Abstract
With a current prevalence of approximately 20%, smoking continues to impact negatively upon health. Tobacco or nicotine use influences the endocrine system, with important clinical implications. In this review we critically evaluate the literature concerning the impact of nicotine as well as tobacco use on several parameters of the endocrine system and on glucose and lipid homeostasis. Emphasis is on the effect of smoking on diabetes mellitus and obesity and the consequences of smoking cessation on these disorders. Understanding the effects of nicotine and cigarettes on the endocrine system and how these changes contribute to the pathogenesis of various endocrine diseases will allow for targeted therapies and more effective approaches for smoking cessation.
Keywords: nicotine, cigarettes, pituitary, hormones, hypogonadism, addiction
Epidemiology of nicotine use
Cigarette smoking is a major public health issue in both the US and worldwide placing an enormous burden on the US economy. Approximately 20% (~60 million) of Americans smoke [1]. In addition, in 2008 in the US, approximately 88 million nonsmokers aged ≥3 years were exposed to second-hand smoke [2]. Previous declines in rates of tobacco use have stalled over the past 5 years [3]. Cigarette smoking (first- and second-hand) and exposure to nicotine are associated with premature death from chronic diseases, economic losses to society, and a substantial public health burden [4]. The Centers for Disease Control (CDC) estimate that, between the years 2000 and 2004, the average annual productivity losses attributable to smoking were approximately $96.8 billion [4]. Tobacco use has remained a particular burden for those below the poverty line [5], thus contributing to some of the health disparities in the US.
Even though notable progress has been made in raising awareness of cigarettes in relation to cardiovascular and lung diseases, much less is known about the endocrine effects of nicotine and smoking. The goal of this article is therefore to review the effects of nicotine (Box 1) and cigarette smoking on the endocrine system, by critically evaluating studies in both humans and animal models, and to address areas in need of further research. Gaining a better understanding of the effects of nicotine on the endocrine system and its subsequent impact upon the pathogenesis of various endocrine diseases will allow targeted therapies and provide useful information for the development of more effective approaches for smoking cessation.
Box 1. Pharmacology and Physiology of Nicotine.
Nicotine sources and pharmacokinetics
Nicotine is a naturally occurring alkaloid found in the tobacco plant, Nicotiana tabacum [100]. In humans, when nicotine is inhaled, it rapidly enters the blood stream, crosses the blood–brain barrier and reaches the central nervous system (CNS) where it acts as a stimulant [100,101]. Nicotine is metabolized in the liver by the cytochrome P450 enzymes CYP2A6 and CYP2B6 to form a variety of metabolites, 70 to 80% of which are converted to cotinine that is then excreted in the urine [102].
Tobacco and cigarette smoke also contain other compounds such as tar, arsenic, 1,3-butadiene and carbon monoxide [103]. In addition, several nitrosamines, aldehydes, and small organics are found in cigarette smoke which may contribute to the cancer risk associated with smoking [103]. The effect of these components on the endocrine system is not known.
Pharmacodynamics
In the brain, nicotine acts by binding to and activating the nicotinic acetylcholine receptors (nAChRs), members of a superfamily of transmembrane ligand-gated ion-channel proteins [104], found in both the CNS, peripheral nervous system (PNS) as well as in some peripheral tissues [105]. Some of the addictive properties of nicotine are attributable to its ability to increase synaptic neurotransmission in the CNS, particularly of dopamine, from the mesolimbic dopaminergic neurons; the neurotransmitter dopamine is involved in the rewarding and reinforcing effects of nicotine and plays a key role in the addictive properties of tobacco [106]. The effects of nicotine on the PNS include skeletal muscle contraction due to activation of nAChRs at the neuromuscular junction and neurotransmission along the autonomic ganglia, which leads to activation of postganglionic adrenergic and cholinergic fibers. Activation of nAChRs in the adrenal medulla leads to increased catecholamine levels with corresponding cardiovascular and metabolic responses.
The predominant effects of nicotine in humans include increased release of catecholamines into the bloodstream that increase pulse rate and blood pressure, the release of plasma free fatty acids, and the mobilization of blood glucose [107]. Decreases in skin temperatures, arousal and relaxation are also noted following nicotine administration [107]. At the cellular level, the effects of nicotine include increased synthesis and release of neurotransmitters and hormones, induction of oxidative stress, activation of transcription factors and the catecholamine-synthesizing enzyme tyrosine hydroxylase, as well as prevention of apoptosis [107]. Serving as the fundamental mediator of neurotransmission in the CNS and PNS, the activation of nAChRs has important physiological consequences for multiple organs, including the endocrine system.
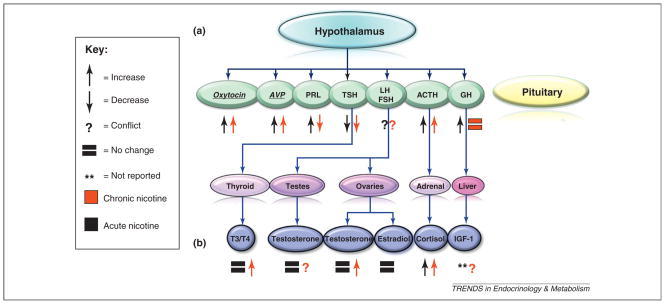
The effects of smoking and nicotine on pituitary/ end-organ endocrine systems (Figure 1 and Table 1)
Figure 1.
Effects of nicotine on hypothalamic–pituitary–end organ axes. Schematic demonstrating the effects of acute and chronic nicotine /cigarette use on hypothalamic–pituitary–end organ axes. (a) Nicotine mediates its effects on the hormonal levels of cortisol and AVP at the level of the hypothalamus or in other brain regions. (b) Nicotine mediates its effects on hormonal levels T3/T4 via direct activation of its end-organ target, the thyroid. Most acute information is from nicotine administration studies whereas chronic information is predominantly from studies on cigarette smokers. The level of regulation of other hormones is either at the pituitary or unknown. It is important to note that many of the effects of nicotine on hormones are not well-understood, and often we can only assess the end result of observed changes in circulating hormone levels. Underlined text in italics indicates hormones originating from the posterior pituitary.
Table 1.
