Abstract
Background
In rodent models of pulmonary hypertension (PH) and right ventricular hypertrophy (RVH), the QTc interval is prolonged, reflecting downregulation of repolarizing Kv channels in RV myocytes. The significance of QTc prolongation in human PH is unknown. We hypothesized that QTc prolongation occurs in human PH, is associated with RVH and decreased RV function, and predicts adverse prognosis.
Methods
Patients receiving a PAH-specific therapy (a prostanoid, endothelin-receptor antagonist and/or a phosphodiesterase-5 inhibitor), who had a 12-lead electrocardiogram (ECG) (n=202) were compared to age- and sex-matched controls (n=100). The duration of QTc on ECG was correlated with invasive hemodynamics (n=156) and with the status of the RV, as measured by Brain Natriuretic Peptide (NT-proBNP, n=145) and magnetic resonance imaging (n=24). Survival of the entire PH cohort and a subgroup with WHO Group 1 and 4 PAH was prospectively determined from the Social Security Death Index.
Results
QTc intervals were longer in PH vs. controls (454.8±29 ms vs. 429.8±18 ms, p<0.001) and did not differ based on PAH-specific therapy. NT-proBNP increased proportionately with QTc and was higher for those in the upper quintile (QTc≥480ms) vs. those with QTc≤480ms (4004±6682 pg/mL vs. 1501±1822 pg/mL, p<0.001). The QTc interval also correlated directly with increasing RV end-diastolic volume (r=.67, p<0.001) and mass (r=.0.51, p<0.05), and inversely with RV ejection fraction (r=−.49, p<0.05). In the entire PH cohort and WHO Group 1 and 4 subgroup, QTc ≥480 ms and cardiac index were independent predictors of mortality.
Conclusions
QTc prolongation in PH patients reflects the status of the RV and is an independent predictor of mortality.
Keywords: Biomarkers, brain natriuretic peptide, cardiac repolarization, pulmonary hypertension, right ventricular failure, right ventricular hypertrophy
Introduction
Pulmonary arterial hypertension (PAH) has an annual incident mortality rate of 15%, with most deaths occurring due to right ventricular (RV) failure[1–3]. It is increasingly recognized that prognosis in PAH is best predicted by RV function[4–5]. Simple screening tests for RV failure and hypertrophy include N-Terminal pro Brain Natriuretic Peptide (NT-proBNP) and echocardiography; however, early detection of RVH is difficult and patients with severe PAH and dyspnea have a mean delay to diagnosis of > 1 year[6]. Although the surface electrocardiogram (ECG) is specific for RVH, its sensitivity is quite low[7–8]. Recent studies of experimental PAH and RVH demonstrate that there are additional electrophysiologic changes in the RV that might offer insights into the status of the RV, notably prolongation of the duration of the QTc interval on the surface ECG[9].
In rodent models of RVH, whether accompanied by PAH (monocrotaline) or not (pulmonary artery banding), there is prolongation of the RV monophasic action potential duration and the QTc interval on the surface ECG[9]. Mechanistically, this reflects downregulation of repolarizing voltage-gated potassium channels in RV myocytes, including Kv1.5 and Kv4.2[9]. The QTc prolongation observed in these rodents with RVH was reversed with agents that regressed PAH and RVH, notably the pyruvate dehydrogenase kinase inhibitor, dichloroacetate, and these electrical changes were associated with improved RV function[9].
Few studies have examined whether humans with PAH manifest prolongation of the QTc interval or increased QRS duration and no studies have assessed whether QTc prolongation or increased QRS duration in patients with PAH are associated with indices of RV size and function or predict clinical outcomes[10–11].
We thus performed the current study to determine:
whether PAH is associated with QTc prolongation or increased QRS duration.
whether such ECG abnormalities correlate with established indices of RV dysfunction.
whether such ECG abnormalities predict worse clinical outcomes.
Methods
Patient Selection
The institutional review board at the University of Chicago Medical Center approved the conduct of this study. We initially performed a retrospective chart review of patients diagnosed at the University of Chicago with pulmonary hypertension (PH) between January 2004 and February 2010. The following inclusion criteria were applied:
Presence of PH, defined as a mean pulmonary artery pressure (PAP) ≥25 mmHg and an elevated pulmonary vascular resistance (PVR), measured during cardiac catheterization.
An expert from the University of Chicago Pulmonary Hypertension Program confirmed the PH diagnosis and directed the management.
The patient was prescribed at least one or more of the following FDA approved, PAH-specific therapies: a prostacyclin analogue (epoprostenol or treprostinil), a phosphodiesterase-5 inhibitor (sildenafil or tadalafil), and/or an endothelin receptor antagonist (bosentan or ambrisentan).
A resting 12-lead ECG was performed at the time of the PH diagnosis.
After applying these criteria, we arrived at a final PH cohort consisting of 202 patients. The majority of these patients had WHO Group 1 PH (148/202). However, we also included patients from other WHO Groups only if they had pulmonary vascular disease of sufficient severity to warrant prescription of a PAH-specific drug by one of our PH specialists[1–2]. Regardless of the WHO PH Group, all patients in the study had not only elevated PA pressures but had significantly elevated PVR, reflective of pulmonary vascular disease. The severity of pulmonary vascular disease in those with non-Group 1 PH led the PH specialist to justify prescribing a PAH-specific medication for off label use. The rationale for this design reflects the fact that we intended to test the ability of the ECG to evaluate the status of the right ventricle, a key predictor of outcomes in all forms of PH.
