Abstract
Objectives
We have previously reported that transcranial direct current stimulation (tDCS) delivered to the occipital cortex enhances visual functional recovery when combined with 3 months of computer-based rehabilitative training in patients with hemianopia. The principal objective of this study was to evaluate the temporal sequence of effects of tDCS on visual recovery as they appear over the course of training and across different indicators of visual function.
Methods
Primary objective outcome measures were i) shifts in visual field border and ii) stimulus detection accuracy within the affected hemifield. These were compared between patients randomized to either vision restoration therapy (VRT) combined with active tDCS or VRT paired with sham tDCS. Training comprised of 2 half hour sessions, 3 times a week for 3 months. Primary outcome measures were collected at baseline (pretest), monthly interim intervals, and at posttest (3 months). As secondary outcome measures, contrast sensitivity and reading performance were collected at pretest and posttest time-points only.
Results
Active tDCS combined with VRT accelerated the recovery of stimulus detection as between-group differences appeared within the first month of training. In contrast, a shift in the visual field border was only evident at posttest (after 3 months of training). TDCS did not affect contrast sensitivity or reading performance.
Conclusions
These results suggest that tDCS may differentially affect the magnitude and sequence of visual recovery in a manner that is task- specific to the type of visual rehabilitative training strategy employed.
Keywords: transcranial direct current stimulation (tDCS), brain stimulation, hemianopia, visual field, rehabilitation, vision restoration therapy (VRT)
Introduction
Unilateral damage to post-chiasmal and occipital regions of the brain (e.g. from stroke or trauma) typically leads to contralateral visual field defects referred to as hemianopia 1. This visual deficit greatly impacts upon an individual’s sense of independence, well-being, and ability to carry out important activities of daily living 2. In a minority of cases, spontaneous recovery has been reported shortly after the insult however, recovery is considered generally to be minimal over time 3.
Numerous groups have been developing computer-based training approaches aimed at improving visual function within the impaired visual field 4–8. Using customized training algorithms and repeated visual stimulus presentation, patients learn to detect and identify the targets presented. Over time, visual performance gradually improves in the area of the impaired visual field. One approach, called vision restoration therapy (VRT; Novavision Inc. Boca Raton, FL, USA), trains patients to detect repeated flashing light stimuli presented within the area of residual vision bordering the blind and the intact visual fields (referred to as the “transition zone” 6,7). Following long-term repeated training (usually daily sessions lasting up to 6 months), a mean shift in the visual field border of approximately 5o 6,9 and an increase in stimulus detection accuracy 10,11 have been reported (see also 12 for a comprehensive review).
It has been proposed that focused, repetitive, and systematic training of these areas of residual vision promotes localized changes in visual cortical circuitry through synaptic- and network-level reactivation of surviving peri-lesional and higher-order networks 6,7,11–13. Potentiating these mechanisms by enhancing the activity of these residual cortical networks may represent a useful startegy in improving the clincal applicability and efficacy of VRT. This premise is based on previous evidence from stroke motor recovery suggesting that inherent neuroplastic mechanisms associated with rehabilitation may be enhanced through the delivery of concurrent cortical stimulation 14–19. In this direction, we have previously reported that up-regulating the excitability of surviving visual networks within the occipital cortex (specifically, using transcranial direct current stimulation or tDCS) during VRT promotes visual rehabilitative outcomes following 3 months of training. Compared to VRT delivered alone, its combination with tDCS showed better stimulus detection accuracy in the affected visual field and a greater shift in visual field border (i.e. expansion in intact visual field) 20.
Studies in stroke motor recovery have further suggested that there is a temporal separation in terms of functional recovery when assessing varying phases of training between groups receiving combined brain stimulation and rehabilitation versus rehabilitative training alone 16,21. Intriguingly, the sequence of functional recovery appears to vary between task-specific versus generalizable types of rehabilitative outcomes 16,21,22. Returning to the findings from our pilot study 20, it remains unknown whether tDCS accelerates recovery or simply increases the overall magnitude of functional recovery achieved with VRT. Further, it is uncertain whether the visual functional improvements observed generalize to other measures of visual performance including contrast sensitivity and more complex visuo-cognitive skills such as reading. In this study, we investigated these questions using interim, serial assessments along with pre- and post-training comparisons of visual performance.
