Abstract
Mounting evidence has established that diet-induced obesity (DIO) is associated with deficits in hippocampus-dependent memory. The bulk of research studies dealing with this topic have utilized rats fed a high-fat diet as an experimental model. To date, there has been a paucity of research studies that have established whether the memory deficits exhibited in DIO rats can be recapitulated in mice. Moreover, the majority of experiments that have evaluated memory performance in rodent models of DIO have utilized memory tests that are essentially aversive in nature (i.e., Morris water maze). The current study sought to fill an empirical void by determining if mice maintained on a high-fat diet exhibit deficits in two non-aversive memory paradigms: novel object recognition (NOR) and object location memory (OLM). Here we report that mice fed a high-fat diet over 23 weeks exhibit intact NOR, albeit a marked impairment in hippocampus-dependent OLM. We also determined the existence of corresponding aberrations in gene expression within the hippocampus of DIO mice. DIO mice exhibited significant reductions in both SIRT1 and PP1 mRNA within the hippocampus. Our data suggest that mice maintained on a high-fat diet present with impaired hippocampus-dependent spatial memory and a corresponding alteration in the expression of genes that have been implicated in memory consolidation.
Keywords: Obesity, Memory, Insulin Resistance, Object Location Memory, SIRT1
1. Introduction
Obesity is a global epidemic that is marked by myriad medical complications and comorbidities, not the least of which is insulin resistant diabetes (Lazar, 2005). Both cross-sectional and epidemiological studies have found that obesity is correlated with an increased likelihood of developing age-related cognitive decline (Cohen, 2010; Cournot et al., 2006; Gunstad, Lhotsky, Wendell, Ferrucci, & Zonderman, 2010). Empirical evidence has revealed that diet-induced obesity (DIO) contributes to memory impairments in adult rodents (Stranahan & Mattson, 2011). Rats maintained on a high-fat, high-sucrose diet exhibit impaired hippocampus-dependent spatial learning and memory, as measured using the Morris water maze (Molteni, Barnard, Ying, Roberts, & Gomez-Pinilla, 2002; Stranahan, Norman, Lee, Cutler, Telljohann, Egan, & Mattson, 2008b). These aforementioned studies also revealed that the diet-induced memory impairments were associated with reductions in molecules linked to hippocampus-dependent memory formation (e.g., BDNF) (Molteni et al., 2002). Furthermore, rats maintained on a high-fat diet, in the absence of high sucrose, exhibited a similar impairment in water maze performance (Wu, Ying, & Gomez-Pinilla, 2004). Surprisingly, in contrast to rats, mice maintained on a similar high-fat diet did not present with impaired water maze performance (Mielke et al., 2006). The failure of mice maintained on a high-fat diet to exhibit spatial memory deficits might reveal species differences, with rats being more vulnerable to obesity-induced memory impairments than mice (Stranahan & Mattson, 2011). Alternatively, since mice are apparently less adept at learning the water maze task than rats, it might be more difficult to detect a subtle spatial memory deficit in mice on this task (Frick, Stillner, & Berger-Sweeney, 2000; Stranahan & Mattson, 2011).
With only a few exceptions, diet-induced hippocampus-dependent memory deficits have been measured using the traditional “gold standard” test of spatial memory, the Morris water maze. The water maze is considered to be an aversive test, and is particularly stressful (Harrison, Hosseini, & McDonald, 2009). Using the water maze to gauge obesity-induced memory impairments might turn out to be a nontrivial consideration, since there is conjecture in the field regarding whether or not obese rodents present with enhanced HPA-axis activity, a physiological abnormality that might skew performance on an aversive task (Lindqvist et al., 2006; Morton, 2010; Stranahan, Arumugam, Cutler, Lee, Egan, & Mattson, 2008a; Tannenbaum et al., 1997). Addressing these concerns, a recent study reported that mice maintained on a high-fat diet exhibited impaired spatial learning on the radial arm maze (Valladolid-Acebes et al., 2011).