The control group consisted of 100 consecutive, age- and sex-matched patients at the University of Chicago Medical Center with normal ECGs (defined as normal sinus rhythm without evidence of bundle branch block, ST-segment depression or elevation, left or right ventricular hypertrophy, or previous myocardial infarction). The clinical indication for the ECG in these controls was not determined; however the normalcy of their ECG was confirmed.
We recorded the following ECG variables: QT interval, QTc interval, QRS duration, presence of RVH, and presence of right axis deviation. All intervals were determined by commercial ECG software algorithm (Marquette™ 12SL™ ECG Analysis Program). The exact details of the algorithm can be found elsewhere[12]. Briefly, the onsets and offsets of all intervals are determined by an analysis of the simultaneous slopes in all 12 leads. Onsets are defined as the earliest deflection in any lead, and offsets are defined as the latest deflection in any lead. The QT interval is measured from the earliest depolarization in any lead to the latest detection of repolarization in any lead. The QTc interval was determined by the Fridericia formula (corrected QT interval = QT/RR⅓). In order to account for the possible impact of a RBBB on the QTc interval, we subtracted 40 ms from the measured QTc in the 14 patients from the PH cohort with a RBBB[13]. Right axis deviation was defined as a frontal plane QRS axis ≥90 degrees. The ECG criteria for RVH were achieved if there was right axis deviation and the R-wave in V1 was both greater than 0.5 mV and greater than the V1 S-wave[8].
Diuretic therapy is not uncommon in PH patients and can result in hypokalemia, which may prolong the QTc. Therefore, we recorded potassium values in all PH patients who had a measured level within 30 days of the index ECG (n=185).
Right Heart Catheterization
Hemodynamic data from right heart catheterization were included if performed within six months of the index ECG (n=156). Hemodynamics recorded included: mean right atrial pressure, mean pulmonary artery pressure, PVR, pulmonary capillary wedge pressure, and cardiac index.
Measures of Right Ventricular Status
NT-proBNP may predict clinical outcomes in PH, likely reflecting the status of the RV[14]. Thus, we recorded NT-proBNP levels in all PH patients who had a value within 30 days of their index ECG (n=145). We also identified 24 subjects among our PH cohort who underwent Cardiac Magnetic Resonance Imaging (CMR) within 6 months of the index ECG. All patients were imaged on a 1.5 Tesla scanner (Achieva, Philips, Best, Netherlands) using a phased-array surface coil with electrocardiographic gating. After scout imaging, retrospectively gated cine images were obtained in the short axis plane using a steady-state free precession (SSFP) sequence (TR 2.9ms, TE 1.5ms, flip angle 60°, and temporal resolution ~40ms). Images were analyzed using commercial software (Philips ViewForum, Best, Netherlands). Short axis slices were used to calculate right ventricular end-diastolic volume (RVEDV), end-systolic volume (RVESV), ejection fraction (RVEF), and mass via the method of disks.
Clinical Outcomes
>We prospectively followed subjects in the PH cohort to determine whether QTc prolongation or QRS duration predicted mortality during a subsequent follow-up period of one year. We also performed a subgroup analysis of clinical outcomes in patients restricted to Group 1 and 4 PH. Vital statistics were collected for all patients by searching the Social Security Death Index as previously described[15]. The vital status of patients who were not identified as deceased in the Social Security Death Index was determined and confirmed by chart review. Thus, no patients were lost to follow-up.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation and categorical variables as frequency and percentages. Continuous variables were compared using the unpaired Students t-test and multi-way ANOVA with Bonferroni’s testing for post hoc analysis. Categorical variables were compared using the Chi-square test. Pearson’s correlation coefficients were determined when comparing continuous variables. We compared survival in patients with a QTc interval ≥480 ms versus QTc interval ≤480 ms, which represented the highest quintile of QTc prolongation, using the Kaplan–Meier method and log-rank test. We used the multivariable Cox proportional hazard regression method to determine independent predictors of mortality. All baseline, clinical, and hemodynamic characteristics were included in our univariable analysis, including quintiles of QTc interval and QRS duration, which were entered as categorical predictors (Table 1). Covariates with a p-value <0.06 from our univariable analysis were initially entered into our multivariate model; covariates with p-values >0.10 were removed sequentially to arrive at a final multivariate model. All statistical analyses were performed using STATA11.1 (StataCorp LP, College Station, TX).
Table 1.