Materials and Methods
1. Subjects and Study Design
Twelve patients [7 females; mean age of 59.58 ± 3.47 years] previously diagnosed with unilateral post-chiasmal visual field loss (hemianopia: 7; quadrantanopia: 5) due to stroke (n=10) or surgical trauma (n=2) participated in the study. Subjects were enrolled in the study following a comprehensive neurological and ophthalmological examination. All patients were in the chronic stage of recovery (mean time since event: 39.83 ± 16.16 months). Exclusion criteria included any ocular visual pathology or contraindication to noninvasive brain stimulation 23 and tDCS 24. Specific criteria drawn from safety guidelines pertaining to the use of non-invasive cortical stimulation include: 1) presence of any metallic, mechanical or magnetic implant in the head or implantable device (e.g. cardiac pacemaker), 2) prior history of seizure or familial history of seizure disorder in a first degree relative, and 3) chronic use of neuro-active medication (e.g. neuro-stimulants, anticonvulsants or antidepressants). For further details see20.
Following a double-blind, pilot clinical study design, participants were randomized to one of two possible study arms: 1) VRT combined with active tDCS (VRT+active tDCS) or 2) VRT combined with sham tDCS (VRT+sham tDCS). Experimental blinding to stimulation (i.e. active or sham) was maintained at the level of the patient and the investigators analyzing visual field outcomes (see below). All patients provided written informed consent prior to participation. The study was approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center and registered with www.clinicaltrials.gov (NCT00921427).
2. Visual Rehabilitative Training (VRT) and Brain Stimulation (tDCS)
We employed a contracted VRT regimen lasting 3 months (2 half-hour sessions twice a day, 3 days a week) conducted in a controlled laboratory environment. Details regarding VRT training have been described in detail elsewhere 25,26. Briefly, patients were seated in front of a computer screen at a constant viewing distance and instructed to detect and report (using a key press) the presence of a flashed light stimulus while maintaining fixation on a central target (Fig. 1A). Built-in fixation monitoring required patients to respond to a color change of the central fixation target occurring at random intervals. Target stimuli were presented primarily in the region bordering the blind and intact visual field identified by a prior visual field test using high resolution perimetry (HRP; for further details see 27). The spatial parameters of the customized therapy were determined based on progress recorded by monthly interval HRP testing and weekly improvements noted in performance during VRT.
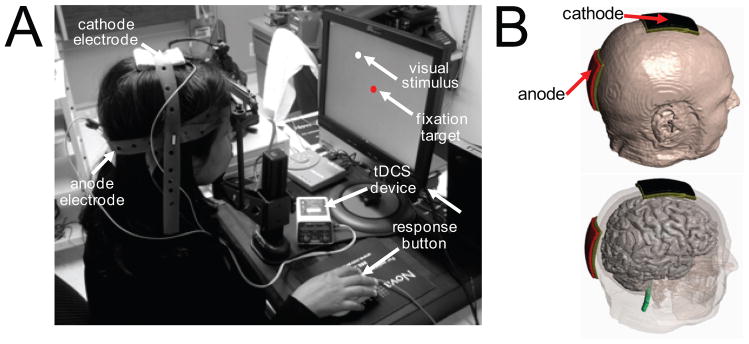
Figure 1.
Experimental set-up demonstrating combination of tDCS montage with VRT. (A) For VRT, the patient is seated in front of a computer screen and instructed to fixate upon a central fixation target and respond (using button press) to the detection of visual stimuli. For both active and sham tDCS, a montage consisting of an anodal electrode (placed over the occipital pole; Oz) and cathodal electrode (placed over the vertex; Cz) is used. (B) Three dimensional head and montage renderings illustrating the relative location of the anodal and cathodal tDCS electrodes.
TDCS was delivered using two 5x7 cm2 saline-soaked sponge electrodes connected to a 9 V battery-driven stimulator (IOMED Inc., Salt Lake City, UT), delivering a constant current of 2mA. Surface anodal polarization of the cortex is associated with an increase in spontaneous neuronal activity 28. In this experimental protocol, the anode electrode was placed along the midline (i.e. overlying the damaged and intact occipital poles). Following the international 10–20 EEG coordinate system, the anode electrode was placed overlying the Oz position and the cathode (reference) was positioned at the vertex Cz (see Fig 1B). Electrodes were then secured using non-latex rubber straps and the same montage was worn by all patients throughout training. This electrode configuration was chosen to optimally enhance bilateral occipital cortical excitability (including lesioned and non-lesioned hemispheres) 29,30. Experimental blinding with respect to active or sham tDCS was implemented according to standard protocol guidelines 31,32 and has been described in detail previously 25.