Another candidate memory test that is relatively non-aversive is the novel object recognition paradigm (NOR)—a hippocampus-independent object memory test. To date, a single study has gauged the extent of diet-induced memory impairments in two rat models of obesity using NOR (Jurdak & Kanarek, 2009). Moreover, a non-aversive test of spatial memory test that has yet to be utilized to assess obesity-induced memory impairments is the object location memory paradigm (OLM)—a test of hippocampus-dependent spatial memory (Murai, Okuda, Tanaka, & Ohta, 2007). In designing the present experiments we considered that employing OLM to assay the extent of obesity-induced hippocampus-dependent spatial memory impairments might prove to be a more effective way of detecting subtle spatial memory impairments in mice.
The lion’s share of experiments that have investigated the memory-disrupting effects of obesity have used rats as a model system. With the widespread utilization of genetically engineered mouse models and considering potential species-specific disparities in obesity-induced spatial memory impairments and molecular profiles in the hippocampus, results that have been found in rats need to be recapitulated using a mouse model of obesity. To address the aforementioned concerns, we set out to determine if mice maintained on a high-fat diet exhibit impaired performance on the NOR and OLM memory paradigm. In undertaking these studies we complemented the behavioral approach with a molecular assessment, evaluating memory-associated gene expression profiles in our mouse model of diet-induced obesity as well.
2. Methods
2.1. Animals
12 week old C57BL/6 male mice from Jackson Labs (Taconic, Hudson, NY) were singly housed, with food and water available ad libitum, on a 12:12 h light:dark schedule. Animals were randomly assigned to either a standard lab chow (control animals, Harlan) or a high-fat (diet-induced obese (DIO), 60% fat by calories from lard, Research Diets #D12492) chow and were maintained on this diet until death. The mice were weighed at the onset of the diet and on the day they were euthanized. After having been maintained on their respective diets for 22 weeks mice were subjected to various behavioral tests (described below) before being sacrificed.
2.2 Euthanization and serum collection
After having been maintained on their respective diets for 27 weeks, both control and DIO mice were randomly assigned to one of two groups. Both groups were sacrificed at roughly the same starting time on consecutive days so as to ensure all blood samples would be collected at comparable times during the circadian cycle. Before euthanization mice were food deprived an average of 2 hours, as opposed to over-night fasting, so as to mitigate the possibility of inducing changes in gene expression that might be associated with the stress of over-night fasting. Mice were rapidly decapitated and immediately afterwards trunk blood glucose levels were measured using an Alphatrak glucometer (Abbott, Abbott Park, IL). Serum samples for insulin, leptin, corticosterone, cholesterol and triglyceride measurements were taken from trunk blood following euthanasia and decapitation. After decapitation whole hippocampi were removed by gross dissection, and immediately submerged in ice-cold RNAlater (Life Technologies, Grand Island, NY) before being stored in −80°C.
2.3. Behavior
2.3.1. Open Field
Animals were handled for at least 3 days before the onset of behavioral testing, and they were transported to the laboratory at least one hour prior to the start of each experiment. Locomotion and time in center-square behavior were measured in an open-field arena (43.2 cm × 43.2 cm × 30.5 cm) for 30 min by an automatic video tracking system (Med Associates, St Albans, VT).
2.3.2. Elevated Plus Maze
For the elevated plus-maze, mice were placed on the central platform of an opaque Plexiglass maze that is one meter high with two open arms and two closed arms (Med Associates, St. Albans, VT). Throughout the course of the 5 min trial, entries and time spent in the open and closed arms were measured by an automatic video tracking system (Med Associates, St. Albans, VT).
2.3.3. Novel object recognition task
The experimental apparatus consisted of four separate identical white rectangular open fields (39 cm × 19 cm × 21 cm (height)), and blackened cardboard was taped to the four walls of each arena. On each day of testing, before having been transferred to the room designated for NOR, all mice were initially stored in a nearby storage room within the behavioral facility for at least an hour prior to the start of the NOR paradigm, to promote acclimation to the test facility environment. After having been acclimated in the storage room, four mice at a time were carted to the NOR testing room. Each mouse cage was fitted with blackened foam sleeve during the transfer process. Once in the NOR room, mice were habituated to their respective NOR arenas in the absence of objects for 25 min. Habituation was carried out for three consecutive days. During the training phase, mice were placed in their arenas in the presence of two new identical objects, and were allowed to explore for 20 min. After a 24 h retention period mice were placed again in the apparatus, where this time one of the objects was replaced by a novel one, and allowed to explore for 5 min. During the NOR test the two objects used were the head of a toothbrush and a single square Lego®. This time both variables related to the identity (toothbrush head vs. Lego) and the positioning (left side vs. right side) of the novel object were balanced between groups.