Baseline demographic and clinical characteristics of PH patients and controls
| Variable | PH cohort (n=202) |
Controls (n=100) |
p-value |
|---|---|---|---|
| Demographics | |||
| Age (yrs) | 53.5±14 | 52.2±15 | 0.48 |
| Female, n (%) | 160 (79) | 74 (74) | 0.31 |
| ECG Findings | |||
| Heart rate | 82.9±15 | 75.7±10 | <0.001 |
| QT interval (ms) | 391.7±37 | 385.1±21 | 0.10 |
| QTc interval (ms) | 454.8±29 | 429.8±18 | <0.001 |
| QRS duration (ms) | 96.5±16 | 84.4±8 | <0.001 |
| RVH | 86 (42.6%) | 0 | N/A |
| Right axis deviation | 129 (63.9%) | 0 | N/A |
| WHO PH Etiology n (%) | N/A | ||
| Group 1 | 148 (73.3%) | ||
| Group 2 | 16 (7.9%) | ||
| Group 3 | 7 (3.5%) | ||
| Group 4 | 15 (7.4%) | ||
| Group 5 | 16 (7.9%) | ||
| WHO Functional Class n (%) | N/A | ||
| Functional Class I | 4 (2%) | ||
| Functional Class II | 79 (39%) | ||
| Functional Class III | 104 (51%) | ||
| Functional Class IV | 15 (7%) | ||
| PAH Specific Therapy | N/A | ||
| Prostacyclin (IV/SQ) | 42 (20.8%) | ||
| PDE5 inhibitor | 76 (37.6%) | ||
| ERA | 23 (11.4%) | ||
| Prostacyclin + PDE5 | 35 (17.3%) | ||
| PDE5 inhibitor + ERA | 26 (12.9%) | ||
| Laboratory Tests | |||
| Serum Potassium mEq/L (n=185) | 4.02±0.51 | NR | |
| Serum NT-proBNP pg/mL (n=144) | 1973.3±3379.5 | NR | |
| Invasive Hemodynamics (n=156) | N/A | ||
| Right atrial pressure, mm Hg | 8.8±5.5 | ||
| Pulmonary artery systolic pressure, mm Hg | 78.1±18.7 | ||
| Mean pulmonary artery pressure, mm Hg | 47.4±11.4 | ||
| PVR, Wood units | 9.2±5.2 | ||
| Pulmonary capillary wedge pressure, mm Hg | 10.7±4.0 | ||
| Cardiac output, L/min | 4.7±1.7 | ||
| Cardiac index, L/min/m^2 | 2.6±0.9 |
NR=not recorded; NA=not available; ECG=electrocardiogram; RVH=right ventricular hypertrophy; PH=pulmonary hypertension; PDE5=phosphodiesterase 5; ERA=endothelin receptor antagonist; NT-proBNP=n-terminal pro brain natriuretic peptide
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology: Shewan LG and Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol 2010;144:1-2.
Results
Patient demographic, electrocardiographic, clinical, laboratory, and invasive hemodynamic data can be seen in Table 1. There was no difference in age (54±14 years vs. 52±15 years, p=0.48) or sex (79% female vs. 74% female, p=0.31) between PH subjects and controls, reflecting the effective case matching. However, subjects with PH had a significantly longer QTc interval (454.8±29 ms vs. 429.8±18 ms, p<0.001) and QRS duration (96.5±16 ms vs. 84.4±8 ms, p<0.001), as compared to controls (Figure 1AB).
Figure 1. Prolongation of QTc and QRS in PH Patients Versus Controls.
A: The QTc interval is significantly longer in PH compared to age and sex matched controls (454.8 ms vs. 429.8 ms, p<0.001)
B: QRS duration is prolonged in PH compared to age and sex matched controls (96.5 ms vs. 84.4 ms, p<0.001).
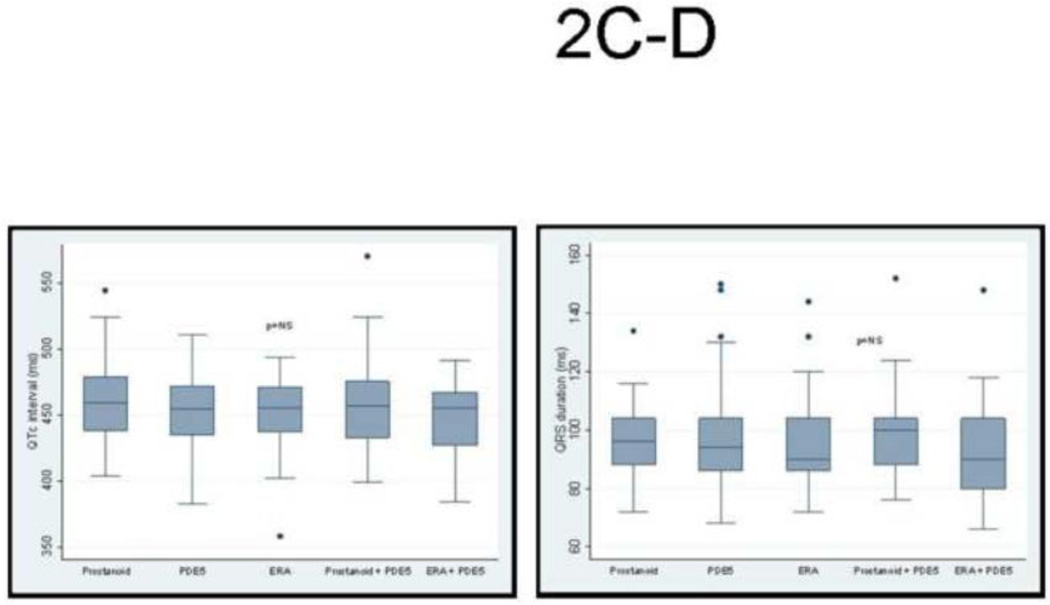
Given that ECG changes in patients with PH likely reflects the status of the RV, it is not surprising that we found no difference in QTc interval or QRS duration based on WHO PH Group classification (Figure 2A–B), nor did the QTc or QRS vary according to the type of PAH-specific therapy. Among those with PH, both the QTc interval and QRS duration, respectively, were more prolonged in those with electrocardiographic evidence of RVH (458.8±3 ms vs. 449.8±3 ms, p<0.05) and (100.9±2 ms vs. 90.9±1 ms, p<0.01). A similar observation was seen in those with right axis deviation on the ECG (458.4±2 ms vs. 446.1±3 ms, p<0.01) and (99.2±1 ms vs. 90.1±2 ms, p<0.01). Interestingly, there was a poor correlation between the QTc interval and QRS duration in PH patients (r=0.21, p<0.01).