3. Outcome Measures
Primary objective outcome measures were derived from visual field assessments using high resolution perimetry (HRP) (for complete details see 6,25. Similar to VRT, patients were seated in front of a computer screen and instructed to detect (using a key press) the appearance of transient suprathreshold (95 cd/m2) visual stimuli presented throughout the visual field while maintaining fixation on a central target. Stimuli appeared at random intervals and within an area spanning 43° x 32° corresponding to an imaginary grid of 284 cells each subtending roughly 2° of visual angle. Fixation monitoring was the same as described for VRT above. To ensure optimal test validity, a priori defined criteria ensured that only tests where 95% fixation and false positive responses below 3% were included. Three consecutive HRP tests were compiled to generate a composite visual field map characterizing stimulus detection probability 27,33. HRP-based visual field maps were collected at baseline (pretest) and at regular monthly intervals (interim test 1, interim test 2) up until the completion of training at 3 months (posttest) (see Fig 2 for representative examples from the study). Recovery of visual field function was evaluated by comparing differences in 1) the position of visual field border and 2) stimulus detection accuracy. The visual field border was defined as the horizontal distance between the central vertical meridian and the medial edge of two consecutive blind cells along each row of the imaginary grid 25;Romano, 2008 #85}. Stimulus detection accuracy was expressed as a percentage of stimuli detected versus total number of targets presented in the affected field 9,25. As a secondary objective outcome measure, contrast sensitivity was tested using a Pelli-Robson letter chart at a viewing distance of 1 m under recommended luminance conditions 34. Briefly, the chart consists of letters of constant size and arranged in 16 groups of three. The contrast of the first triplet is 100% and is subsequently reduced (for each subsequent triplet) by a factor of approximately 0.7 (0–15 log unit). The contrast of the last triplet is 0.56% (2.25 log units below 100%). Each eye was tested separately at baseline (pretest). To avoid the possibility of ceiling effects on contrast sensitivity due to excitability-enhancing paradigms such as anodal tDCS 35, we chose to assess changes in contrast sensitivity using performance from the worse eye. Contrast sensitivity was quantified at baseline and then tested again after training (posttest; 3 months).
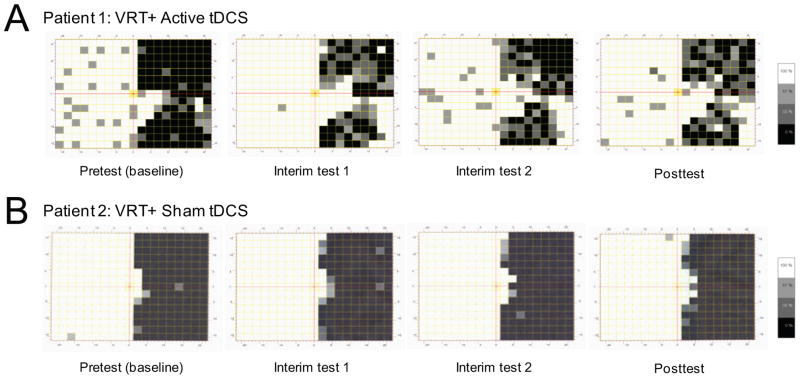
Figure 2.
Temporal Profile of Visual Field Functional Recovery in Representative Subjects. Comparing HRP assessments at pretest, interim test 1, interim test 2, and at posttest between representative patients from the VRT+active tDCS (patient 1) and VRT+sham tDCS (patient 2) groups. (A) For patient 1, the position of overall visual field border shifted from 3.37° at baseline to 7.17°, 6.76° and 6.92° at interim test 1, interim test 2, and at posttest respectively. Stimulus detection accuracy within the hemianopic field increased from 22.82% at pretest and reaching up to 48.32%, 44.52% and 50.11% at corresponding testing time points. (B) For patient 2, the position of overall visual field border shifted from 1.99° at baseline to 2.49°, 2.54° and 2.89° at interim test 1, interim test 2, and at posttest respectively. The stimulus detection accuracy within the hemianopic field increased from 13.42% at pretest and reaching up to 15.88%, 15.88% and 17.00% at the corresponding testing time points.
Finally, as another secondary outcome measure, the Minnesota Reading (MNREAD) standardized test was used to evaluate reading performance. The MNREAD acuity chart assesses reading of continuous text (60 characters per sentence) at varying print sizes 36. At a reading distance of 40 cm and under binocular and appropriate spectacle correction, the maximum reading speed (expressed in words per minute; wpm) was calculated at three tested print sizes: “large” (5M; typical size of newspaper headlines), “medium” (2M; for large-print text) and “small” (1M; for newspaper print). Similar to contrast sensitivity, MNREAD data was collected at baseline (pretest) and following the 3 month training period (posttest).
4. Statistical Analysis
Baseline differences between groups were compared using independent samples t-test and α level of significance was set at 0.05. Planned comparisons included 3, separate, two-way [group X time (interval 1: pretest vs. interim test 1, interval 2: pretest vs. interim test 2 and post-interval: pretest vs. posttest)] repeated measures analyses of variance (RMANOVA) for the primary objective outcome measures. Post-hoc analyses included within-group, pair-wise comparisons and between-group analysis of interval difference scores (interval 1: interim test 1 minus pretest, interval 2: interim test 2 minus pretest and post-interval: posttest minus pretest). Secondary objective outcome measures were analyzed using two-way [group X time (pretest vs. posttest)] RMANOVA. Statistical analyses were carried out using SPSS software (SPSS Inc. version 18, Chicago, IL).