Summary of the effects of acute and chronic nicotine use on the endocrine systems of rodents and humans
| Hormone | Acute | Chronic | Potential physiological outcome | Refs |
|---|---|---|---|---|
| Prolactin (PRL) | ↑ | ↓ | Fertility, sexual behavior, lactation | [6,7] |
| Thyroid hormones (T4/T3) | = | ↑ | Increased metabolism | [9–11] |
| ACTH/cortisol | ↑ | ↑ | Increased catabolism (breakdown of protein), increased ability to handle stress, altered immune response | [17,19] |
| Estradiol | = | ↑ | Endometrial build-up | [35] |
| Testosterone | = | ↑ or ↑↓ | In women: irregular periods and problems with ovulation. In men: increased aggression | [32,33,35] |
| Growth hormone (GH)/IGF-1 | ↑ | ↓ | Changes in anabolism (build-up of protein) and cell proliferation, low birth-weight neonates | [8,39] |
| Vasopressin (AVP) | ↑ | = | Changes in blood pressure, serum sodium levels and cognition | [8,42] |
| β-Endorphin | ↑ | ↑↓ | Pain threshold, immune responses | [97–99] |
| POMC | ↑ | Suppressed appetite | [55,56] | |
| NPY | ↑ | ↑↓ | Increased appetite | [56,57] |
| Orexins | ↑ | Increased appetite | [56] | |
| Ghrelin | ↑↓ | Suppressed appetite | [59,60] | |
| Leptin | ↓ | Weight gain | [62] | |
| Insulin | ↓ | Increased energy expenditure, weight loss | [62] |
Most acute data are from nicotine administration whereas chronic data are predominantly from studies on cigarette smokers. Note: the effects of nicotine on the endocrine system are complex and the expected physiological outcomes may or may not be manifested depending on the dose and duration of exposure, as well on changes in other known and unknown hormone systems. Symbols: ↑, stimulation; ↓, inhibition; ↑↓, conflicting; =, no change; no symbol, not reported.
Brief overview of the endocrine system
The endocrine system is a group of glands that maintain body homeostasis via the secretion of different hormones. Many of these hormones are regulated via various regulatory axes including the hypothalamic–pituitary–adrenal axis (HPA), the hypothalamic–pituitary–gonadal axis (HPG), and the hypothalamic–pituitary–thyroid axis (HPT).
The HPA axis is activated with the release of corticotropin-releasing hormone (CRH) from the hypothalamus, typically in response to psychological or physical stress. CRH then stimulates the anterior pituitary to produce adrenocorticotropic hormone (ACTH), which activates the production of cortisol by the adrenal gland. Cortisol mediates the physiological effects of this axis which include effects on the cardiovascular system, control of metabolic homeostasis, effect on connective tissue, modulation of the immune system, and effects on behavior and cognition. The HPG axis is activated by gonadotropin-releasing hormone (GnRH), which is released from the hypothalamus and then stimulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. FSH and LH stimulate the testes and ovaries to release sex hormones, estradiol in females and testosterone in males. The HPT axis is activated by thyrotopin-releasing hormone (TRH) that is secreted from the hypothalamus which then stimulates the release of thyroid-stimulating hormone (TSH) from the anterior pituitary. TSH induces the production and release of triiodothyronine (T3) and thyroxine (T4) from the thyroid gland.
Two additional anterior pituitary hormones are prolactin (PRL), which is regulated by dopamine, and growth hormone (GH), which is in part is regulated by growth hormone releasing hormone (GHRH) secreted from the hypothalamus. PRL regulates lactation. GH has many physiological functions including stimulating growth, cell regeneration and promoting gluconeogenesis. Vasopressin and oxytocin (OT) are hormones released from the posterior pituitary gland.
Cigarettes and prolactin
PRL secretion from the anterior pituitary is primarily inhibited by dopamine. Acute cigarette smoke significantly increased PRL secretion and the increase in PRL levels correlated with increased plasma nicotine levels [6]. When subjects smoked nicotine-free cigarettes, PRL levels were unchanged [6]. Significant increases in PRL levels in response to opioid blockade have also been observed [7]. However, the response is significantly diminished in smokers, relative to nonsmokers [7]. Because dopamine inhibits PRL secretion, opioids increase dopamine secretion that results in an inhibition of PRL secretion. Therefore, these data suggest that smokers may have blunted opioid-mediated dopamine release or dysregulated interactions between dopamine and PRL [7]. Considering the importance of dopamine levels in nicotine addiction, dysregulation of dopamine-release may play a role in the mechanism of addiction associated with nicotine. In light of the stimulatory effects of nicotine on dopamine levels one would expect a decrease in PRL secretion, given the inverse relation between dopamine and PRL. Decreased PRL levels are observed in long-term, but not acute smoking, possibly due to desensitization of the nAChRs [8].
Cigarettes and the HPT axis
The HPT axis maintains thyroid hormone production and disruption of this axis can result in either hypothyroidism or hyperthyroidism. Serum TSH levels are lower, whereas T3 and T4 levels are higher, in smokers versus non-smokers [9] and in both active smokers and passive smokers [10,11]. This suggests that there is a stimulatory effect of cigarette smoke exposure on thyroid hormone release, with resultant suppression of TSH. This may have important clinical relevance when making the already difficult evaluation and management of smokers with subclinical hyperthyroidism. Multiple studies have shown that there is a lower prevalence of thyroid auto-antibodies in smokers compared with nonsmokers [11,12] and smokers have lower rates of hypothyroidism [13]. However, these studies only offer evidence of an association between smoking and lower rates of hypothyroidism, and do not provide confirmation on causality or potential mechanisms behind the association between smoking and decreased rates of hypothyroidism. One recent study showed that discontinuation of smoking might increase the risk of developing thyroid peroxidase (TPO) antibodies [14]. In patients with Graves’ disease, smoking clearly worsens thyroid-associated ophthalmopathy (TAO) [15]. The pathogenesis of TAO involves inflammation, excess production of glycosaminoglycans, and adipogenesis resulting in an increase in the volume of both the orbital fatty connective tissues and the extraocular muscle bodies [16]. Interestingly, in an in vitro model, cigarette smoke extract increased both glycosaminoglycan production and adipogenesis, presenting a possible mechanism by which cigarette smoke worsens TAO [16].
Nicotine and cigarettes and the HPA axis
Studies conducted in rats illustrate that nicotine and smoking stimulate the HPA axis and proceed via a central mechanism to stimulate CRH and/or arginine-vasopressin (AVP) which then leads to the release of ACTH from the anterior pituitary [17]. Smoking acutely activates the HPA axis resulting in increased cortisol levels [6,18], and nicotine is the principal component of tobacco responsible for the stimulation of the HPA axis [19]. Some of the effects of smoking on the HPA axis are given in Box 2.
Box 2. Smoking and the HPA axis.