Figure 2. No Difference in QTc and QRS According to WHO PH Diagnosis or PH-Specific Therapy.
There was no difference in (A) QTc interval or (B) QRS duration in subgroups based on WHO clinical classification of PH. Likewise, there was no difference in (C) QTc interval or (D) QRS duration in subgroups based on type of PAH-specific therapy receiving.
The mean serum potassium level in the 185 PH patients with available levels at the time of the index ECG was 4.01±0.50 mEq/L. Serum potassium was not different in those with a normal versus prolonged QTc (3.99±.54 mEq/L vs. 4.08±.43 mEq/L, p=0.27). There was also no difference in serum potassium levels in those with QTc prolongation in the upper quintile (≥480 ms) compared to those with a QTc ≤480 ms (4.04±0.47 mEq/L vs. 3.89±0.62 mEq/L, p=0.13).
Right Heart Catheterization
Hemodynamic status reflected a prevalence cohort with severe PH, as evidenced by a markedly elevated PVR (Table 1). The QTc interval and mean PA pressure were directly, but poorly correlated (r=0.18, p<0.05). However, there were no other statistically significant correlations between hemodynamics and the QTc interval and/or the QRS duration.
Measures of Right Ventricular Status
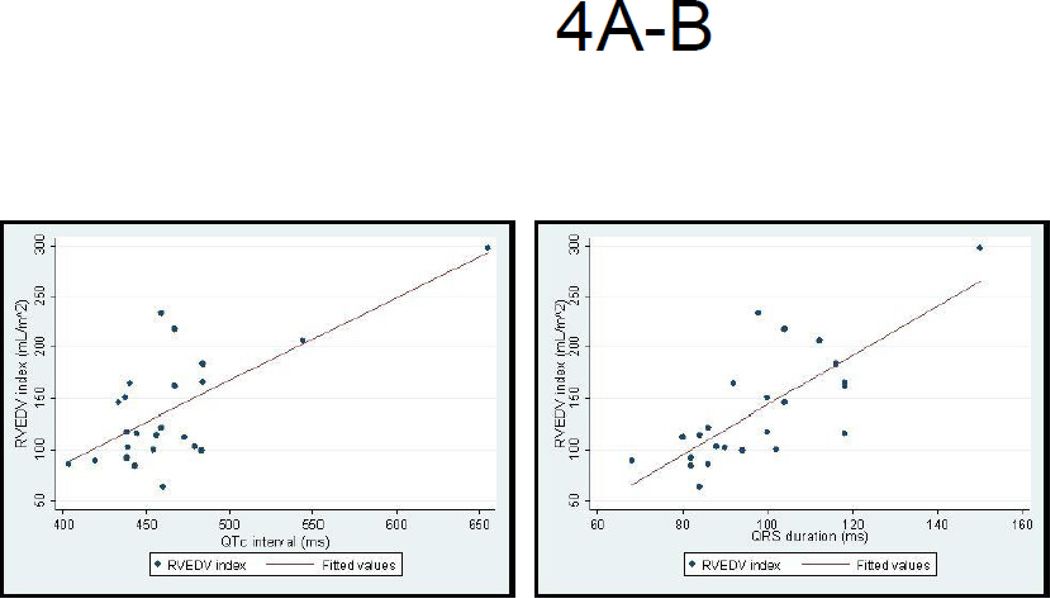
The mean NT-proBNP level was elevated at 1959±3401 pg/mL (normal <125 pg/mL) in the 145 PH patients with available tests. There was a significant but poor correlation between QTc and NT-proBNP levels (r=0.24, p<0.01). In addition, NT-proBNP levels were significantly higher in those with a QTc≥480 ms compared to those with a QTc≤480 ms (4004.0±6682.1 pg/mL vs. 1501.2±1822.3 pg/mL, p<0.001) (Figure 3). Mean RV size and mass were markedly enlarged and RV systolic function was reduced in the PH cohort (Table 2). In these patients, the QTc interval and QRS duration were both strongly correlated with all measured indices of RV size and function (Table 3 and Figures 4A–B).
Figure 3. Serum NT-proBNP levels stratified by quintiles of QTc interval in PH patients.
Increasing serum NT-proBNP levels, reflecting severity of RV dysfunction, is associated with progressive increases in the QTc interval in PH patients.
Table 2.
Clinical characteristics and indices of RV size and function among PH patients undergoing cardiac MRI
| Variable | PH cohort (n=24) | |
|---|---|---|
| Demographics | ||
| Age, yrs | 57±14 | |
| Female, n (%) | 20 (83) | |
| QTc Interval (ms) | 464.9±49 | |
| QRS Duration (ms) | 98.2±18 | |
| PH diagnosis, n (%) | ||
| Group 1 | 17 (70.8) | |
| Groups 2–5 | 7 (29.2) | |
| CMR variable | ||
| RVEDV (mL) | 258.3±98 | |
| RVEDV index (mL/m2) | 139.3±56 | |
| RVESV (mL) | 180.6±93 | |
| RVESV index (mL/m2) | 97.1±53 | |
| RV mass (g) | 52.8±24 | |
| RV mass index (g/m2) | 26.7±14 | |
| RVEF (%) | 33.0±12 |
PH=pulmonary hypertension; CMR=cardiac magnetic resonance imaging; RVEDV=right ventricular end diastolic volume; RVESV=right ventricular end systolic volume; RVEF=right ventricular ejection fraction
Table 3.