Results
All participants were able to interact successfully with the computerized VRT system and no adverse events were associated with combining active/sham tDCS with VRT within the laboratory setting (e.g. skin burn, headaches) 20,25. Additionally, experimental blinding with respect to the active/sham status of tDCS was successful and was verified during subject exit interviews. A total of four subjects (two each from the VRT+active tDCS group and VRT+sham tDCS group) were excluded from final analysis for reasons including other unrelated medical issues, medication use that precluded further participation, and inadvertent technical variation in tDCS delivery during training. The final data analysis therefore included four patients from each group.
i) Primary Objective Outcome Measures
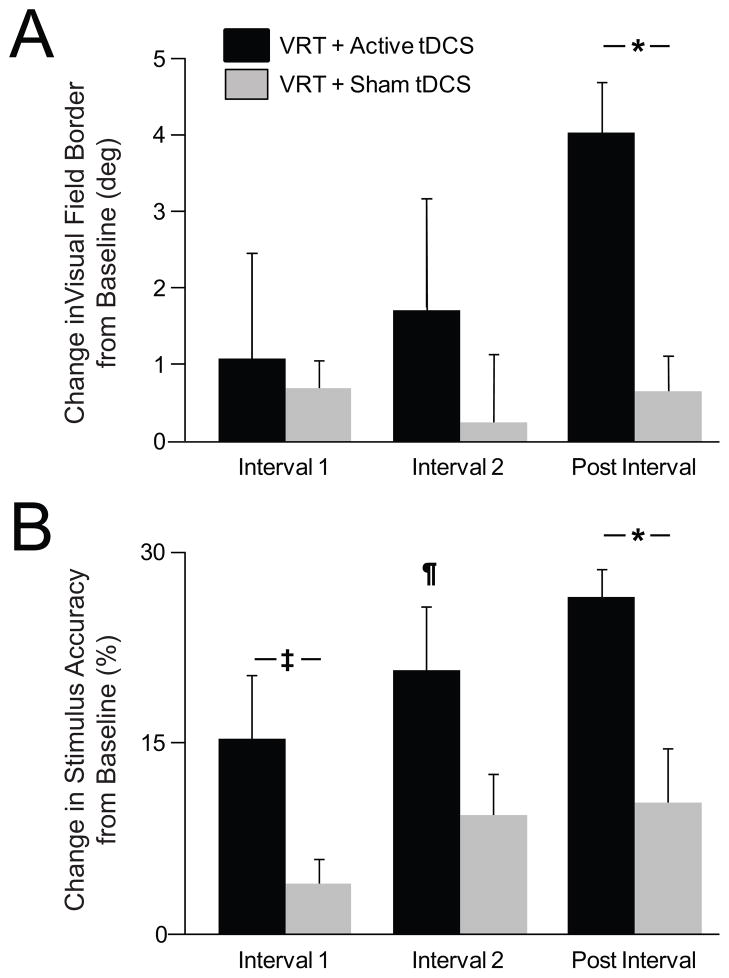
A two-way (Group X Time) RMANOVA was used to characterize the temporal profile of recovery of visual field border. Whereas a within- and between-group change for interval 1 and interval 2 was not significant [(interval 1: time- F1,6=1.49, p=0.267; group- F1,6=0.54, p=0.492; group x time- F1,6=0.07, p=0.804) and (interval 2; time- F1,6=1.3, p=0.3; group- F1,6=0.31, p=0.60; group x time- F1,6=0.7, p=0.44)], differences emerged for post-interval (time- F1,6=28.18, p=0.002; group- F1,6=0.02, p=0.89; group X time F1,6=14.51, p=0.009) (Fig. 3A). It is important to note that this effect at post-test was noted previously 20 however, differences in the magnitude of recovery between the two groups did not manifest any earlier based on the analysis carried out here. Post-hoc between-group comparisons confirmed that the shift in the visual field border demonstrated by the VRT+active tDCS group from pretest to posttest (4.11 ± 1.50° to 8.37 ± 2.29°) was significantly greater than that in the VRT+sham tDCS group (6.33 ± 2.59° to 7.03 ± 2.51°) (t6, 0.05= 3.81, p= 0.009) (Fig. 2A, B for representative examples and Fig. 3A for group effects). Differences in visual field recovery between groups were not likely attributed to differences in baseline performance (t6, 0.05= 0.74, p=0.487).
Figure 3.