2.3.4. Object location task
24 h following the time of the 24 h NOR test marked the beginning of the object location task paradigm. The experimental apparatus contexts were virtually identical to the ones previously used for NOR except for one important addition—visual cues (electrical tape) were placed on two arena walls. During the OLM training phase mice were placed in their respective arenas in the presence of two identical objects and allowed to explore for 15 min. The objects were all 2 inch long light bulbs fixed to a stabilizing base. After a 24 h retention period mice were placed again in the apparatus, where this time one of the objects was displaced to a novel spatial location, and allowed to explore for 5 min. Both the object being displaced and the spatial location of the displaced object were balanced between groups.
All phases of NOR and OLM were recorded using TopScan (Clever Sys, Reston, VA). In both tasks, each group’s ability to recognize the novel object was determined by dividing the mean time exploring the novel object by the mean of the total time exploring the novel and familiar objects during the test session. This value was multiplied by 100 to obtain a percentage preference for the novel object (Tnovel/[Tnovel + Tfamiliar] × 100).
In both tasks, objects were rinsed with ethanol between trials and before the first trial to remove residual odors. The first 2 min of each test phase were hand-scored by a researcher blinded to the experimental conditions, as there is evidence to suggest that the discrimination between two objects is best in the first 2 minutes of the preference test (Dix & Aggleton, 1999). Mice were deemed to be interacting with an object when facing and sniffing the objects within a very close proximity.
2.3.5. Fear Conditioning
For cue and contextual fear conditioning, animals were placed in the fear-conditioning apparatus for 2 min, and then exposed to a 30 sec acoustic conditioned stimulus (CS; tone). During the last 2 sec of the tone, a single 0.75 mA shock [unconditioned stimulus (US)] was applied to the floor grid. To assess contextual learning, the animals were placed back into the training context 24 hr after training and scored for freezing for 5 min. To assess cue learning, the animals were placed in a different context (novel odor, cage floor, and visual cues) 24 hr after training. Baseline behavior was measured for 3 min in the novel context, and then the acoustic CS was presented for 3 min. All trials were recorded using an automated video tracking system (Med Associates, St. Albans, VT), and scored by a researcher blinded to the experimental conditions. Learning was assessed by measuring freezing behavior (i.e., motionless position). Freezing was scored during conditioning as well as testing. The behavior of each mouse was sampled at 3 s intervals, and the percentage of those intervals in which the mouse froze was calculated.
2.4. Measurement of Endocrine Profiles
Insulin analysis was performed via ultra-sensitive mouse insulin ELISA kit (Crystal Chem, Downers Grove, IL). Insulin resistance was assessed with the homeostasis model: HOMA-IR = fasting glucose level (mg/dl) x fasting insulin level (ng/ml) ÷ 22.5 (Matthews et al., 1985). Corticosterone analysis was accomplished by radioimmunoassay using the ImmuChem Corticosterone DA for rats and mice kit (MP Biomedicals, Costa Mesa, CA). Leptin analysis was accomplished by radioimmunoassay using the Mouse Leptin RIA kit (EMD Millipore, Technical, Billerica, MA). Both cholesterol and triglyceride serum quantification was achieved using the colorimetric method on a Sirrus analyzer (Stanbio Laboratory, Boerne, TX).
2.5. Isolation of RNA
RNA was isolated using the AllPrep DNA/RNA Mini Kit (Qiagen, Venlo, Netherlands). Concentrations were determined spectrophotometrically using the NanoDrop 2000c (Thermo Scientific, Waltham, Massachusetts).