Correlation between QTc interval, QRS duration, and indices of RV size and function in PH
| Variable | r-value | p-value | |
|---|---|---|---|
| QTc interval (ms) | |||
| RVEDV (mL) | 0.67 | <0.001 | |
| RVEDV index (mL/m2) | 0.71 | <0.001 | |
| RVESV (mL) | 0.70 | <0.001 | |
| RVESV index (mL/m2) | 0.75 | <0.001 | |
| RV mass (g) | 0.51 | <0.05 | |
| RV mass index (g/m2) | 0.53 | <0.01 | |
| RVEF (%) | −0.49 | <0.05 | |
| QRS duration (ms) | |||
| RVEDV (mL) | 0.76 | <0.001 | |
| RVEDV index (mL/m2) | 0.76 | <0.001 | |
| RVESV (mL) | 0.74 | <0.001 | |
| RVESV index (mL/m2) | 0.75 | <0.001 | |
| RV mass (g) | 0.57 | <0.01 | |
| RV mass index (g/m2) | 0.59 | <0.01 | |
| RVEF (%) | −0.46 | <0.05 |
RVEDV=right ventricular end diastolic volume; RVESV=right ventricular end systolic volume; RVEF=right ventricular ejection fraction
Figure 4. Correlation between the QTc and QRS duration and RV Diastolic Dimension on CMR.
A: The QTc interval correlates strongly with indices of RV end diastolic dimension
B: QRS duration in PH correlates strongly with indices of RV end diastolic dimension.
Survival Analysis
There were 23 deaths in the PH cohort during the 12 month prospective follow-up period. Compared to patients with QTc interval < 480 ms, PH patients in the highest quintile (QTc ≥ 480 ms) had decreased survival (Figure 5A). The univariable and multivariable predictors of mortality are shown in Table 4. On multivariate analysis, those in the highest QTc quintile (QTc≥480 ms) were at increased risk of death compared to those with shorter QTc (HR 3.09; 95% CI 1.14 – 8.38; p<0.05). However, despite a trend, there was no statistically significant difference in absolute QTc interval (453.8±29 ms vs. 462.3±30 ms, p=0.17) or QRS duration (95.9±15 ms vs. 100.7±19 ms, p=0.15) in PH survivors compared to those who died. When we restricted our analyses to only those patients with WHO Group 1 and 4 PH (n=163), a QTc≥480 ms continued to be an independent predictor of mortality (HR 4.38; 95% CI 1.41 – 13.61; p<0.05) (Figure 5B).
Figure 5. Kaplan-Meier plots of QTc Interval and Risk of Death in PH.
A: A QTc≥480 ms Predicts Increased Mortality in the entire PH cohort
B: A QTc≥480 ms Predicts Increased Mortality in WHO Groups 1 and 4 PAH
Table 4.
Univariate and Multivariate Predictors of Mortality
| Characteristics | Univariate Survival Analysis | Multivariate Survival Analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| QTc ≥480 ms | 2.51 (1.61 – 14.23) | 0.042 | 3.09 (1.14 – 8.38) | 0.022 |
| NT-proBNP (pg/mL) | 1.0002 (1.0001 – 1.0003) | 0.005 | NS | |
| Cardiac index (L/min/m^2) | 0.54 (0.29 – 0.99) | 0.048 | 0.50 (0.26 – 0.94) | 0.032 |
| Right atrial pressure (mmHg) | 0.97 (0.89 – 1.06) | 0.530 | NS | |
| Pulmonary vascular resistance (Wood units) | 1.06 (0.99 – 1.13) | 0.072 | NS | |
Discussion
In the present study, we found that both the QTc interval and QRS duration are prolonged in patients with PH. Moreover, increasing QTc predicts increasing NT-pro BNP levels, suggesting a dose-effect of QTc on an important biomarker of RV function (Figure 3). Also, both prolonged QTc interval and increased QRS duration are associated with accepted indices of RV dysfunction in PH, including increased RV mass and depressed systolic function, as measured by CMR. Finally, a severely prolonged QTc interval (≥480 ms) predicted mortality in the entire PH cohort (n=202) as well as in the subgroup of patients with WHO Group 1 and 4 PH (n=163). These findings are biologically plausible based on the observed prolongation of RV monophasic action potential duration and QTc interval observed in experimental RVH induced by monocrotaline or pulmonary artery banding[9].