Between-group differences in temporal profile of visual field functional recovery. Bar graphs representing change in (A) visual field border and (B) stimulus detection accuracy in affected field for interval 1 (pretest to interim test 1), interval 2 (pretest to interim test 2) and post interval (pretest to posttest). Findings of 2-way (group X time) RMANOVA with post-hoc 2 sample t-tests and within-group, pair-wise comparisons are shown. Significant and trend towards significance between-groups at a given interval are represented using “*” (p< 0.05) and “‡” symbols between horizontal bars respectively. Within-group differences at an interval (signifying effect of time) is denoted by the symbol “¶”(p< 0.05). Error bars represent standard error.
In comparison, the RMANOVA comparing stimulus detection accuracy within the affected hemifield suggested an earlier separation between the two groups (Fig. 3B). For interval 1, a RMANOVA confirmed a significant time (F1,6=12.73, p=0.012) and a trend towards group X time interaction effect (F1,6=4.48, p=0.079). Post-hoc 2-sample t-test revealed a mean change in stimulus detection accuracy in the VRT+active tDCS group from pretest to interim test 1 (27.96 ± 9.8 to 42.56 ± 7.17%) and showed a trend towards for a greater effect as compared to the VRT+sham tDCS group (27.00 ± 8.1 to 30.73 ± 9.03%) (t6, 0.05= 2.12, p= 0.079). Between-group differences over time were non-significant for interval 2 (group X time: F1,6=3.10, p=0.129) but an effect for time remained significant (F1,6=22.10, p=0.003). Within-group analysis showed that although the improvement in VRT+active tDCS group from pretest to interim test 2 (27.96 ± 9.8 to 47.42 ± 6.15%) was significant (t3, 0.05= 3.98, p= 0.028), the observed improvement for the VRT+sham tDCS group (27.00 ± 8.1% to 35.85 ± 11.43%) was not significant (t3, 0.05= 2.52, p= 0.087). Finally, RMANOVA comparing stimulus detection accuracy for the post-interval demonstrated a significant effect for time (F1, 6=48.71, p<0.001) and interaction (F1,6=9.06, p=0.024). Furthermore, stimulus perception showed a greater improvement in the VRT+active tDCS group from pretest to posttest (27.96 ± 9.80 to 52.98 ± 8.21%) compared to VRT+sham tDCS group (27 ± 8.06 to 36.95 ± 11.71%) (t6, 0.05=3.01, p=0.024) (Fig. 2A, B and 3B). Differences in baseline performance likely did not explain significant differences between groups upon stimulus detection (t6, 0.05= 0.08, p=0.942).
Unlike assessment of visual field function, contrast sensitivity (assessed in the poorer eye at baseline) remained unchanged from pretest to posttest between as well as within groups. The effect of time (F1, 6=1.26, p=0.31), group (F1, 6=1.54, p=0.26) and their interaction (F1, 6=0.56, p=0.48) were all non-significant. Similarly, performance on the MNREAD was not different between or within groups across time. The effect of time (5M: F1, 6=1.69, p=0.241; 2M: F1,6 =1.93, p=0.214; 1M: F1, 6=0, p=0.958), group (5M: F1, 6=0.05, p=0.837; 2M: F1, 6=0.28, p=0.614; 1M: F1, 6=0.01, p=0.915) and their interaction (5M: F1, 6=0, p=0.984; 2M: F1, 6=0.01, p=0.915; 1M: F1, 6=0.26, p=0.630) were all non-significant for all three print sizes tested.
Discussion
In this study, we explored whether adjunctive tDCS improves the efficiency of visual rehabilitative training by influencing its temporal profile of recovery. We have previously demonstrated that (analogous to findings from stroke sensorimotor recovery) visual field rehabilitative outcomes in hemianopic patients were facilitated by anodal tDCS delivered to the occipital cortex following three months of training 20. In this report, we reveal that besides enhancing the overall magnitude of visual function after 3 months of training, tDCS also appears to accelerate progress towards this overall recovery. Intriguingly, this enhancement is not manifested equally across different measures of visual field function. While an improvement in stimulus detection accuracy in affected visual field was apparent early on in training, the translation to visual field gain (i.e. shift in visual field border) was relatively delayed and did not manifest until after completion of 3 months of training. Importantly, however, these improvements in visual field outcomes did not generalize to either simple nor complex measures of visual performance (contrast sensitivity and reading performance, respectively) suggesting that the concurrent delivery of tDCS was effective in modulating outcomes that were task-specific to the rehabilitative training, but not to those that tested the generalizability of benefits of the training.