2.6. Real-Time, Quantitative Reverse Transcription PCR (qPCR)
mRNA was reverse transcribed using the SuperScript® VILO™ cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). We used the following Taqman probes for qPCR mRNA analysis: Brain derived neurotrophic factor (BDNF), Sirtuin 1 (SIRT1), Glycogen synthase kinase 3 beta (GSK3b), Insulin-like growth factor 1 (IGF1), Insulin-like growth factor 2 (IGF2), DNA (cytosine-5)-methyltransferase 1 (DNMT1), Histone Deacetylase 5 (HDAC5), Calcineurin A (PPP3CA), Protein phosphatase 1 (PP1), and Beta-actin (ACTB) (Applied Biosystems, Foster City, CA). Probe and primer sequences used to perform the analyses are available upon request. All probes ensured the amplification of only mRNA as they were designed to span exon boundaries. Beta-actin quantification was used as an internal control for normalization. The comparative CT method was used to calculate differences in gene expression between samples (Livak & Schmittgen, 2001; Pfaffl, 2001).
2.7. Statistics
All comparisons were conducted using two-tailed, Student’s unpaired t-tests, with alpha = 0.05 in all cases. N of each experimental group was between 5 and 8.
3. Results
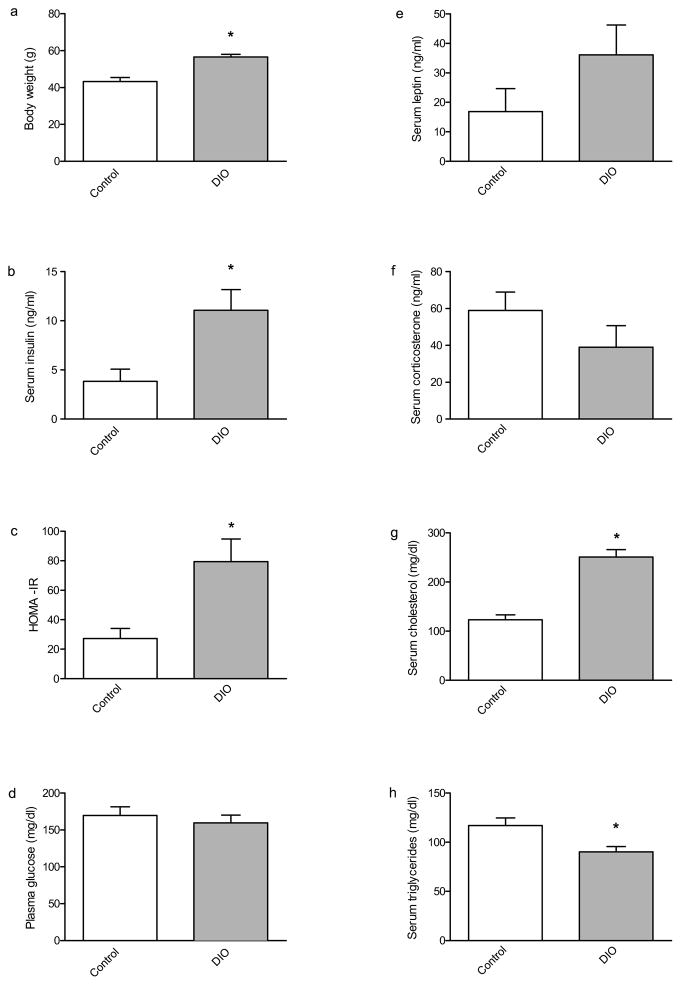
We sought to utilize a mouse model of DIO in order to perform a behavioral and molecular characterization of the effects of high-fat diet on learning and memory and its underlying mechanisms. In our first series of studies we replicated prior work (Winzell & Ahrén, 2004) demonstrating that a high-fat diet causes obesity. Thus, we used C57BL6 mice and compared animals fed normal lab chow (control) versus animals fed a high-fat diet (DIO). There were no differences in weight between control and DIO mice at the start of the experiment (data not shown). However, immediately prior to being euthanized, after having been maintained on their diet for 27 weeks, DIO mice weighed significantly more than their chow-fed counterparts (Fig. 1A).
Fig. 1.