In nicotine-dependent male smokers, smoking cigarettes containing higher but not lower nicotine content leads to increased peak nicotine levels followed by an increase of HPA axis hormones (ACTH and cortisol), suggesting a dose-dependent effect of nicotine or a threshold response to nicotine in activating the HPA axis [108].
Nicotine levels increase significantly after smoking each of three high-nicotine cigarettes at 1 h intervals, attributable to the cumulative effect of the three consecutive cigarettes smoked [108]. The cumulative increase in nicotine levels seen after second and third smoked cigarettes is not accompanied by progressive increases in peak positive subjective effects ratings, or peak heart rate, ACTH, cortisol, or DHEA levels, suggesting tolerance to the effects of nicotine despite increased nicotine levels [108].
In over 150 adolescents followed from 6 months to 5 years, higher basal cortisol levels (measured using nocturnal free urinary cortisol and salivary cortisol levels) correlated with an increased risk of smoking initiation and smoking persistence [109]. In addition, exposure to stressful life-events had an additive effect on smoking [109].
In a recent study of 106 healthy smokers, a significant increase in cortisol concentrations was found following exposure to the stressor in daily smokers, occasional smokers, and nonsmokers, and the magnitude of the cortisol increase in the daily smoker group was lower than in the occasional smokers and nonsmoker groups [110].
A positive correlation has been identified between stress-induced changes in salivary cortisol concentrations and increased cigarette-craving, but not with the quantity of cigarettes smoked [111].
The role of stress in affecting the HPA axis and how this pertains to smoking habits is important. Smokers cite stress-relief as one of the major reasons for smoking [20]. Grunberg and Shafer postulated the mechanisms on why stress is associated with increased smoking as follows: (a) smoking decreases stress levels because nicotine or some other chemical in tobacco relieves some aspects of stress; (b) stress decreases the action of nicotine, thereby resulting in increased nicotine self–administration to reach the levels of activity that occur in non–stressful circumstances; (c) stress decreases the availability of nicotine, precipitating withdrawal and resulting in subsequent increased tobacco use; (d) tobacco smoking (and nicotine) enhance cognitive functions which overcome the deleterious effects of stress on cognition [21]. Stress is one of the leading causes of smoking relapse [22,23]. Stress also potentiates the rewarding and reinforcing actions of smoking [22,24]. Alternatively, nicotine deprivation between cigarettes involves withdrawal symptoms including irritability, frustration, poor concentration, insomnia, and restlessness, all of which may exacerbate stress [25] and lead to craving for continued nicotine use. Use of nicotine lozenges reduces affective withdrawal symptoms in dependent smokers who recently quit [26]. Interestingly, in dependent smokers who report that smoking helps cope with stress, smoking cessation is associated with lowering of stress [27]. Despite the acute effects smoking may have on perceived stress, overall it appears that smoking may worsen negative emotional states [27]. It is likely that this overall state of elevated stress in smokers, as measured by elevated resting cortisol, is a reflection of a dysregulated HPA axis.
Cigarette use and the reproductive axis
Cigarette smoking is associated with decreased fertility in both males and females [28,29]. Cigarette smoking also has a significant negative effect on the clinical outcome of assisted reproduction treatment (ART) [30].
The data on the effects of cigarette smoking on testosterone levels in men are conflicting. A cross-sectional study including a total of 255 men failed to show any significant association between cigarette smoking and several markers of male reproductive hormones, including total, free and bioavailable testosterone, sex-hormone-binding globulin (SHBG), LH and FSH [31]. By contrast, in a cross-sectional population-based study of 3427 men, Svartberg and Jorde found that men who smoked had significantly higher levels of total and free testosterone, compared with men who never smoked, and that testosterone levels were correlated with the number of cigarettes smoked daily [32]. Furthermore, Wu and colleagues found that the total but not free testosterone, SHBG, and LH were also significantly higher in current smokers compared with nonsmokers [33]. The conflicting data on the effects of smoking on testosterone may make the evaluation of testosterone levels in smokers difficult. It may skew the clinical evaluation of hypogonadism in men, and make the diagnosis and management of hypogonadism in older men more difficult. Cigarette smoking is also associated with erectile dysfunction and it was estimated that 23% of cases of erectile dysfunction can be attributed to smoking [34].
A cross-sectional study conducted in postmenopausal women revealed that testosterone levels were higher in current smokers, compared with nonsmokers, and testosterone, estradiol and SHBG levels increased as the extent of cigarette exposure increased [35]. After women stopped smoking for one year, levels of estradiol and total and free testosterone returned to those of nonsmokers [35]. Because high testosterone is part of the polycystic ovarian syndrome (PCOS) that is associated with infertility, lack of ovulation, menstrual irregularities and insulin resistance, the resolution of hormonal changes following smoking cessation suggests that smoking cessation could help ameliorate some of the PCOS-related complications.
Cord-blood levels of LH and FSH in maternal smokers were not significantly different between smokers and non-smokers [36]. However, placental estriol, human placental lactogen (HPL) and β-human chorionic gonadotropin (β-HCG) negatively correlated with the number of cigarettes smoked per day [36]. These changes may be significant because these placental hormones may affect fetal brain development.
Cigarettes and the GH/insulin-like growth factor (IGF-1) axis
Cigarette use in pregnancy is associated with intrauterine growth restriction (IUGR) [37]. Cord plasma concentrations of IGF-I and IGF binding protein-3 were lower in the babies of mothers who had smoked, and this may contribute to fetal IUGR [38]. This suggests that smoking might have some attenuating effects on growth in babies as a result of lower IGF-1. IGF-1 was decreased in female but not male fetuses of asthmatic mothers who smoked cigarettes [39]. A lower birth-weight of female but not male neonates of mothers with asthma who smoked during pregnancy correlated with lower IGF-1 levels [39]. Unfortunately few data are available regarding the effects of smoking on IGF-1 levels in men or women, although older data show that smoking acutely raises GH levels [8]. Based on the available literature on how smoking affects IGF-1 and GH in utero we conclude that smoking cessation during pregnancy is a prudent measure and beneficial to the health and growth of the fetus.