Previous work in developing computer-based visual rehabilitative training programs have also noted that the mechanisms associated with residual visual function in hemianopia have specific spatial and temporal parametric properties, and further, may also have different profiles of recovery following training 8,37. The finding of an adjunctive benefit of tDCS on stimulus detection accuracy preceding changes in the visual field border is consistent with a continuum of visual field recovery. This sequential pattern (though not explicitly stated) is also evident in previous studies of VRT alone employing contracted therapy regimens 11,38. Gains in visual detection accuracy may initially be a function of improved psychophysical performance wherein patients show improved performance as they become more familiar with the training task. This improvement may manifest within larger and larger regions of residual vision and ultimately translate into an overall gain in visual field following longer periods of training. This conjecture appears to be consistent with our preliminary findings as well as evidence presented by other groups. For example, following approximately 24 sessions of VRT (compared to a more typical regimen of 144 sessions, 6,9) , an improvement in psychophysical response time was noted by other investigators 38. However, an associated shift in visual field border was not apparent at that stage. On the other hand, improvements in stimulus detection as well as modest gains in visual field were apparent following 72 sessions of VRT 11. In our study, patients underwent on average 36 sessions of training, but more importantly, serial testing allowed us to delineate this continuum of visual recovery that was not previously reported implicating early improvements in detection followed by long-term shifts in visual field border. Intriguing however, is the fact that we observed changes in visual performance that were comparable with other (and much longer) studies 9,27 despite only 36 sessions of training. We postulate that the combination of tDCS with VRT not only accelerated visual functional gains but also within a time frame that was substantially shorter than gains reported from VRT alone.
It is important to note however that the accelerated recovery in stimulus detection accuracy may not be solely a consequence of psychophysical performance-related gains. The group specific effect (active tDCS and VRT) of higher detection accuracy early on compared to the control group suggests that anodal tDCS delivered to the occipital cortex may modulate visual performance-related factors differentially. Indeed, previous studies using a similar electrode montage in healthy subjects have shown an increase in occipital cortical excitability within a few minutes of tDCS application 39 that is associated with transient improvements in perceptual visual function including enhanced contrast sensitivity 30,40. It is also possible that the observed differences could be explained by differences in attention performance between patients receiving active tDCS. However, this is unlikely given that we verified experimental blinding during patient exit interviewing and confirmed that they could not perceive the stimulating current. Comparing performance from the application of active tDCS targeting other cortical areas (such as frontal or parietal) may help address this possible confound in the future.
The adjunctive benefits of tDCS were highly task-specific and not all measures of visual performance were differentially affected by tDCS. The fact that we did not note a similar translation to performance-oriented outcomes (particularly with contrast sensitivity and reading) further reiterates previously reported disconnect between objective and subjective outcomes in visual recovery 12,26,41,42. Upon further analysis, this discrepancy is perhaps best explained by a combination of both conceptual and methodological factors. Conceptually, based on prior evidence in sensorimotor recovery it has been shown in translational 43 and clinical studies 16 that cortical stimulation improves those specific outcomes that are most similar to the trained rehabilitation tasks when coupled with stimulation. In contrast, transfer of performance to untrained tasks may remain limited. Methodologically, the lack of transfer on contrast sensitivity and reading performance may be related to the outcome measures used (e.g. Pelli-Robson chart compared to the assessment of a contrast sensitivity function 44) or may be simply due to the fact that training ended at 3 months thus not allowing for sufficient time for improvement. This latter notion is confirmed by evidence from previous VRT studies. Following standard VRT delivered daily for 6 months (equivalent to more than 4 times the training regimen used in this study), both low and higher-order visual functions have been shown to improve including color perception 45–47. Future studies should explore the effect of longer training regimens delivered with concurrent tDCS and the impact of different cortical targets as well as training parameters such as the visual stimuli used.
Conclusions
The results presented here suggest that tDCS may differentially affect the magnitude and sequence of visual recovery in a manner that is task-specific and related to the visual rehabilitative training strategy employed. Though preliminary, these findings help to lay the foundation for future more rigorous investigations regarding the duration of training, site of tDCS delivery, and type of rehabilitative training. Careful considerations of these variables may prove to not only disentangle the continuum of visual recovery, but also further enhance the efficacy of visual rehabilitative training strategies.
Acknowledgments
Sources of Funding: This work was supported by an investigator-initiated pilot grant from Novavision VRT Inc. and by the National Institutes of Health (K23-EY016131 to LBM).
Footnotes
Conflict of Interest statement: The authors report no conflict of interests
Authorship: All authors contributed to the preparation of this manuscript and approved the submitted version.