Weight and serum chemistry profile of DIO and control mice. After 27 weeks on the high-fat diet DIO mice weighed significantly more than chow-fed controls; [t(12) = 5.022, p < 0.001]. (b) DIO mice had a significantly higher concentration of serum insulin than controls [t(12) = 2.978, p < 0.05], as well as higher insulin resistance index HOMA-IR (c) than controls [t(12) = 2.983, p < 0.05]. (d) There was no difference in serum glucose levels between control and DIO mice. (e) There was no difference in serum leptin between control and DIO mice. (f) There was no difference in serum corticosterone between control and DIO mice. (g) DIO mice had a significantly higher concentration of serum cholesterol than controls [t(11) = 6.848, p < 0.0001]. (h) DIO mice had a significantly lower concentration of serum triglycerides than controls [t(11) = 2.867, p < 0.05]. Asterisks indicate significant group differences between DIO mice compared to the chow-fed control group, as determined by unpaired t-tests. Error bars indicate s.e.m.
3.1. DIO mice presented with hyperinsulinemia
It was expected that the DIO mice would exhibit blood glucose and other endocrine profiles that are comparable to those typically observed in human patients with metabolic syndrome. Therefore we assessed 2 hour fasting blood levels of glucose, insulin, leptin, corticosterone, cholesterol, and triglycerides. Of particular interest were serum insulin levels, as obesity tends to be comorbid with hyperinsulinemia. DIO insulin levels were significantly higher than those of control mice (Fig. 1B). Furthermore, DIO mice scored significantly higher on the homeostatic assessment index of insulin resistance (Fig. 1C). Surprisingly, there was no difference between the 2 hour fasting blood glucose levels of control and DIO mice (Fig. 1D). No significant difference was detected in either serum leptin or corticosterone between DIO and control mice (Fig. 1E; 1F). DIO mice did present with both a significant elevation in serum cholesterol, and a reduction in triglycerides, compared to controls (Fig. 1G; 1H). Therefore, it was apparent that the DIO mice exhibited many of the characteristic traits associated with obesity.
3.2 Behavioral Characterization
Interested in whether DIO mice would present with impaired memory, we opted to observe performance on both the NOR and OLM behavioral tests of memory. Prior to performing these memory tests we performed a basic behavioral battery to detect potential behavioral abnormalities that might influence performance on NOR and OLM and consequently confound any interpretations drawn from these memory tests.
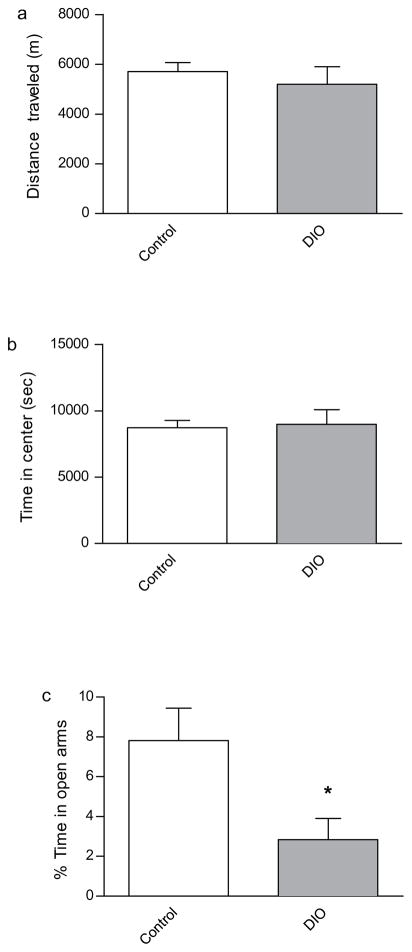
Therefore, we began our behavioral characterization of the DIO mice by gauging their performance on various baseline behavioral tests relative to control mice. To this end, we observed the mice in an open field as a means of assessing locomotor activity. DIO and control mice did not differ in terms of their performance on the open field task, traveling the same distance and spending similar amounts of time in the center of the camber (Fig. 2A; 2B). We interpreted this to indicate that DIO mice did not exhibit locomotor deficits. Furthermore, it is reasonable to suggest that the DIO mice would not have their performance on subsequent behavioral tests beset by locomotor impairments.
Fig. 2.
Open-field and elevated plus-maze behaviors in control and DIO mice. DIO mice did not differ from controls in terms of (a) distance traveled during the open field test or (b) in terms of thigmotaxis. (c) DIO mice spent significantly less time within the open arms of the elevated plus maze than did controls [t(11) = 2.455, p < 0.05]. Asterisks indicate significant group differences between DIO mice compared to the chow-fed control group, as determined by unpaired t-tests. Error bars indicate s.e.m.
