Biomarkers of steady-state platelet count and risk for thrombocytopenia post-chemotherapy have not been previously identified. Thrombocytopenia can interfere with chemotherapy by limiting doses and delaying therapy[1], potentially resulting in poorer outcomes.[2] Therefore strategies to predict risk and mitigate chemotherapy-induced thrombocytopenia (CIT) are needed. Additionally, the ability to identify patients at high risk of thrombocytopenia and new strategies to influence platelet production may permit more intensive therapy, while avoiding severe thrombocytopenia and its sequelae.
To date, no therapies are established for treatment of CIT. Use of thrombopoietin (TPO), the cytokine most related to megakaryocyte and platelet production, is not recommended because of risk of immunogenicity.[3] Other cytokines [e.g., stromal derived factor-1 (SDF-1, CXCL12), interleukin (IL)-3 and IL-6] have limited utility due to toxicity.[4] TPO receptor agonists are in clinical trials to examine their efficacy in this setting; however, experience with recombinant human (h) TPO suggests that in settings where bone marrow suppression is severe, these agents may have limited efficacy.[3] Another candidate to ameliorate thrombocytopenia, especially after marrow injury[5, 6], is platelet factor 4 (PF4, CXCL4), a platelet and megakaryocyte-specific chemokine stored in alpha granules and released from activated platelets. In vitro studies have shown that PF4 is a negative regulator of human and murine megakaryopoiesis via the LDL-receptor related protein-1 (LRP-1).[6–8] In mice, we have shown that steady-state platelet counts are inversely related to platelet (p) PF4 content.[6] In CIT and radiation-induced thrombocytopenia (RIT) models, mice with high levels of pPF4 developed significantly worse thrombocytopenia and delay in platelet count recovery.[5,6] Blocking PF4 using anti-PF4 antibodies was able to improve platelet count recovery in both CIT and RIT; however, PF4's importance in defining platelet counts in humans has not been examined.
We present the first evidence that pPF4 levels inversely correlate with steady-state platelet counts in individuals and recovery after human bone marrow injury. By studying the relationship between pPF4 and recovery from CIT in standard risk acute lymphoblastic leukemia (SR-ALL), we found that steady-state and post-CIT nadir platelet counts were inversely related to pPF4. There was a significant difference in pPF4 content for those patients requiring transfusions compared to those that did not. The clinical implications of these findings are discussed.
In a single institution, cohort study of patients with National Cancer Institute SR-ALL (age >1 year or <10 years; white blood cell count <50,000/mcL at diagnosis; morphological, flow cytometry and surface marker studies consistent with SR-ALL) who had completed therapy since January 1999, blood samples were collected during a routine follow-up visit 4 months to 10 years after completion of chemotherapy. Eligible patients were off therapy and were treated at our institution so that medical records were complete and available for review. Patients with SR-ALL were selected because of the relative uniformity of therapy. Medical records were examined to determine platelet count nadir and transfusion requirements during delayed intensification (DI) by investigators (AR, AG) blinded to pPF4 levels. Transfusion data were obtained from the hospital blood bank; only transfusions given during DI were analyzed. Control subjects were recruited from the healthy pediatric population referred for routine laboratory studies or minimal risk surgical procedures through the Anesthesia Resource Center. This study was approved by the Institutional Review Board.
One hundred and one patients with SR-ALL were enrolled between October 2008 and September 2011. Ninety-seven had evaluable PF4 levels and were used in analysis of PF4 with respect to race, gender and age. Ninety subjects had complete medical records available for review and were used in the transfusion and nadir platelet count analysis. Fifteen subjects who were seen more than once during the study period also had repeated PF4 measurements. The gender/age/racial distribution within this study group reflected the SR-ALL patient population at our institution. Ninety-seven healthy, control subjects were recruited to compare their PF4 levels to those of SR-ALL patients (Supplemental Table 1).