Cigarettes, nicotine, AVP and OT
Tobacco smoke and nicotine interact with the hormones AVP and OT secreted from the posterior pituitary (neurohypophysis). Direct application of nicotine to rodent hypothalamic tissue was shown to increase AVP levels [40]. In humans, cigarette smoking increased plasma AVP [8] and OT [41] levels. Most interestingly, the responses of AVP and cortisol to smoking are highly correlated, suggesting that the cortisol response to smoking may be attributable to the AVP increase [42]. Significantly higher levels of OT receptor mRNA were found in preterm myometrial strips in human and rats receiving cigarette-smoke extract compared to control groups that did not receive the extract [43]. These results, in both rats and humans, suggest that smoking may be related to increased contractile sensitivity of preterm myometrium in response to OT, which leads to increased risk of preterm delivery in women who smoke during pregnancy [43].
Cigarette smoking and osteoporosis
The association between smoking and osteoporosis is well established [44] with increased hip fractures seen in smokers [45]. Calcium absorption and vitamin D levels are lower in smokers [46], with variable reports on PTH levels [47,48]. In addition, cigarette smoking may reduce the efficacy of estradiol therapy in increasing bone mass [49]. The literature in humans clearly shows a relation between smoking and osteoporosis, and smoking cessation efforts should help decrease the rate of osteoporosis in smokers.
Metabolic homeostasis and cigarettes/nicotine
Obesity
It has long been recognized that smokers generally have reduced body-weight compared to nonsmokers [50], that smoking cessation leads to weight gain [50], and that weight loss recurs when cigarette smoking is resumed [51]. Studies have reported a U-shaped relation between body mass index (BMI) and number of cigarettes smoked [52], with weight gain in the heavy smokers that correlates positively with number of cigarettes smoked [53], possibly influenced by poorer lifestyle habits among heavy smokers [54].
The mechanisms that underlie this weight phenomenon are complex and involve multiple neurochemical pathways that govern hunger and satiety. Recent studies in rodents have shown that nicotine suppresses appetite ultimately via the activation of melanocortin-4 receptors (MC4R) expressed on hypothalamic pro-opiomelanocortin (POMC) neurons [55,56] that signals satiety. However, animals studies found that hypothalamic neuropeptide Y (NPY), a potent orexigenic neuropeptide that increases food intake, may be enhanced (either acutely [56] or after 7 weeks of nicotine exposure [57]) or, conversely, suppressed after 12 weeks of exposure [58]. Orexins, a family of hypothalamic hormones which also enhance feeding, were found to increase following nicotine exposure, in rodents [56]. Data with regards to the gut peptide ghrelin that induces satiety are unclear, and it has been reported that ghrelin may [59] or may not [60] be affected acutely by cigarette exposure in habituated smokers. However, it may fall with smoking cessation [61], and it may also be acutely reduced by cigarette exposure of nonsmokers [60]. The levels of the fat-derived hormone, leptin, and its hypothalamic receptor OBRb [62] may be reduced with nicotine exposure [62] and increased with cessation in humans [61], and this might be related to the changes observed in weight following nicotine exposure. Nicotine exposure also increases energy expenditure [50], which is reversible with nicotine withdrawal [63].
Prenatal smoking exposure studies have found an association with subsequent childhood obesity [64,65] and have also suggested additional ‘imprinting’ mechanisms [66] that contribute to obesity in the child, occurring dose-dependently and even in association with paternal smoking (i.e. passive exposure) [67]. Animal models have shown that nicotine-exposed neonates which have experienced growth retardation in utero display ‘catch-up’ growth and an expansion of adipose stores when provided with calorie excess, and this contributes to insulin resistance and glucose intolerance later in life [68]. An observed association between maternal smoking and short stature in the child [67] might be consistent with this theory, but the role of low birth-weight in the pathogenesis of this phenomenon has been questioned [66].
In practice, fear of weight gain may attenuate the adherence of patients to smoking cessation programs, particularly among female smokers [69]. Greater abstinence may be achieved with smoking cessation programs that are combined with a variety of weight loss interventions, at least in the short-term [70,71]; long-term efficacy is less certain [70,71], and adjunctive pharmacotherapies for smoking cessation that also attenuate weight gain appear to be more effective than behavioral interventions alone [71]. Another related, troubling phenomenon in clinical practice is the use of cigarette smoking as an intentional means to lose weight [72].
Cigarette smoking/nicotine and insulin resistance and diabetes mellitus
Despite weight loss, cigarette exposure worsens insulin resistance [73], even with passive exposure, in a dose-dependent manner [74], predisposing to the metabolic syndrome as a consequence [73]; alternatively, smoking cessation may also lead to changes that favor the development of insulin resistance, depending on the resulting variations in body weight [75]. Smoking is also associated with worsening visceral adiposity independently of changes in BMI [76], which helps explain the paradox of increased metabolic risk associated with visceral adiposity, despite overall weight loss. However, the incidence of metabolic syndrome may not be increased if weight loss is associated with a net loss of central adiposity [77]. Other potential mechanisms of nicotine-induced insulin resistance, born out of cell culture, rodent and human studies, are listed in Box 3.
Box 3. Potential mechanisms of nicotine-induce insulin resistance.
Increased expression of tumor necrosis factor-α leading to increased reactive oxygen species and impairment of Akt phosphorylation and GLUT4 translocation (seen in myocytes when in the presence of palmitate [112]).
Increased saturation of intramyocellular triglyceride and diacylglycerol associated with increased serine-phosphorylation of the insulin-receptor substrate-1 (IRS-1) [113].
Adiponectin levels that fall with smoking [114] and rise with smoking cessation [115], but may also fall with smoking cessation in the presence of post-cessation weight gain [75].
Maternal cigarette exposure during pregnancy and breastfeeding, ‘imprinting’ on the child, leading to insulin resistance later in childhood [66,74].
Nicotine activates an innate, anti-inflammatory pathway via the macrophage α7-nicotinic acetylcholine receptor, and interruption of this pathway exacerbates nicotine-associated inflammation and insulin resistance in mice [116], which would be consistent with a co-existing anti-inflammatory effect of nicotine on obesity-related inflammation [117].
Studies have found that nicotine can dose-dependently reduce body-weight gain in mice that consume either a high-fat diet (HFD) or a normal chow diet, but this effect is significantly greater in mice in the HFD group. Computed tomography analysis for fat distribution demonstrated that nicotine effectively reduced abdominal fat in mice that consumed the HFD, resulting in lower visceral fat [78]. The effect of nicotine on weight loss in mice on a HFD was completely blocked by mecamylamine, a nonselective nAChR antagonist, but was only partially blocked by the α4β2 nAChR partial agonist/antagonist, varenicline, a drug known as Chantix that is currently used in the clinic for smoking cessation [78].