Next we assessed performance on the elevated plus maze as a means of acquiring an index of basal anxiety. DIO mice spent significantly less time within the open arms of the maze, compared to control mice, thereby exhibiting a considerable aversion to brightly lit areas—a behavior that is indicative of increased anxiety in DIO mice (Fig. 2C). Therefore, we have reason to suspect the anxiety phenotype exhibited by DIO mice might influence their performance on anxiety-evoking memory tests that are aversive in nature. The potential for this confound further validates the importance of using non-aversive behavioral paradigms to gauge memory performance in DIO mice.
3.3. DIO mice exhibit a selective impairment in hippocampus-dependent spatial memory
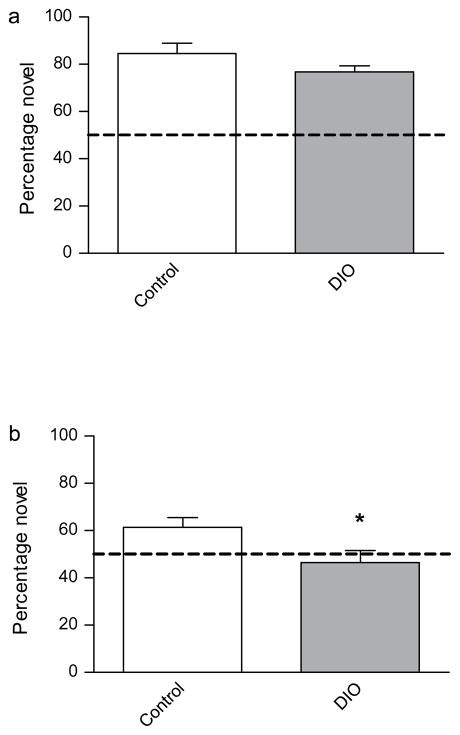
We then sought to access memory using a 24 h version of the NOR paradigm. To our surprise both groups exhibited robust 24 h object recognition memory (Fig. 3A).
Fig. 3.
Tests of novel object recognition (NOR) and object location memory (OLM) in control and DIO mice. (a) No significant difference was observed between control and DIO mice during the NOR test. (b) DIO mice exhibited a significant reduction in OLM relative to control mice [t(12) = 2.292, p < 0.05]. The dotted lines indicate values for random chance (50%). Asterisks indicate significant group differences between DIO mice compared to the chow-fed control group, as determined by unpaired t-tests. Error bars indicate s.e.m.
Suspecting that diet-induced obesity might selectively, and subtly, impair the capacity of the hippocampus to consolidate memory we opted to utilize a variant of NOR that has a considerable spatial component to it and recruits the hippocampus more heavily than NOR—object location memory (OLM) (Balderas et al., 2008). When hippocampus-dependent spatial memory was assessed using the OLM paradigm DIO mice exhibited a marked impairment in the task relative to control mice (Fig 3B). Therefore, we established that DIO mice exhibited a hippocampus-dependent spatial memory deficit.