Cytokine/chemokines measurement
Because it is not possible to directly measure megakaryocyte PF4 levels, we used platelet PF4 content as a surrogate marker for megakaryocyte PF4 content. Immediately after collection, a platelet count was measured on citrate-anticoagulated whole blood using a Hemavet HV950FS set for human samples. The remaining specimen was centrifuged[6] to produce platelet-rich plasma (PRP). Prostaglandin E1 (0.5 μM) was added and the platelets were pelleted at 930 × g for an additional 10 minutes. The platelet-poor plasma (PPP) was collected and stored at −80°C and used to measure SDF-1 and TPO levels using commercially available ELISA kits from R&D Systems (Minneapolis, MN). Plasma PF4 levels were also measured to monitor specimen quality (data not shown). The platelet pellet was lysed using 3 cycles of freeze/thaw and then resuspended in phosphate-buffered saline (Invitrogen, Carlsbad, CA). PF4 and β-thromboglobulin [βTG, an N-terminal cleavage product of platelet basic protein (PBP or CXCL7)] were measured in the supernatant using an Asserachrom ELISA kit (Diagnostica Stago, Inc., Parsipanny, NJ). PRP platelet counts and concurrent PF4 and βTG levels were used for calculation of concentration/platelet. PF4 and other cytokines and chemokine levels were compared between groups using Student's t test (for normally distributed variables) or Mann-Whitney test (for pPF4 comparisons). Pearson correlation was used to assess correlations between PF4 and linear parameters. Statistical analysis was performed using GraphPad Prism (La Jolla, CA). P values were considered significant if <0.05.
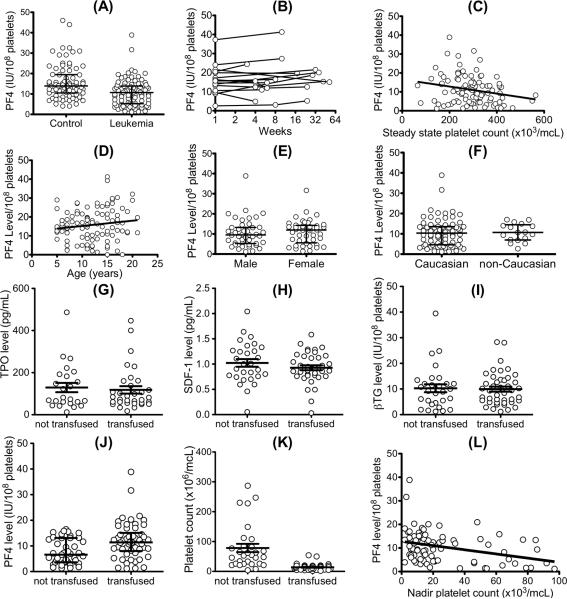
There was a 40-fold difference in pPF4 levels between individuals in both the control arm and the SR-ALL subjects (Fig. 1A), similar to the range we previously described in a healthy adult blood donor population[6]. As in the prior adult study, these values are not normally distributed[6], but rather showed a Chi-squared distribution (data not shown). Although the ranges were the same, pPF4 levels in the post-therapy SR-ALL group were lower when compared to controls (median 10.74 IU/108 platelets (range 1.16 to 46.23) in SR-ALL subjects versus median 13.92 IU/108 platelets (range 4.06 to 46) in controls, p<0.001, Fig. 1A). This difference may be related to age and/or ethnic differences between the groups or to the underlying SR-ALL disease or its treatment. Further prospective studies need be done to explain this observed difference, but we show that in the SR-ALL patients tested, pPF4 levels remain stable over time (SD was 0.3–6.9 IU/108 platelets) when we examined serial measurements of 15 post-therapy patients who had at least two samples separated by 4 weeks to 12 months (Fig. 1B) selected because they were seen more than once during the study period and prior to any pPF4 level measurements. In agreement with animal studies,[5,9] pPF4 levels were inversely related to steady-state platelet count (r=−0.22, 95% CI −0.40 to −0.04, p=0.02, Fig. 1C), suggesting a role for PF4 in the regulation of steady-state platelet levels. We assessed the influence of demographic variables on PF4 levels in the patients with SR-ALL, and found that age (Fig. 1D), gender (Fig. 1E), and ethnicity (Fig. 1F) did not have an effect, although the numbers of subjects in different racial groups were very small and so a difference due to race may not be appreciable.
Figure 1. PF4 levels in the pediatric SR-ALL population and the effect of cytokines on transfusion, platelet nadir and duration of therapy.