Consistent with the adverse effects of nicotine on insulin sensitivity, there is a clear, dose-dependent relation between diabetes or glucose intolerance and both active and passive cigarette exposure [79]. However, in keeping with the paradox of smoking-associated weight loss and greater insulin-resistance, some studies found that the amount of smoking interacts with BMI to produce either adverse or favorable effects on diabetes [80]. The incidence of diabetes may be lower with smoking if the weight loss leads to a net improvement in central adiposity [77]. With smoking cessation, the risk of diabetes decreases over time [81], but it may also paradoxically increase in association with the weight gain that occurs during the first 3–5 years following smoking cessation [82].
Development of glucose intolerance requires impairment of β cell function. Recent animal studies have shown that prenatal nicotine exposure, which is toxic to β cell mitochondria and leads to apoptosis, impairs pancreatic islet development [66,68], thus limiting β cell reserve; however, in one rat study, if nicotine exposure was not continued through both gestation and lactation, β cell recovery was still possible [83]. Maternal smoking during pregnancy has also been implicated in diabetes secondary to nonselective damage to the pancreas [84], and in association with β cell auto-antibodies, in young children [85].
In patients with established diabetes, nicotine exposure may paradoxically increase the incidence of severe hypoglycemia in insulin-treated patients [86], possibly via reduced clearance of subcutaneous insulin and enhancement of insulin action [87]. In addition, the kinetics of pulmonary absorption of inhaled insulin increase substantially in the smoking versus the nonsmoking state [88]. In diabetes care, smoking cessation is crucial to facilitating glycemic control and limiting the development of complications [23].
Smoking and fatty liver
Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steato-hepatitis (NASH) have emerged as important complications of insulin resistance. In humans, cigarette exposure is independently associated with ultrasound-defined NAFLD [89] as well as with advanced hepatic fibrosis [90], and fatty liver synergizes with smoking in its association with metabolic derangements [91]. In obese rats, the severity of NAFLD is worsened with long-term cigarette exposure, in association with increased oxidative stress and apoptosis [92]. In a mouse model of diet-induced obesity, increased abdominal lipolysis coupled with exacerbation of hepatic steatosis [93] and increased skeletal muscle [94] fat accumulation was observed following nicotine treatment, This is of potentially serious clinical relevance because the clinical consequences of further progression of NASH and NAFLD to hepatic cirrhosis can be life-threatening.
Smoking and dyslipidemia
The dyslipidemia observed with cigarette smoking mirrors the classic dyslipidemia of insulin-resistance that is characterized by elevated triglycerides (TG) and reduced high-density lipoprotein cholesterol (HDL-C) [95], with lesser effects on total and low-density lipoprotein cholesterol (LDL-C). Enzymes that modulate this dyslipidemic profile, such as hepatic lipase and cholesterol ester transfer protein, are involved in mediating these effects [95]. Favorable changes are seen with smoking cessation, particularly increased HDL-C [96]. These effects are closely associated with changes in insulin resistance, and probably contribute to the overall cardiovascular risks of smoking.
Concluding remarks
We have examined how nicotine exposure from smoking affects hormonal levels and metabolic homeostasis, using information from both humans and animal studies. The effect of smoking in thyroid disease, osteoporosis, and lipid levels has also been discussed. With the high prevalence of smoking throughout the world and the growing burden of diabetes, smoking cessation of patients should be actively pursued as part of prevention and management of diabetes regiments. However, the challenge of weight gain following smoking cessation remains, and this current review points out the impact of underlying hormonal changes, as well as the psychological factors that are associated with this weight gain. Furthermore, as smoking by pregnant women continues, hormonal changes in response to nicotine exposure might gravely impact upon fetal health, and thus smoking cessation is highly recommended.
Additional research is needed to tease out the differences between the effects of nicotine and cigarettes on the endocrine system and to decipher the physiological and clinical relevance of these hormonal changes; this information is lacking in the current literature. In addition, the effects of second-hand smoke and of other forms of nicotine (nicotine replacement therapies such as gums and patches, as well as chewless tobacco, Hookah, and electronic cigarettes) on the endocrine and metabolic systems also need to be explored. Future studies should focus on providing useful information on the mechanisms of smoking-associated endocrine and metabolic diseases and give additional credence to smoking cessation.
Acknowledgments
This work was supported by a grant from the Minority Institutions’ Drug Abuse Research Development Program (MIDARP, grant R24DA017298).
References
- 1.Garrett BE, et al. Cigarette smoking – United States, 1965–2008. MMWR Surveill Summ. 2011;60 (Suppl):109–113. [PubMed] [Google Scholar]
- 2.Vital signs: nonsmokers’ exposure to secondhand smoke –United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2010;59:1141–1146. [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration: Office of Applied Studies. NSDUH Series H-34, DHHS Publication No SMA 08-4343. 2008. Results from the 2007 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- 4.Center for Disease Control and Prevention. Cigarette smoking among adults – United States 2007. MMWR. 2008;57:1221–1226. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged > or = 18 years – United States, 2009. Morb Mortal Wkly Rep. 2010;59:1135–1140. [PubMed] [Google Scholar]
- 6.Xue Y, et al. Venous plasma nicotine correlates of hormonal effects of tobacco smoking. Pharmacol Biochem Behav. 2010;95:209–215. doi: 10.1016/j.pbb.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw D, al’Absi M. Blunted opiate modulation of prolactin response in smoking men and women. Pharmacol Biochem Behav. 2010;95:1–5. doi: 10.1016/j.pbb.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuxe K, et al. Neuroendocrine actions of nicotine and of exposure to cigarette smoke: medical implications. Psychoneuroendocrinology. 1989;14:19–41. doi: 10.1016/0306-4530(89)90054-1. [DOI] [PubMed] [Google Scholar]