Few studies have examined whether QTc prolongation or increased QRS duration occur in PH and none have examined the potential clinical implications of these changes. In 73 sickle cell patients, Akgul et al found QTc prolongation among the 26 with sickle-cell associated PH (QTc=459ms) versus those with sickle disease without PH (QTc=436 ms) and both groups were longer than controls (QTc=402ms) [10]. Important limitations of their study included the small sample size and the use of Doppler to estimate PA pressure rather than right heart catheterization. The reliance on Doppler to diagnose PAH in a sickle cell disease cohort with mild PH (PA systolic pressure 38±5 mmHg) is problematic because of the possibility of a high cardiac output state and the inability to quantify PVR[16]. Moreover, extrapolation to the broader PH population is quite limited by their exclusive use of a sickle cell disease population. More recently, Hong-Liang et al studied a cohort of PH patients and found the QTc was higher in those with severe PH versus controls (428.6±32.8 ms vs. 411.1±28.4 ms, p=0.02)[11]. However, the QTc in their PH cohort, though higher than controls, was within normal limits, unlike our study (where QTc was prolonged for the entire PH group). In addition, they used Bazett’s formula for QTc correction, which over-corrects QTc at high heart rates and under-corrects at low heart rates. This is important because Bazett's formula systematically overestimates QTc relative to the other formulae (Fridericia, Hodges and Framingham)[17]. Indeed the upper limit of normal QTc, as defined as the upper 2% of values in 10,303 normal EKGs, is 483ms for Bazett's formula vs. 460ms for Fridericia and 457ms for Hodges and Framingham[17]. Thus, none of the QTc prolongations reported previously would fall outside the normal range and may simply reflect the sicker patients within their cohort (those with PH) having higher heart rate. Finally, and perhaps most importantly, they did not correlate QTc intervals with indices of RV function or determine the impact on survival.
Even less is known about QRS duration in adult PH. In a study that evaluated for the presence of intraventricular dyssynchrony in patients with PH, Kalogeropoulos et al found that PH patients had an increased mean QRS duration compared to controls, but associations with PH outcomes or QTc interval were not assessed[18]. To our knowledge, the present study represents the largest study to evaluate and confirm the presence of both QTc prolongation and increased QRS duration in a relatively sick cohort of patients with PH and the first study to determine the individual predictive values of each on survival.
The close correlation between QTc and QRS duration with NT-proBNP and measures of RV mass and function on CMR suggest these ECG abnormalities reflect the function of the RV and/or the severity of RVH. In this regard there are lessons to be learned from LVH. Both QRS duration and QTc interval are prolonged in patients with LVH[19–24]. LVH also results in increased arrhythmic vulnerability[25–26]. The impairment of depolarization and repolarization of cardiomyocytes in ventricular hypertrophy accounts for the ECG changes known as “electrical remodeling”. Similar ECG changes have been described in experimental RVH[9]. Impaired active depolarization-repolarization in hypertrophy reflects (in part) downregulation of repolarizing potassium channels, such as those which account for the transient outward current, ITO (Kv4.2 and Kv4.3) or the ultra-rapid delayed rectifier K+ current (IKur) (Kv1.5)[9]. In addition, ventricular conduction can be slowed by pathological processes that reduce cell-to-cell coupling, including impaired expression/function of gap junctions and myocardial fibrosis. In a double-transgenic, angiotensin II-induced rat model (overexpressing the human renin and angiotensinogen genes), Fisher et al showed that QTc prolongation and prolonged QRS duration accompanied LVH. This was due to a combination of factors: downregulation of cardiac Kv4.3, decreased expression of the gap junction protein Connexin 43 and myocardial fibrosis. The electrical remodeling led to arrhythmogenic early- and delayed after-depolarizations and reentry and inducible ventricular tachycardia[27]. In rodent RVH QTc prolongation results (in part) from downregulation of repolarizing Kv channels in RV myocytes (Kv1.5 and Kv4.2) and is reversed when RVH is experimentally regressed. This was associated with re-expression of repolarizing Kv channels[9]. The fact that rats with RVH induced by pulmonary artery banding (who have no PH) display the QTc prolongation indicates the effect is related to RVH not PH. The reversibility of QTc prolongation in experimental RVH suggests this simple noninvasive marker could potentially be used to follow the progression/regression of RVH. However, it remains to be determined whether effective PH treatment in patients with PH would result in shortening of the QTc interval with a concomitant improvement in survival.
The consequence of long QTc in patients with PH has not previously been studied. In RVH it is not clear whether the consequence of QTc prolongation is arrhythmia, as in LVH, or impaired ventricular RV function. One of the more important aspects of this study is that the highest quintile of QTc prolongation was an independent predictor of mortality in patients with PH. The other two predictors of mortality in our PH cohort, NT-proBNP and cardiac index, respectively, both reflect the status of the RV in PH and both have been shown to be predictive of adverse outcomes in PH[3, 14, 28]. PH patients are not thought to be particularly susceptible to ventricular arrhythmias and are usually thought to die from RV failure and bradyarrhythmia[28]. However, many deaths are sudden and occur outside the hospital and this is an important area for future study[29].
Our study was not specifically designed to understand molecular mechanisms or investigate what initiates the QTc prolongation observed in patients with PH. However, emerging evidence supports the presence of chronic RV ischemia in many PH patients with RVH[30–31]. This ischemia may reflect microvascular rarefaction[32] and/or impaired right coronary artery perfusion pressure[31, 33]. Ischemia can prolong the QT interval by several mechanisms, including impaired function and expression of repolarizing Kv channels. Ischemia may also prolong QTc by increasing intracellular calcium during the cardiac action potential or through acidification of intracellular pH, both of which inhibit repolarizing potassium current[9]. Thus, chronic RV ischemia could be a potential mechanism responsible for QTc prolongation in PH patients and could possibly explain why those with the greatest QTc prolongation had the highest NT-proBNP levels (Figure 3) and were at the highest risk of death (Figure 5A). In addition, QTc interval duration is significantly affected by changes in the sympathetic nervous system and sympathetic tone[34]. PH patients with advanced RV failure are known to have neuroendocrine activation similar to that which is seen in LV failure and the QTc prolongation may be a marker of advanced disease with neurohormonal activation[35–36].