3.4. DIO have intact fear memory
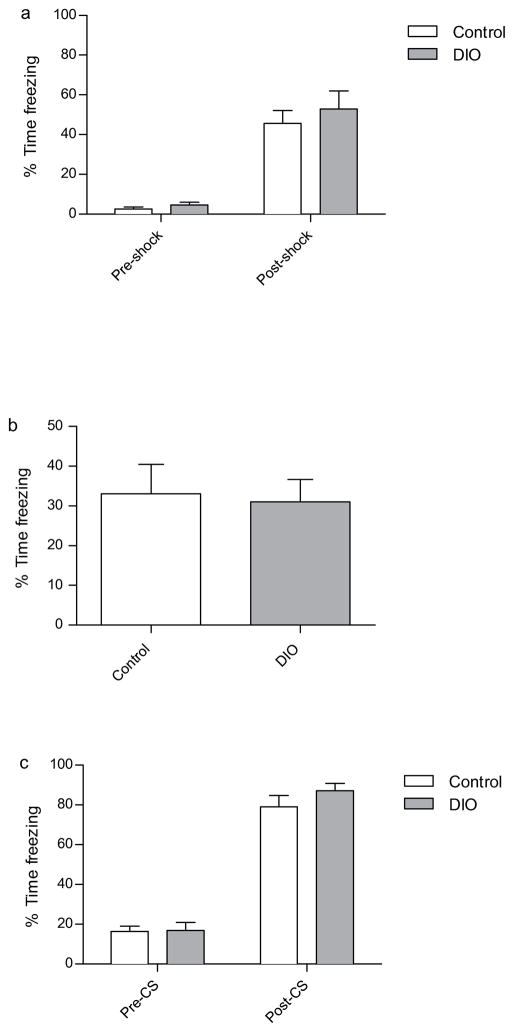
It then became necessary to determine whether DIO mice suffered from a selective deficit in hippocampus-dependent spatial memory, versus a more generalized deficit in overall hippocampus-dependent memory function. If the latter were true, DIO mice should exhibit impaired contextual fear conditioning, a form of hippocampus-dependent memory (Anagnostaras, Gale, & Fanselow, 2001). To this end, mice were tested in 24 h contextual and cued fear conditioning. DIO and control mice froze to an equivalent degree throughout the training trial (figure 4A). DIO and control mice exhibited intact contextual fear memory, and there was no discernible difference between groups (Fig. 4B). This result was interpreted to indicate that DIO mice had intact hippocampus-dependent context memory.
Fig. 4.
Assessment of fear-conditioned memories in control and DIO mice. (a) There was no significant difference between the percent freezing exhibited by control and DIO mice either prior to, or immediately after, cued-fear conditioning. (b) Tests of 24-hour contextual and cued fear conditioned (c) memory failed to detect a significant difference between control and DIO mice. Error bars indicate s.e.m.
Furthermore, DIO mice demonstrated intact cued fear conditioning, which suggests that DIO mice have intact hippocampus-independent memory (Fig. 4C). Upon considering the behavioral data collectively it became apparent that DIO mice possessed a selective deficit in hippocampus-dependent spatial memory.
3.5. DIO mice have reduced hippocampal SIRT1 mRNA expression
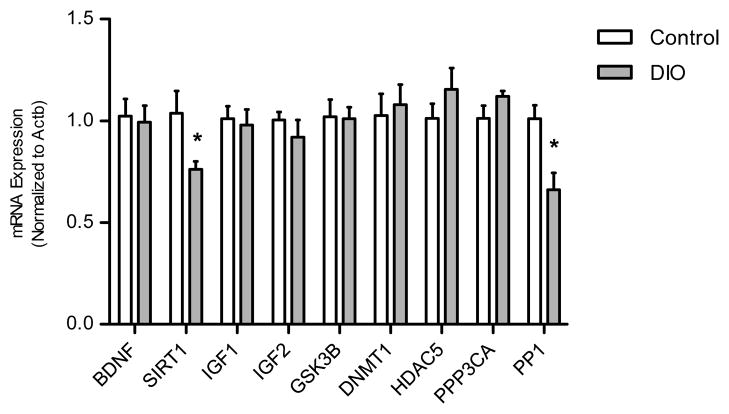
Having obtained evidence that DIO mice present with impaired hippocampus-dependent spatial memory we sought to identify corresponding changes in gene expression, within the hippocampus, that might account for said impairments. To achieve this end, we measured mRNA expression for a variety of gene targets that have been associated with hippocampus-dependent memory consolidation. The gene expression analysis revealed that both SIRT1 and PP1 mRNA were significantly reduced in DIO mice compared to controls (Fig. 5).
Fig. 5.
Hippocampal mRNA expression profile. DIO mice hippocampi had reduced Sirtuin 1 (SIRT1) mRNA relative to that of controls [t(12) = 2.346, p < 0.05], in addition reduced protein phosphatase 1 (PP1) mRNA relative to controls [t(12) = 3.298, p < 0.01]. Other genes evaluated include brain derived neurotrophic factor (BDNF), Glycogen synthase kinase 3 beta (GSK3b), Insulin-like growth factor 1 (IGF1), Insulin-like growth factor 2 (IGF2), DNA (cytosine-5)-methyltransferase 1 (DNMT1), Histone Deacetylase 5 (HDAC5), Calcineurin A (PPP3CA) and Beta-actin (ACTB). Asterisks indicate significant group differences between DIO mice compared to the chow-fed control group, as determined by unpaired t-tests. Error bars indicate s.e.m.