- 9.Asvold BO, et al. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. 2009;94:5023–5027. doi: 10.1210/jc.2009-1180. [DOI] [PubMed] [Google Scholar]
- 10.Soldin OP, et al. Thyroid hormone levels associated with active and passive cigarette smoking. Thyroid. 2009;19:817–823. doi: 10.1089/thy.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorde R, Sundsfjord J. Serum TSH levels in smokers and non-smokers. The 5th Tromso study. Exp Clin Endocrinol Diabetes. 2006;114:343–347. doi: 10.1055/s-2006-924264. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen IB, et al. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: a population study. Eur J Endocrinol. 2008;158:367–373. doi: 10.1530/EJE-07-0595. [DOI] [PubMed] [Google Scholar]
- 13.Effraimidis G, et al. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: a prospective study. Eur J Endocrinol. 2011;164:107–113. doi: 10.1530/EJE-10-0785. [DOI] [PubMed] [Google Scholar]
- 14.Effraimidis G, et al. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: a prospective study. J Clin Endocrinol Metab. 2009;94:1324–1328. doi: 10.1210/jc.2008-1548. [DOI] [PubMed] [Google Scholar]
- 15.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cawood TJ, et al. Smoking and thyroid-associated ophthalmopathy: a novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92:59–64. doi: 10.1210/jc.2006-1824. [DOI] [PubMed] [Google Scholar]
- 17.Matta SG, et al. Nicotine elevates rat plasma ACTH by a central mechanism. J Pharmacol Exp Ther. 1987;243:217–226. [PubMed] [Google Scholar]
- 18.Seyler LE, Jr, et al. The effects of smoking on ACTH and cortisol secretion. Life Sci. 1984;34:57–65. doi: 10.1016/0024-3205(84)90330-8. [DOI] [PubMed] [Google Scholar]
- 19.Rohleder N, Kirschbaum C. The hypothalamic–pituitary–adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 20.McEwen A, et al. Motives for smoking and their correlates in clients attending Stop Smoking treatment services. Nicotine Tob Res. 2008;10:843–850. doi: 10.1080/14622200802027248. [DOI] [PubMed] [Google Scholar]
- 21.Grunberg NE, Shafer ST. Stress, smoking, and 9/11/01. Psychiatry. 2005;68:311–315. doi: 10.1521/psyc.2005.68.4.311. [DOI] [PubMed] [Google Scholar]
- 22.McKee SA, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Public Health Service. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2010. [Google Scholar]
- 24.Childs E, de Wit H. Effects of acute psychosocial stress on cigarette craving and smoking. Nicotine Tob Res. 2010;12:449–453. doi: 10.1093/ntr/ntp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paolini M, De Biasi M. Mechanistic insights into nicotine withdrawal. Biochem Pharmacol. 2011;82:996–1007. doi: 10.1016/j.bcp.2011.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiffman S. Effect of nicotine lozenges on affective smoking withdrawal symptoms: secondary analysis of a randomized, double-blind, placebo-controlled clinical trial. Clin Ther. 2008;30:1461–1475. doi: 10.1016/j.clinthera.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Hajek P, et al. The effect of stopping smoking on perceived stress levels. Addiction. 2010;105:1466–1471. doi: 10.1111/j.1360-0443.2010.02979.x. [DOI] [PubMed] [Google Scholar]
- 28.Practice Committee of American Society for Reproductive Medicine. Smoking and infertility. Fertil Steril. 2008;90:S254–S259. doi: 10.1016/j.fertnstert.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Gaur DS, et al. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010;53:35–40. doi: 10.4103/0377-4929.59180. [DOI] [PubMed] [Google Scholar]
- 30.Farhi J, Orvieto R. Influence of smoking on outcome of COH and IUI in subfertile couples. J Assist Reprod Genet. 2009;26:421–424. doi: 10.1007/s10815-009-9330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halmenschlager G, et al. Evaluation of the effects of cigarette smoking on testosterone levels in adult men. J Sex Med. 2009;6:1763–1772. doi: 10.1111/j.1743-6109.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 32.Svartberg J, Jorde R. Endogenous testosterone levels and smoking in men. The fifth Tromso study. Int J Androl. 2007;30:137–143. doi: 10.1111/j.1365-2605.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu FC, et al. Hypothalamic–pituitary–testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 34.Wu C, et al. The association of smoking and erectile dysfunction: results from the Fangchenggang Area Male Health and Examination Survey (FAMHES) J Androl. 2012;33:59–65. doi: 10.2164/jandrol.110.012542. [DOI] [PubMed] [Google Scholar]
- 35.Brand JS, et al. Cigarette smoking and endogenous sex hormones in postmenopausal women. J Clin Endocrinol Metab. 2011;96:3184–3192. doi: 10.1210/jc.2011-1165. [DOI] [PubMed] [Google Scholar]
- 36.Varvarigou AA, et al. Effect of maternal smoking on cord blood estriol, placental lactogen, chorionic gonadotropin, FSH, LH, and cortisol. J Perinat Med. 2009;37:364–369. doi: 10.1515/JPM.2009.028. [DOI] [PubMed] [Google Scholar]
- 37.Villalbí JR, et al. Maternal smoking, social class and outcomes of pregnancy. Paediatr Perinat Epidemiol. 2007;21:441–447. doi: 10.1111/j.1365-3016.2007.00845.x. [DOI] [PubMed] [Google Scholar]
- 38.Pringle PJ, et al. The influence of cigarette smoking on antenatal growth, birth size, and the insulin-like growth factor axis. J Clin Endocrinol Metab. 2005;90:2556–2562. doi: 10.1210/jc.2004-1674. [DOI] [PubMed] [Google Scholar]
- 39.Clifton VL, et al. Effect of maternal asthma, inhaled glucocorticoids and cigarette use during pregnancy on the newborn insulin-like growth factor axis. Growth Horm IGF Res. 2010;20:39–48. doi: 10.1016/j.ghir.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 40.McKlveen JM, et al. Sexually diergic, dose-dependent hypothalamic–pituitary–adrenal axis responses to nicotine in a dynamic in vitro perfusion system. J Pharmacol Toxicol Methods. 2010;61:311–318. doi: 10.1016/j.vascn.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiodera P, et al. Gamma-aminobutyric acid mediation of the inhibitory effect of endogenous opioids on the arginine vasopressin and oxytocin responses to nicotine from cigarette smoking. Metabolism. 1993;42:762–765. doi: 10.1016/0026-0495(93)90246-k. [DOI] [PubMed] [Google Scholar]
- 42.Chiodera P, et al. Abnormal effect of cigarette smoking on pituitary hormone secretions in insulin-dependent diabetes mellitus. Clin Endocrinol. 1997;46:351–357. doi: 10.1046/j.1365-2265.1997.1470945.x. [DOI] [PubMed] [Google Scholar]
- 43.Nakamoto T, et al. Cigarette smoke extract enhances oxytocin-induced rhythmic contractions of rat and human preterm myometrium. Reproduction. 2006;132:343–353. doi: 10.1530/rep.1.00908. [DOI] [PubMed] [Google Scholar]
