009;19(2):96–102. doi: 10.1016/j.annepidem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Stewart LV, Weigel NL. Role of insulin-like growth factor binding proteins in 1alpha,25-dihydroxyvitamin D(3)-induced growth inhibition of human prostate cancer cells. Prostate. 2005;64(1):9–19. doi: 10.1002/pros.20212. [DOI] [PubMed] [Google Scholar]
- 8.Swami S, Krishnan AV, Feldman D. Vitamin D metabolism and action in the prostate: Implications for health and disease. Mol Cell Endocrinol. 2011;347(1–):61–69. doi: 10.1016/j.mce.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 10.Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Mol Aspects Med. 2008;29(6):388–396. doi: 10.1016/j.mam.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan AV, Moreno J, Nonn L, Malloy P, Swami S, Peng L, Peehl DM, Feldman D. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol. 2007;103(3–5):694–702. doi: 10.1016/j.jsbmb.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan AV, Peehl DM, Feldman D. Inhibition of prostate cancer growth by vitamin D: Regulation of target gene expression. J Cell Biochem. 2003;88(2):363–371. doi: 10.1002/jcb.10334. [DOI] [PubMed] [Google Scholar]
- 13.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42(1):73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan AV, Moreno J, Nonn L, Swami S, Peehl DM, Feldman D. Calcitriol as a chemopreventive and therapeutic agent in prostate cancer: role of anti-inflammatory activity. J Bone Miner Res. 2007;22(Suppl 2):V74–V80. doi: 10.1359/jbmr.07s213. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan AV, Srinivas S, Feldman D. Inhibition of prostaglandin synthesis and actions contributes to the beneficial effects of calcitriol in prostate cancer. Dermatoendocrinol. 2009;1(1):7–11. doi: 10.4161/derm.1.1.7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65(17):7917–7925. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 18.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125(3 Suppl):777S–783S. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 19.Constantinou A, Huberman E. Genistein as an inducer of tumor cell differentiation: possible mechanisms of action. Proc Soc Exp Biol Med. 1995;208(1):109–115. doi: 10.3181/00379727-208-43841. [DOI] [PubMed] [Google Scholar]
- 20.Perabo FG, Von Low EC, Ellinger J, von Rucker A, Muller SC, Bastian PJ. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2008;11(1):6–12. doi: 10.1038/sj.pcan.4501000. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Sarkar FH. Gene expression profiles of genistein-treated PC3 prostate cancer cells. J Nutr. 2002;132(12):3623–3631. doi: 10.1093/jn/132.12.3623. [DOI] [PubMed] [Google Scholar]
- 22.Swami S, Krishnan AV, Moreno J, Bhattacharyya RS, Gardner C, Brooks JD, Peehl DM, Feldman D. Inhibition of prostaglandin synthesis and actions by genistein in human prostate cancer cells and by soy isoflavones in prostate cancer patients. Int J Cancer. 2009;124(9):2050–2059. doi: 10.1002/ijc.24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao A, Coan A, Welsh JE, Barclay WW, Koumenis C, Cramer SD. Vitamin D receptor and p21/WAF1 are targets of genistein and 1,25-dihydroxyvitamin D3 in human prostate cancer cells. Cancer Res. 2004;64(6):2143–2147. doi: 10.1158/0008-5472.can-03-3480. [DOI] [PubMed] [Google Scholar]
- 24.Rao A, Woodruff RD, Wade WN, Kute TE, Cramer SD. Genistein and vitamin D synergistically inhibit human prostatic epithelial cell growth. J Nutr. 2002;132(10):3191–3194. doi: 10.1093/jn/131.10.3191. [DOI] [PubMed] [Google Scholar]