References
- 1.Zihl J. Rehabilitation of Visual Disorders After Brain Injury. East Sussex, UK: Psychology Press Ltd; 2000. [Google Scholar]
- 2.Kerkhoff G. Restorative and compensatory therapy approaches in cerebral blindness - a review. Restor Neurol Neurosci. 1999;15(2–3):255–271. [PubMed] [Google Scholar]
- 3.Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Natural history of homonymous hemianopia. Neurology. 2006 Mar 28;66(6):901–905. doi: 10.1212/01.wnl.0000203338.54323.22. [DOI] [PubMed] [Google Scholar]
- 4.Huxlin KR, Martin T, Kelly K, et al. Perceptual relearning of complex visual motion after V1 damage in humans. J Neurosci. 2009 Apr 1;29(13):3981–3991. doi: 10.1523/JNEUROSCI.4882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Julkunen L, Tenovuo O, Jaaskelainen S, Hamalainen H. Rehabilitation of chronic post-stroke visual field defect with computer-assisted training: a clinical and neurophysiological study. Restor Neurol Neurosci. 2003;21(1–2):19–28. [PubMed] [Google Scholar]
- 6.Kasten E, Wust S, Behrens-Baumann W, Sabel BA. Computer-based training for the treatment of partial blindness. Nat Med. 1998 Sep;4(9):1083–1087. doi: 10.1038/2079. [DOI] [PubMed] [Google Scholar]
- 7.Sabel BA, Kasten E. Restoration of vision by training of residual functions. Curr Opin Ophthalmol. 2000 Dec;11(6):430–436. doi: 10.1097/00055735-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Sahraie A, Macleod MJ, Trevethan CT, et al. Improved detection following Neuro-Eye Therapy in patients with post-geniculate brain damage. Exp Brain Res. 2010 Sep;206(1):25–34. doi: 10.1007/s00221-010-2395-z. [DOI] [PubMed] [Google Scholar]
- 9.Romano JG, Schulz P, Kenkel S, Todd DP. Visual field changes after a rehabilitation intervention: Vision restoration therapy. J Neurol Sci. 2008 Oct 15;273(1–2):70–74. doi: 10.1016/j.jns.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Jobke S, Kasten E, Sabel BA. Vision restoration through extrastriate stimulation in patients with visual field defects: a double-blind and randomized experimental study. Neurorehabil Neural Repair. 2009 Mar-Apr;23(3):246–255. doi: 10.1177/1545968308324221. [DOI] [PubMed] [Google Scholar]
- 11.Kasten E, Bunzenthal U, Sabel BA. Visual field recovery after vision restoration therapy (VRT) is independent of eye movements: an eye tracker study. Behav Brain Res. 2006 Nov 25;175(1):18–26. doi: 10.1016/j.bbr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Sabel BA, Henrich-Noack P, Fedorov A, Gall C. Vision restoration after brain and retina damage: the "residual vision activation theory". Prog Brain Res. 2011;192:199–262. doi: 10.1016/B978-0-444-53355-5.00013-0. [DOI] [PubMed] [Google Scholar]
- 13.Pleger B, Foerster AF, Widdig W, et al. Functional magnetic resonance imaging mirrors recovery of visual perception after repetitive tachistoscopic stimulation in patients with partial cortical blindness. Neurosci Lett. 2003 Jan 2;335(3):192–196. doi: 10.1016/s0304-3940(02)01153-9. [DOI] [PubMed] [Google Scholar]
- 14.Edwards DJ, Krebs HI, Rykman A, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restor Neurol Neurosci. 2009;27(3):199–207. doi: 10.3233/RNN-2009-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol. 2007 Feb;6(2):188–191. doi: 10.1016/S1474-4422(07)70032-7. [DOI] [PubMed] [Google Scholar]
- 16.Huang M, Harvey RL, Stoykov ME, et al. Cortical stimulation for upper limb recovery following ischemic stroke: a small phase II pilot study of a fully implanted stimulator. Top Stroke Rehabil. 2008 Mar-Apr;15(2):160–172. doi: 10.1310/tsr1502-160. [DOI] [PubMed] [Google Scholar]
- 17.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006 Aug;5(8):708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 18.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010 Dec 14;75(24):2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plautz EJ, Barbay S, Frost SB, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003 Dec;25(8):801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 20.Plow EB, Obretenova SN, Fregni F, Pascual-Leone A, Merabet LB. Comparison of Visual Field Training for Hemianopia With Active Versus Sham Transcranial Direct Cortical Stimulation. Neurorehabilitation and Neural Repair. 2011 doi: 10.1177/1545968311431963. In Press. [DOI] [PubMed] [Google Scholar]
- 21.Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006 Mar;58(3):464–473. doi: 10.1227/01.NEU.0000197100.63931.04. [DOI] [PubMed] [Google Scholar]