- 44.Dimai HP, Chandran M. Official Positions for FRAX(R) clinical regarding smoking from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX(R) J Clin Densitom. 2011;14:190–193. doi: 10.1016/j.jocd.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Jenkins MR, Denison AV. Smoking status as a predictor of hip fracture risk in postmenopausal women of northwest Texas. Prev Chronic Dis. 2008;5:A09. [PMC free article] [PubMed] [Google Scholar]
- 46.Need AG, et al. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int. 2002;13:83–88. doi: 10.1007/s198-002-8342-9. [DOI] [PubMed] [Google Scholar]
- 47.Jorde R, et al. Serum parathyroid hormone (PTH) levels in smokers and non-smokers. The fifth Tromso study. Eur J Endocrinol. 2005;152:39–45. doi: 10.1530/eje.1.01816. [DOI] [PubMed] [Google Scholar]
- 48.Rapuri PB, et al. Smoking and bone metabolism in elderly women. Bone. 2000;27:429–436. doi: 10.1016/s8756-3282(00)00341-0. [DOI] [PubMed] [Google Scholar]
- 49.Bjarnason NH, et al. The influence of smoking on bone loss and response to nasal estradiol. Climacteric. 2009;12:59–65. doi: 10.1080/13697130802587689. [DOI] [PubMed] [Google Scholar]
- 50.Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mozaffarian D, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiolero A, et al. Association of cigarettes smoked daily with obesity in a general adult population. Obesity (Silver Spring) 2007;15:1311–1318. doi: 10.1038/oby.2007.153. [DOI] [PubMed] [Google Scholar]
- 53.Watari M, et al. A longitudinal study of the influence of smoking on the onset of obesity at a telecommunications company in Japan. Prev Med. 2006;43:107–112. doi: 10.1016/j.ypmed.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Chiolero A, et al. Clustering of risk behaviors with cigarette consumption: A population-based survey. Prev Med. 2006;42:348–353. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Mineur YS, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H, et al. Nicotine excites hypothalamic arcuate anorexigenic proopiomelanocortin neurons and orexigenic neuropeptide Y neurons: similarities and differences. J Neurophysiol. 2011;106:1191–1202. doi: 10.1152/jn.00740.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen H, et al. Detrimental metabolic effects of combining long-term cigarette smoke exposure and high-fat diet in mice. Am J Physiol Endocrinol Metab. 2007;293:E1564–E1571. doi: 10.1152/ajpendo.00442.2007. [DOI] [PubMed] [Google Scholar]
- 58.Chen H, et al. Regulation of hypothalamic NPY by diet and smoking. Peptides. 2007;28:384–389. doi: 10.1016/j.peptides.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 59.Bouros D, et al. Smoking acutely increases plasma ghrelin concentrations. Clin Chem. 2006;52:777–778. doi: 10.1373/clinchem.2005.065243. [DOI] [PubMed] [Google Scholar]
- 60.Kokkinos A, et al. Differentiation in the short- and long-term effects of smoking on plasma total ghrelin concentrations between male nonsmokers and habitual smokers. Metabolism. 2007;56:523–527. doi: 10.1016/j.metabol.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 61.Lee H, et al. Increased leptin and decreased ghrelin level after smoking cessation. Neurosci Lett. 2006;409:47–51. doi: 10.1016/j.neulet.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Al Mutairi SS, et al. Effect of smoking habit on circulating adipokines in diabetic and non-diabetic subjects. Ann Nutr Metab. 2008;52:329–334. doi: 10.1159/000151487. [DOI] [PubMed] [Google Scholar]
- 63.Hur YN, et al. High fat diet altered the mechanism of energy homeostasis induced by nicotine and withdrawal in C57BL/6 mice. Mol Cells. 2010;30:219–226. doi: 10.1007/s10059-010-0110-3. [DOI] [PubMed] [Google Scholar]
- 64.Ino T. Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatr Int. 2010;52:94–99. doi: 10.1111/j.1442-200X.2009.02883.x. [DOI] [PubMed] [Google Scholar]
- 65.Oken E, et al. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obesity. 2008;32:201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruin JE, et al. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116:364–374. doi: 10.1093/toxsci/kfq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koshy G, et al. Dose response association of pregnancy cigarette smoke exposure, childhood stature, overweight and obesity. Eur J Public Health. 2011;21:286–291. doi: 10.1093/eurpub/ckq173. [DOI] [PubMed] [Google Scholar]
- 68.Somm E, et al. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology. 2008;149:6289–6299. doi: 10.1210/en.2008-0361. [DOI] [PubMed] [Google Scholar]
- 69.Klesges RC, et al. Factors associated with participation, attrition, and outcome in a smoking cessation program at the workplace. Health Psychol. 1988;7:575–589. doi: 10.1037//0278-6133.7.6.575. [DOI] [PubMed] [Google Scholar]
- 70.Spring B, et al. Behavioral intervention to promote smoking cessation and prevent weight gain: a systematic review and meta-analysis. Addiction. 2009;104:1472–1486. doi: 10.1111/j.1360-0443.2009.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parsons AC, et al. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev. 2009:CD006219. doi: 10.1002/14651858.CD006219.pub2. [DOI] [PubMed] [Google Scholar]
- 72.Potter BK, et al. Does a relationship exist between body weight, concerns about weight, and smoking among adolescents?. An integration of the literature with an emphasis on gender. Nicotine Tob Res. 2004;6:397–425. doi: 10.1080/14622200410001696529. [DOI] [PubMed] [Google Scholar]
- 73.Chiolero A, et al. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 74.Thiering E, et al. Prenatal and postnatal tobacco smoke exposure and development of insulin resistance in 10 year old children. Int J Hyg Environ Health. 2011;214:361–368. doi: 10.1016/j.ijheh.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Inoue K, et al. Early effects of smoking cessation and weight gain on plasma adiponectin levels and insulin resistance. Intern Med. 2011;50:707–712. doi: 10.2169/internalmedicine.50.4600. [DOI] [PubMed] [Google Scholar]
- 76.Berlin I. Smoking-induced metabolic disorders: a review. Diabetes Metab. 2008;34:307–314. doi: 10.1016/j.diabet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Onat A, et al. Prospective epidemiologic evidence of a ‘protective’ effect of smoking on metabolic syndrome and diabetes among Turkish women – without associated overall health benefit. Atherosclerosis. 2007;193:380–388. doi: 10.1016/j.atherosclerosis.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Mangubat M, et al. Effect of nicotine on body composition in mice. J Endocrinol. 2012;212:317–326. doi: 10.1530/JOE-11-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Willi C, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 80.Nagaya T, et al. Heavy smoking raises risk for type 2 diabetes mellitus in obese men; but, light smoking reduces the risk in lean men: a follow-up study in Japan. Ann Epidemiol. 2008;18:113–118. doi: 10.1016/j.annepidem.2007.07.107. [DOI] [PubMed] [Google Scholar]