- 25.Wietzke JA, Welsh J. Phytoestrogen regulation of a Vitamin D3 receptor promoter and 1,25-dihydroxyvitamin D3 actions in human breast cancer cells. J Steroid Biochem Mol Biol. 2003;84(2–3):149–157. doi: 10.1016/s0960-0760(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 26.Swami S, Krishnan AV, Peehl DM, Feldman D. Genistein potentiates the growth inhibitory effects of 1,25-dihydroxyvitamin D3 in DU145 human prostate cancer cells: role of the direct inhibition of CYP24 enzyme activity. Mol Cell Endocrinol. 2005;241(1–2):49–61. doi: 10.1016/j.mce.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Swami S, Krishnan AV, Moreno J, Bhattacharyya RB, Peehl DM, Feldman D. Calcitriol and genistein actions to inhibit the prostaglandin pathway: potential combination therapy to treat prostate cancer. J Nutr. 2007;137(1 Suppl):205S–210S. doi: 10.1093/jn/137.1.205S. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan AV, Trump DL, Johnson CS, Feldman D. The Role of Vitamin D in Cancer Prevention and Treatment. Endocrinology and metabolism clinics of North America. 2010;39(2):401–418. doi: 10.1016/j.ecl.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleet JC, Gliniak C, Zhang Z, Xue Y, Smith KB, McCreedy R, Adedokun SA. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J Nutr. 2008;138(6):1114–1120. doi: 10.1093/jn/138.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, Lamb DJ, Marcelli M, Belldegrun A, Witte ON, Sawyers CL. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3(4):402–408. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 31.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42(4):586–592. [PubMed] [Google Scholar]
- 32.Muindi JR, Modzelewski RA, Peng Y, Trump DL, Johnson CS. Pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 in normal mice after systemic exposure to effective and safe antitumor doses. Oncology. 2004;66(1):62–66. doi: 10.1159/000076336. [DOI] [PubMed] [Google Scholar]
- 33.Peng L, Malloy PJ, Feldman D. Identification of a functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol Endocrinol. 2004;18(5):1109–1119. doi: 10.1210/me.2003-0344. [DOI] [PubMed] [Google Scholar]
- 34.Beer TM, Javle MM, Ryan CW, Garzotto M, Lam GN, Wong A, Henner WD, Johnson CS, Trump DL. Phase I study of weekly DN-101, a new formulation of calcitriol, in patients with cancer. Cancer Chemother Pharmacol. 2007;59(5):581–587. doi: 10.1007/s00280-006-0299-1. [DOI] [PubMed] [Google Scholar]
- 35.Scher HI, Jia X, Chi K, de Wit R, Berry WR, Albers P, Henick B, Waterhouse D, Ruether DJ, Rosen PJ, Meluch AA, Nordquist LT, Venner PM, Heidenreich A, Chu L, Heller G. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol. 2011;29(16):2191–2198. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- 36.Yang ES, Burnstein KL. Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem. 2003;278(47):46862–46868. doi: 10.1074/jbc.M306340200. [DOI] [PubMed] [Google Scholar]
- 37.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 38.Bektic J, Berger AP, Pfeil K, Dobler G, Bartsch G, Klocker H. Androgen receptor regulation by physiological concentrations of the isoflavonoid genistein in androgen-dependent LNCaP cells is mediated by estrogen receptor beta. Eur Urol. 2004;45(2):245–251. doi: 10.1016/j.eururo.2003.09.001. discussion 251. [DOI] [PubMed] [Google Scholar]