(A) Comparison of pPF4 for healthy pediatric controls compared to subjects that have recovered from SR-ALL. Median and interquartile range are indicated by long and short horizontal bars, respectively, and in (E) and (F). (B) Variability of PF4 expression in patients over time. Each series of lines/circles represents one patient. Maximal intra-individual variability was 6.8 IU/108 platelets. (C) through (L) are for the SR-ALL cohort. (C) Data for the relationship between pPF4 and steady-state platelet count at the time of specimen collection (r=−0.22, 95% CI −0.4013 to −0.03691, p=0.02). (D) The distribution of pPF4 by age t (r=0.16, 95% CI −0.09708 to 0.2831, p=0.14) (E) Distribution of pPF4 for males compared to females. (F) Distribution of pPF4 for Caucasian subjects compared to non-Caucasian subjects. In (G) and (H), mean ± 1 SD for transfused versus non-transfused are shown. (G) Steady-state plasma TPO levels. (H) Steady-state plasma SDF-1 levels. In both (G) and (H), p>0.3 comparing transfused versus not transfused. (I) β-TG and (J) PF4 per platelet in patients that were transfused versus those that were not transfused. In (J) median and interquartile range are shown. (K) Nadir platelet counts separated into patients who received transfusion during DI and those not transfused. (L) Platelet PF4 level versus nadir platelet count (r=−0.3; 95% CI −0.48 to −0.08; p=0.004). The patients with nadir platelet counts >100 were excluded.
We examined whether either of the two positive cytokines affecting platelet counts, TPO and SDF-1, or the negative paracrine PF4 or its related chemokine β-TG that does not affect megakaryopoiesis in vitro,[8] might be associated with transfusion needs. There were no differences in the levels of TPO, SDF-1 (in plasma) or β-TG (in platelets) between patients who did or did not require platelet transfusions (Figs. 1G, 1H and 1I, respectively). However, patients who did not require platelet transfusion during DI had significantly lower pPF4 when compared to patients who required transfusion (7.7 ± 4.8 versus 11.9 ± 7.1 IU/108 platelets, respectively, p<0.001) (Fig. 1J). Patients requiring platelet transfusions had significantly lower nadir platelet counts when compared to those not requiring platelet transfusion (p<0.001, Fig. 1K), and nadir platelet counts were inversely correlated with pPF4 (p=0.004, Fig. 1L).
Unlike TPO and SDF-1, steady-state pPF4 correlated with transfusion needs. Patients with pPF4 >1 SD below the mean (≤4 IU/108 platelets) were almost twice as likely to go through DI without needing a platelet transfusion (76% untransfused versus 42% overall, RR 0.4, 95% CI 0.2 to 0.98; p=0.02). Patients with PF4 levels >1 SD above the mean (≥18 IU/108 platelets) were all transfused (p<0.001 versus 42% overall) and were almost 2 times more likely to need transfusion (RR 1.8; 95% CI 1.5 to 2.2; p=0.01).
In conclusion, prior studies have shown in vitro that both human and murine megakaryopoiesis is inhibited by PF4 and that in vivo, murine steady-state platelet counts and severity of CIT and RIT inversely correlated with pPF4 levels. We now show that these findings also apply to humans and that PF4 may be a useful biomarker for risk of significant thrombocytopenia with chemotherapy. Our data suggest that those individuals with the highest pPF4 level may benefit from therapy directed to prevent PF4-related CIT. Those with low pPF4 levels may better tolerate chemotherapy. Prescreening pPF4 levels in these patients may permit more intensive dosing of chemotherapy or shorter intervals between cycles, which have been associated with better event-free survival in some cancer types.[10,11] Additionally, strategies to block PF4 have been effective in altering the duration and severity of thrombocytopenia in animal models of CIT and RIT.[6,7] Therefore, prospective validation of pPF4 linkage to CIT in a variety of cancers is important as a first step in eventually developing strategies that block PF4's negative paracrine effect on megakaryocytes as a therapeutic strategy for CIT. It is not yet known if pPF4 levels can be measured accurately with low platelet counts or if pPF4 levels are altered during active chemotherapy. These issues will be addressed in future studies. Megakaryocyte apoptosis has also been observed in other disorders including RIT, immune thrombocytopenias and myelodysplastic syndromes, and we propose that studies of these disorders would support a causative as well as predicative role of pPF4 levels in the thrombocytopenia associated with these disorders and offer a novel therapeutic pathway for intervention in these thrombocytopenic disorders.