- 81.Hur NW, et al. Smoking cessation and risk of type 2 diabetes mellitus: Korea Medical Insurance Corporation Study. Eur J Cardiovasc Prev Rehabil. 2007;14:244–249. doi: 10.1097/01.hjr.0000239474.41379.79. [DOI] [PubMed] [Google Scholar]
- 82.Yeh HC, et al. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152:10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruin JE, et al. Fetal and neonatal nicotine exposure and postnatal glucose homeostasis: identifying critical windows of exposure. J Endocrinol. 2007;194:171–178. doi: 10.1677/JOE-07-0050. [DOI] [PubMed] [Google Scholar]
- 84.Maisonneuve P, et al. Impact of smoking on patients with idiopathic chronic pancreatitis. Pancreas. 2006;33:163–168. doi: 10.1097/01.mpa.0000227916.94073.fc. [DOI] [PubMed] [Google Scholar]
- 85.Wahlberg J, et al. Asthma and allergic symptoms and type 1 diabetes-related autoantibodies in 2.5-yr-old children. Pediatr Diabetes. 2011;12:604–610. doi: 10.1111/j.1399-5448.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 86.Hirai FE, et al. Severe hypoglycemia and smoking in a long-term type 1 diabetic population: Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 2007;30:1437–1441. doi: 10.2337/dc06-2264. [DOI] [PubMed] [Google Scholar]
- 87.Bott S, et al. Impact of smoking on the metabolic action of subcutaneous regular insulin in type 2 diabetic patients. Horm Metab Res. 2005;37:445–449. doi: 10.1055/s-2005-870237. [DOI] [PubMed] [Google Scholar]
- 88.Becker RH, et al. The effect of smoking cessation and subsequent resumption on absorption of inhaled insulin. Diabetes Care. 2006;29:277–282. doi: 10.2337/diacare.29.02.06.dc05-1913. [DOI] [PubMed] [Google Scholar]
- 89.Hamabe A, et al. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol. 2011;46:769–778. doi: 10.1007/s00535-011-0376-z. [DOI] [PubMed] [Google Scholar]
- 90.Zein CO, et al. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:753–759. doi: 10.1016/j.jhep.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiang PH, et al. Synergistic effect of fatty liver and smoking on metabolic syndrome. World J Gastroenterol. 2009;15:5334–5339. doi: 10.3748/wjg.15.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Azzalini L, et al. Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology. 2010;51:1567–1576. doi: 10.1002/hep.23516. [DOI] [PubMed] [Google Scholar]
- 93.Mangubat M, et al. Nicotine induces oxidative stress and exacerbates high fat diet (HFD)-induced hepatic steatosis in a mouse mode of diet-induced obesity. Endocrine Soc. 2011;93:OR04–OR01. [Google Scholar]
- 94.Shin CS, et al. Nicotine plus a high-fat diet leads to intramyocellular fat deposition in a mouse model of diet-induced obesity. Am Diabetes Assoc. 2011;71:2633–PO. [Google Scholar]
- 95.Chelland Campbell S, et al. Smoking and smoking cessation –the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis. 2008;201:225–235. doi: 10.1016/j.atherosclerosis.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 96.Gepner AD, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145–151. doi: 10.1016/j.ahj.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conte-Devolx B, et al. Effect of nicotine on in vivo secretion of melanocorticotropic hormones in the rat. Life Sci. 1981;28:1067–1073. doi: 10.1016/0024-3205(81)90755-4. [DOI] [PubMed] [Google Scholar]
- 98.Pomerleau OF, et al. Neuroendocrine reactivity to nicotine in smokers. Psychopharmacology. 1983;81:61–67. doi: 10.1007/BF00439275. [DOI] [PubMed] [Google Scholar]
- 99.Seyler LE, Jr, et al. Pituitary hormone response to cigarette smoking. Pharmacol Biochem Behav. 1986;24:159–162. doi: 10.1016/0091-3057(86)90062-6. [DOI] [PubMed] [Google Scholar]
- 100.Doolittle DJ, et al. The genotoxic potential of nicotine and its major metabolites. Mutat Res. 1995;344:95–102. doi: 10.1016/0165-1218(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 101.Hukkanen J, et al. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 102.Yildiz D. Nicotine, its metabolism and an overview of its biological effects. Toxicon. 2004;43:619–632. doi: 10.1016/j.toxicon.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 103.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tuesta LM, et al. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol. 2011;82:984–995. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu RH, et al. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther. 2004;310:52–58. doi: 10.1124/jpet.103.065037. [DOI] [PubMed] [Google Scholar]
- 106.Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- 107.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mendelson JH, et al. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic–pituitary–adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33:749–760. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- 109.Rao U, et al. Contribution of hypothalamic–pituitary–adrenal activity and environmental stress to vulnerability for smoking in adolescents. Neuropsychopharmacology. 2009;34:2721–2732. doi: 10.1038/npp.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buchmann AF, et al. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010;24:247–255. doi: 10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- 111.Nakanishi N, et al. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Ann Intern Med. 2000;133:183–191. doi: 10.7326/0003-4819-133-3-200008010-00009. [DOI] [PubMed] [Google Scholar]
- 112.Tatebe J, Morita T. Enhancement of TNF-alpha expression and inhibition of glucose uptake by nicotine in the presence of a free fatty acid in C2C12 skeletal myocytes. Horm Metab Res. 2011;43:11–16. doi: 10.1055/s-0030-1267996. [DOI] [PubMed] [Google Scholar]
- 113.Bergman BC, et al. Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes. 2009;58:2220–2227. doi: 10.2337/db09-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawamoto R, et al. Serum high molecular weight adiponectin is associated with mild renal dysfunction in Japanese adults. J Atheroscler Thromb. 2010;17:1141–1148. doi: 10.5551/jat.5124. [DOI] [PubMed] [Google Scholar]
- 115.Otsuka F, et al. Smoking cessation is associated with increased plasma adiponectin levels in men. J Cardiol. 2009;53:219–225. doi: 10.1016/j.jjcc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 116.Wang X, et al. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology. 2011;152:836–846. doi: 10.1210/en.2010-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lakhan SE, Kirchgessner A. Anti-inflammatory effects of nicotine in obesity and ulcerative colitis. J Transl Med. 2011;9:129. doi: 10.1186/1479-5876-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]