- 39.Lund TD, Munson DJ, Adlercreutz H, Handa RJ, Lephart ED. Androgen receptor expression in the rat prostate is down-regulated by dietary phytoestrogens. Reprod Biol Endocrinol. 2004;2:5. doi: 10.1186/1477-7827-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallay E, Adlercreutz H, Farhan H, Lechner D, Bajna E, Gerdenitsch W, Campbell M, Cross HS. Phytoestrogens regulate vitamin D metabolism in the mouse colon: relevance for colon tumor prevention and therapy. J Nutr. 2002;132(11 Suppl):3490S–3493S. doi: 10.1093/jn/132.11.3490S. [DOI] [PubMed] [Google Scholar]
- 41.Farhan H, Wahala K, Cross HS. Genistein inhibits vitamin D hydroxylases CYP24 and CYP27B1 expression in prostate cells. J Steroid Biochem Mol Biol. 2003;84(4):423–429. doi: 10.1016/s0960-0760(03)00063-3. [DOI] [PubMed] [Google Scholar]
- 42.Farhan H, Cross HS. Transcriptional inhibition of CYP24 by genistein. Ann N Y Acad Sci. 2002;973:459–462. doi: 10.1111/j.1749-6632.2002.tb04683.x. [DOI] [PubMed] [Google Scholar]
- 43.Ly LH, Zhao XY, Holloway L, Feldman D. Liarozole acts synergistically with 1alpha,25-dihydroxyvitamin D3 to inhibit growth of DU 145 human prostate cancer cells by blocking 24-hydroxylase activity. Endocrinology. 1999;140(5):2071–2076. doi: 10.1210/endo.140.5.6698. [DOI] [PubMed] [Google Scholar]
- 44.Peehl DM, Seto E, Hsu JY, Feldman D. Preclinical activity of ketoconazole in combination with calcitriol or the vitamin D analogue EB 1089 in prostate cancer cells. J Urol. 2002;168(4 Pt 1):1583–1588. doi: 10.1097/01.ju.0000030158.18335.84. [DOI] [PubMed] [Google Scholar]
- 45.Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW, Pinkel D. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat Genet. 2000;25(2):144–146. doi: 10.1038/75985. [DOI] [PubMed] [Google Scholar]
- 46.King AN, Beer DG, Christensen PJ, Simpson RU, Ramnath N. The vitamin D/CYP24A1 story in cancer. Anticancer Agents Med Chem. 2010;10(3):213–224. doi: 10.2174/1871520611009030213. [DOI] [PubMed] [Google Scholar]
- 47.Brown AJ. Mechanisms for the selective actions of vitamin D analogues. Curr Pharm Des. 2000;6(7):701–716. doi: 10.2174/1381612003400416. [DOI] [PubMed] [Google Scholar]
- 48.Posner GH, Helvig C, Cuerrier D, Collop D, Kharebov A, Ryder K, Epps T, Petkovich M. Vitamin D analogues targeting CYP24 in chronic kidney disease. J Steroid Biochem Mol Biol. 2010;121(1–l2):13–19. doi: 10.1016/j.jsbmb.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 49.Yee SW, Simons C. Synthesis and CYP24 inhibitory activity of 2-substituted-3,4-dihydro-2H-naphthalen-1-one (tetralone) derivatives. Bioorg Med Chem Lett. 2004;14(22):5651–5654. doi: 10.1016/j.bmcl.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 50.St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141(7):2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 51.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Broking E, Fehrenbach H, Wingen AM, Guran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M. Mutations in CYP24A1 and Idiopathic Infantile Hypercalcemia. N Engl J Med. 2011;365(5):410–421. doi: 10.1056/NEJMoa1103864. [DOI] [PubMed] [Google Scholar]
- 52.Fleet JC, Schoch RD. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci. 2010;47(4):181–195. doi: 10.3109/10408363.2010.536429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoenderop JG, Nilius B, Bindels RJ. Epithelial calcium channels: from identification to function and regulation. Pflugers Arch. 2003;446(3):304–308. doi: 10.1007/s00424-003-1045-8. [DOI] [PubMed] [Google Scholar]
- 54.van de Graaf SF, Boullart I, Hoenderop JG, Bindels RJ. Regulation of the epithelial Ca2+ channels TRPV5 and TRPV6 by 1alpha,25-dihydroxy Vitamin D3 and dietary Ca2+ J Steroid Biochem Mol Biol. 2004;89–90(1–5):303–308. doi: 10.1016/j.jsbmb.2004.03.029. [DOI] [PubMed] [Google Scholar]