Another interesting finding in our study is that the QTc interval correlated better with measures of the status of the RV (NT-proBNP and RV mass and size) than with the PVR. This is consistent with our hypothesis that the QTc interval is primarily a reflection the status of the RV, rather than the pulmonary vasculature, and as in animal models, the CMR data show that the QTc does not prolong until there is severe RVH and a reduction in RV systolic function. Consistent with this, there appeared to be a threshold effect, with QTc only predicting survival at the highest quintile of QTc prolongation. This raises the question as to whether QTc prolongation might be useful to distinguish RVH that is “adaptive” (i.e. concentric RVH without chamber dilatation) versus “maladaptive” (i.e. eccentric RVH associated with reduced RV function and clinical RV failure). It has been reported, for example, that patients with the severest degree of pulmonary hypertension as measured by mean PA pressure and as described from post mortem analyses may not result in premature death if accompanied by concentric RVH[37]. Future studies that examine whether it is possible to discern the presence of concentric or adaptive RVH versus eccentric or maladaptive RVH on the basis of the QTc interval on the surface ECG would be important.
Limitations
The present study is not without limitations. First, this was a retrospective cohort study and is thus subject to potential selection and information bias. However, we prospectively followed the patients in our PH cohort for clinical outcomes which may minimize biases. Second, the PAH-specific treatments were not randomly or prospectively assigned but rather prescribed at the discretion of the PH specialist; thus, we cannot assess the effects (beneficial or deleterious) on the QTc interval. However, these specific treatments did not appear to have an effect on the QTc interval or QRS duration (Figure 2 C–D). Third, heart rate correction of the QT interval by the Fridericia formula (or any other corresponding formula) is appropriate under circumstances where there is a constant inverse relationship between heart rate and QT interval. In this study setting, it is conceivable that in PH patients there were factors present other than those related to the electrophysiology of the RV. Among potential extracardiac causes of QT prolongation, we corrected for electrolyte disturbances and PAH specific medications. However, there may be unknown confounders in PH that affect the QTc interval which are not accounted for by heart rate correction, including the direct effects of excessive neurohormonal stimulation. Nonetheless, it is noteworthy that in rodent models of RVH (where there are few confounders), QTc prolongation occurs in direct proportion to RVH severity and is reversible when an effective PH therapy is administered, as we have previously shown[9]. This is true both in pure RVH created by PA banding and in RVH associated with PAH-induced by monocrotaline. Finally, the inclusion of WHO Groups 2-5 patients might be considered a limitation but they were similar to the Group 1 patients with respect to the severity of the PH, as well as their QTc intervals. This is consistent with the interpretation that QTc prolongation reflects the status of the RV and is not specific for a particular WHO PH Group. Moreover, when we restricted our analyses to only those with Group 1 or Group 1 and 4 PH, a prolonged QTc continued to be an independent predictor of mortality.
Conclusions
In conclusion, both QTc interval and QRS duration are prolonged in patients with PH and this reflects the status of the RV. We show for the first time that a QTc interval ≥480 ms is an independent predictor of worse clinical outcomes. If confirmed by others, the present work identifies an inexpensive and readily detectable abnormality on the surface ECG that can be potentially used to risk stratify PH patients. Whether effective treatment of the underlying PH would shorten the QTc interval and improve clinical outcomes remains to be determined.
Acknowledgements
This work is supported by the National Institute of Health [NIH-RO1-HL071115, 1RC1HL099462-01, UL1RR024999]; the American Heart Association (AHA); and the Roche Foundation for Anemia Research.
Abbreviation List
- CMR
cardiac magnetic resonance imaging
- ECG
electrocardiogram
- LVH
left ventricular hypertrophy
- NT-proBNP
N-Terminal pro Brain Natriuretic Peptide
- PAH
pulmonary arterial hypertension
- PAP
pulmonary artery pressure
- PVR
pulmonary vascular resistance
- QTc
QT interval corrected for heart rate
- RV
right ventricle
- RVEDV
right ventricular end-diastolic volume
- RVEF
right ventricular ejection fraction
- RVESV
right ventricular end-systolic volume
- WHO
world health organization
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35:1079–1087. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 5.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 6.Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 7.Ahearn GS, Tapson VF, Rebeiz A, Greenfield JC., Jr Electrocardiography to define clinical status in primary pulmonary hypertension and pulmonary arterial hypertension secondary to collagen vascular disease. Chest. 2002;122:524–527. doi: 10.1378/chest.122.2.524. [DOI] [PubMed] [Google Scholar]
- 8.Lehtonen J, Sutinen S, Ikaheimo M, Paakko P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest. 1988;93:839–842. doi: 10.1378/chest.93.4.839. [DOI] [PubMed] [Google Scholar]