- 22.Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res. 2003 Nov;28(11):1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- 23.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998 Jan;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 24.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009 Dec;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plow EB, Obretenova SN, Halko MA, et al. Combining visual rehabilitative training and noninvasive brain stimulation to enhance visual function in patients with hemianopia: a comparative case study. Pm R. 2011 Sep;3(9):825–835. doi: 10.1016/j.pmrj.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Poggel DA, Mueller I, Kasten E, Sabel BA. Multifactorial predictors and outcome variables of vision restoration training in patients with post–geniculate visual field loss. Restor Neurol Neurosci. 2008;26(4–5):321–339. [PubMed] [Google Scholar]
- 27.Kasten E, Wuest S, Sabel BA. Residual vision in transition zones in patients with cerebral blindness. J Clin Exp Neuropsychol. 1998 Oct;20(5):581–598. doi: 10.1076/jcen.20.5.581.1129. [DOI] [PubMed] [Google Scholar]
- 28.Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962 Jun;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- 29.Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci. 2004 Feb;45(2):702–707. doi: 10.1167/iovs.03-0688. [DOI] [PubMed] [Google Scholar]
- 30.Antal A, Nitsche MA, Paulus W. External modulation of visual perception in humans. Neuroreport. 2001 Nov 16;12(16):3553–3555. doi: 10.1097/00001756-200111160-00036. [DOI] [PubMed] [Google Scholar]
- 31.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006 Apr;117(4):845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008 Jul;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Sabel BA, Kenkel S, Kasten E. Vision restoration therapy (VRT) efficacy as assessed by comparative perimetric analysis and subjective questionnaires. Restor Neurol Neurosci. 2004;22(6):399–420. [PubMed] [Google Scholar]
- 34.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988;(2):187–189. [Google Scholar]
- 35.Antal A, Paulus W. Transcranial direct current stimulation and visual perception. Perception. 2008;37(3):367–374. doi: 10.1068/p5872. [DOI] [PubMed] [Google Scholar]
- 36.Mansfield JS, Legge GE, Bane MC. Psychophysics of reading. XV: Font effects in normal and low vision. Invest Ophthalmol Vis Sci. 1996 Jul;37(8):1492–1501. [PubMed] [Google Scholar]
- 37.Sahraie A, Trevethan CT, MacLeod MJ. Temporal properties of spatial channel of processing in hemianopia. Neuropsychologia. 2008 Feb 12;46(3):879–885. doi: 10.1016/j.neuropsychologia.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Marshall RS, Ferrera JJ, Barnes A, et al. Brain activity associated with stimulation therapy of the visual borderzone in hemianopic stroke patients. Neurorehabil Neural Repair. 2008 Mar-Apr;22(2):136–144. doi: 10.1177/1545968307305522. [DOI] [PubMed] [Google Scholar]
- 39.Antal A, Kincses TZ, Nitsche MA, Paulus W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp Br Res. 2003 Jun;150(3):375–378. doi: 10.1007/s00221-003-1459-8. [DOI] [PubMed] [Google Scholar]
- 40.Kraft A, Roehmel J, Olma MC, Schmidt S, Irlbacher K, Brandt SA. Transcranial direct current stimulation affects visual perception measured by threshold perimetry. Exp Brain Res. 2010 Dec;207(3–4):283–290. doi: 10.1007/s00221-010-2453-6. [DOI] [PubMed] [Google Scholar]
- 41.Mueller I, Poggel DA, Kenkel S, Kasten E, Sabel BA. Vision restoration therapy after brain damage: Subjective improvements of activities of daily life and their relationship to visual field enlargements. Visual Impairment Research. 2003;5(3):157–178. [Google Scholar]
- 42.Reinhard J, Schreiber A, Schiefer U, et al. Does visual restitution training change absolute homonymous visual field defects? A fundus controlled study. Br J Ophthalmol. 2005 Jan;89(1):30–35. doi: 10.1136/bjo.2003.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp Neurol. 2006 Aug;200(2):356–370. doi: 10.1016/j.expneurol.2006.02.131. [DOI] [PubMed] [Google Scholar]
- 44.Owsley C. Contrast sensitivity. Ophthalmol Clin North Am. 2003 Jun;16(2):171–177. doi: 10.1016/s0896-1549(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 45.Kasten E, Muller-Oehring E, Sabel BA. Stability of visual field enlargements following computer-based restitution training -- results of a follow-up. J Clin Exp Neuropsychol. 2001 Jun;23(3):297–305. doi: 10.1076/jcen.23.3.297.1180. [DOI] [PubMed] [Google Scholar]
- 46.Kasten E, Poggel DA, Muller-Oehring E, Gothe J, Schulte T, Sabel BA. Restoration of vision II: residual functions and training-induced visual field enlargement in brain-damaged patients. Restor Neurol Neurosci. 1999;15(2–3):273–287. [PubMed] [Google Scholar]
- 47.Kasten E, Poggel DA, Sabel BA. Computer-based training of stimulus detection improves color and simple pattern recognition in the defective field of hemianopic subjects. J Cogn Neurosci. 2000 Nov;12(6):1001–1012. doi: 10.1162/08989290051137530. [DOI] [PubMed] [Google Scholar]