Supplementary Material
Acknowledgements
MPL was supported by NIH-NHLBI K23HL092164 and an investigator development fund. MP was supported by P01HL040387. The authors gratefully acknowledge Dr. Susan Rheingold (Division of Oncology) for her review of the manuscript. We thank the staff of the Late Effects Clinic for their help in carrying out this study. We thank the patients and families treated at The Children's Hospital of Philadelphia for their time and participation.
Footnotes
Authorship: MPL designed the study, wrote the paper and edited drafts, and analyzed the PF4 levels and patient data. AR and AG collected all of the clinical data, consented patients and collected the blood samples as well as helped to maintain IRB approval and edited drafts of the paper. YN and LX processed and analyzed PF4 samples and helped to edit the paper. RA guided the statistical analysis in both the design of the study and after completion of data collection and analysis as well as helped to revise the paper. LR developed the technique for specimen processing and collected all of the data on the control population as well as helped to edit the paper. MP provided scientific oversight in all phases of the study from design to final manuscript preparation.
Conflict of interest: The authors have no relevant conflicts of interest to declare.
References
- 1.Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, Kanesan K, Cantor SB, Benjamin RS. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19:1137–46. doi: 10.1200/JCO.2001.19.4.1137. [DOI] [PubMed] [Google Scholar]
- 2.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332:901–6. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 3.Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002;100:3457–69. doi: 10.1182/blood.V100.10.3457. [DOI] [PubMed] [Google Scholar]
- 4.Vadhan-Raj S. Management of chemotherapy-induced thrombocytopenia: current status of thrombopoietic agents. Semin Hematol. 2009;46:S26–32. doi: 10.1053/j.seminhematol.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Lambert MP, Xiao L, Nguyen Y, Kowalska MA, Poncz M. The role of platelet factor 4 in radiation-induced thrombocytopenia. Int J Radiat Oncol Biol Phys. 2011;80:1533–40. doi: 10.1016/j.ijrobp.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert MP, Rauova L, Bailey M, Sola-Visner MC, Kowalska MA, Poncz M. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications. Blood. 2007;110:1153–60. doi: 10.1182/blood-2007-01-067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gewirtz AM, Zhang J, Ratajczak J, Ratajczak M, Park KS, Li C, Yan Z, Poncz M. Chemokine regulation of human megakaryocytopoiesis. Blood. 1995;86:2559–67. [PubMed] [Google Scholar]
- 8.Gewirtz AM, Calabretta B, Rucinski B, Niewiarowski S, Xu WY. Inhibition of human megakaryocytopoiesis in vitro by platelet factor 4 (PF4) and a synthetic COOH-terminal PF4 peptide. J Clin Invest. 1989;83:1477–86. doi: 10.1172/JCI114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert MP, Wang Y, Bdeir KH, Nguyen Y, Kowalska MA, Poncz M. Platelet factor 4 regulates megakaryopoiesis through low density lipoprotein receptor related protein 1 (LRP1) on megakaryocytes. Blood. 2009;114:2290–8. doi: 10.1182/blood-2009-04-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Womer RB, Daller RT, Fenton JG, Miser JS. Granulocyte colony stimulating factor permits dose intensification by interval compression in the treatment of Ewing's sarcomas and soft tissue sarcomas in children. Eur J Cancer. 2000;36:87–94. doi: 10.1016/s0959-8049(99)00236-1. [DOI] [PubMed] [Google Scholar]
- 11.Rytting M, Ravandi F, Estey E, Cortes J, Faderl S, Garcia-Manero G, Jeha S, Ouzounian S, Pierce S, Kantarjian H. Intensively timed combination chemotherapy for the induction of adult patients with acute myeloid leukemia: long-term follow-up of a phase 2 study. Cancer. 2010;116:5272–8. doi: 10.1002/cncr.25516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.