- 9.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med. 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akgul F, Seyfeli E, Melek I, Duman T, Seydaliyeva T, Gali E, et al. Increased QT dispersion in sickle cell disease: effect of pulmonary hypertension. Acta Haematol. 2007;118:1–6. doi: 10.1159/000100929. [DOI] [PubMed] [Google Scholar]
- 11.Hong-liang Z, Qin L, Zhi-hong L, Zhi-hui Z, Chang-ming X, Xin-hai N, et al. Heart rate-corrected QT interval and QT dispersion in patients with pulmonary hypertension. Wien Klin Wochenschr. 2009;121:330–333. doi: 10.1007/s00508-009-1184-9. [DOI] [PubMed] [Google Scholar]
- 12.Healthcare G. Marquette 12SL ECG Analysis Program Physicians'Guide. 2007. [Google Scholar]
- 13.Talbot S. QT interval in right and left bundle-branch block. Br Heart J. 1973;35:288–291. doi: 10.1136/hrt.35.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 15.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004;2:2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 17.Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(Suppl):81–90. doi: 10.1016/j.jelectrocard.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Kalogeropoulos AP, Georgiopoulou VV, Howell S, Pernetz MA, Fisher MR, Lerakis S, et al. Evaluation of right intraventricular dyssynchrony by two-dimensional strain echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr. 2008;21:1028–1034. doi: 10.1016/j.echo.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Shenkman HJ, Pampati V, Khandelwal AK, McKinnon J, Nori D, Kaatz S, et al. Congestive heart failure and QRS duration: establishing prognosis study. Chest. 2002;122:528–534. doi: 10.1378/chest.122.2.528. [DOI] [PubMed] [Google Scholar]
- 20.Hombach V, Merkle N, Torzewski J, Kraus JM, Kunze M, Zimmermann O, et al. Electrocardiographic and cardiac magnetic resonance imaging parameters as predictors of a worse outcome in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2009;30:2011–2018. doi: 10.1093/eurheartj/ehp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085–1091. doi: 10.1067/mhj.2002.122516. [DOI] [PubMed] [Google Scholar]
- 22.Oikarinen L, Nieminen MS, Viitasalo M, Toivonen L, Jern S, Dahlof B, et al. QRS duration and QT interval predict mortality in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2004;43:1029–1034. doi: 10.1161/01.HYP.0000125230.46080.c6. [DOI] [PubMed] [Google Scholar]
- 23.Spargias KS, Lindsay SJ, Kawar GI, Greenwood DC, Cowan JC, Ball SG, et al. QT dispersion as a predictor of long-term mortality in patients with acute myocardial infarction and clinical evidence of heart failure. Eur Heart J. 1999;20:1158–1165. doi: 10.1053/euhj.1998.1445. [DOI] [PubMed] [Google Scholar]
- 24.Vrtovec B, Delgado R, Zewail A, Thomas CD, Richartz BM, Radovancevic B. Prolonged QTc interval and high B-type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation. 2003;107:1764–1769. doi: 10.1161/01.CIR.0000057980.84624.95. [DOI] [PubMed] [Google Scholar]
- 25.Kowey PR, Friechling TD, Sewter J, Wu Y, Sokil A, Paul J, et al. Electrophysiological effects of left ventricular hypertrophy. Effect of calcium and potassium channel blockade. Circulation. 1991;83:2067–2075. doi: 10.1161/01.cir.83.6.2067. [DOI] [PubMed] [Google Scholar]
- 26.Rials SJ, Wu Y, Ford N, Pauletto FJ, Abramson SV, Rubin AM, et al. Effect of left ventricular hypertrophy and its regression on ventricular electrophysiology and vulnerability to inducible arrhythmia in the feline heart. Circulation. 1995;91:426–430. doi: 10.1161/01.cir.91.2.426. [DOI] [PubMed] [Google Scholar]
- 27.Fischer R, Dechend R, Gapelyuk A, Shagdarsuren E, Gruner K, Gruner A, et al. Angiotensin II-induced sudden arrhythmic death and electrical remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H1242–H1253. doi: 10.1152/ajpheart.01400.2006. [DOI] [PubMed] [Google Scholar]
- 28.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 29.Hoeper MM, Galie N, Murali S, Olschewski H, Rubenfire M, Robbins IM, et al. Outcome after cardiopulmonary resuscitation in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:341–344. doi: 10.1164/ajrccm.165.3.200109-0130c. [DOI] [PubMed] [Google Scholar]
- 30.Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, et al. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol. 2001;38:1137–1142. doi: 10.1016/s0735-1097(01)01496-6. [DOI] [PubMed] [Google Scholar]
- 31.van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008;29:120–127. doi: 10.1093/eurheartj/ehm567. [DOI] [PubMed] [Google Scholar]
- 32.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 33.Vlahakes GJ, Turley K, Hoffman JI. The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation. 1981;63:87–95. doi: 10.1161/01.cir.63.1.87. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S, Cao JM, Tebb ZD, Ohara T, Huang HL, Omichi C, et al. Modulation of QT interval by cardiac sympathetic nerve sprouting and the mechanisms of ventricular arrhythmia in a canine model of sudden cardiac death. J Cardiovasc Electrophysiol. 2001;12:1068–1073. doi: 10.1046/j.1540-8167.2001.01068.x. [DOI] [PubMed] [Google Scholar]
- 35.Nootens M, Kaufmann E, Rector T, Toher C, Judd D, Francis GS, et al. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol. 1995;26:1581–1585. doi: 10.1016/0735-1097(95)00399-1. [DOI] [PubMed] [Google Scholar]
- 36.Schrier RW, Bansal S. Pulmonary hypertension, right ventricular failure, and kidney: different from left ventricular failure? Clin J Am Soc Nephrol. 2008;3:1232–1237. doi: 10.2215/CJN.01960408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rich S, Pogoriler J, Husain AN, Toth PT, Gomberg-Maitland M, Archer SL. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest. 2010;138:1234–1239. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]