4. Discussion
Mounting empirical evidence has made the case that consuming a high-fat diet has a deleterious effect on memory. We have established that chronic consumption of a high-fat diet contributes to impaired hippocampus-dependent spatial memory in a mouse model of diet-induced obesity. The DIO mice generated in this study presented with moderate hyperinsulinemia (Fig. 1B) that is indicative of insulin resistance. It should be noted that the DIO mice did not present with hyperglycemia when compared to control mice (Fig. 1D). We suspect that had the mice been fasted longer than 2 hours prior to serum collection, thereby allowing for postprandial glucose homeostasis to be achieved, the DIO serum glucose levels might have reflected hyperglycemia. Upon analyzing the relationship between glucose and insulin levels, using the homeostatic assessment index of insulin resistance (HOMA-IR), the DIO mice scored significantly higher than controls (Fig. 1C), a result that is suggestive of the DIO mice having been insulin resistant.
The current study is the first to establish that diet-induced obesity impairs hippocampus-dependent object location memory. We posit that systemic insulin resistance is what drives the spatial memory deficits exhibited by DIO mice. It has previously been established that insulin resistant DIO mice have impaired spatial learning and spatial working memory (McNay et al., 2010; Valladolid-Acebes et al., 2011). McNay et al., (2010), recently published results that strongly implicate insulin resistance as being a critical contributor to high-fat diet-induced memory impairment. McNay et al., (2010) made comparisons between regular chow-fed mice, diet-resistant (DR) mice, which consumed a high-fat diet (HFD) but did not experience systemic hyperinsulinemia and increased body weight, and DIO mice that did experience systemic hyperinsulinemia and increased body weight in response to HFD. In their experiment, control and DR mice did not suffer spatial working memory impairments, whereas DIO mice did; moreover, the dose-response curve for insulin-mediated spatial memory enhancement was similar for both control and DR mice, whereas it was shifted to the right for DIO mice (McNay et al., 2010). Again, these results are consistent with the assertion that obesity-induced insulin resistance contributes to impaired hippocampus-dependent spatial memory. We believe our results serve to echo the above assertion, using a novel behavioral paradigm, object location memory.
It was surprising to find that DIO have a selective impairment in object location memory, while their object recognition memory is spared. Yet, another recent study also reported that mice maintained on a high-fat diet have intact NOR memory exactly as we have observed (Lavin et al., 2011). To account for this finding, it is possible that the hippocampus is more vulnerable to insulin resistance than other brain regions that mediate object recognition. Converging evidence supports the existence of a double dissociation between the perirhinal cortex and the hippocampus for object recognition and object location memory (Balderas et al., 2008; Barker & Warburton, 2011; Mumby, 2002). It is conceivable that insulin resistance would modulate any cognitive process that is mediated by a neural region that is, susceptible to insulin resistance. Furthermore, there is evidence to suggest that the hippocampus is a brain region whose integrity is compromised and whose physiological function is impaired as a result of diet-induced insulin resistance (Stranahan, Norman, Lee, Cutler, Telljohann, Egan, & Mattson, 2008b). Therefore, it would stand to reason that within the context of insulin resistance a task which recruits the hippocampus heavily (i.e., OLM) would be executed suboptimally. Alternatively, a task such as NOR that requires a neural region that is less vulnerable to the effects of systemic insulin resistance than the hippocampus (e.g., possibly the perirhinal cortex) would be executed properly despite systemic insulin resistance. This is merely speculative, but has the potential to account for the impaired hippocampus-dependent spatial memory, but preserved object recognition memory, in our insulin-resistant DIO mice.
Additionally, it stands to reason that high-fat diet induced insulin resistance may simply fail to sufficiently insult brain regions that mediate NOR enough to impaired object recognition, whereas an even more obesogenic diet (e.g., combined high-sucrose and high-fat) might do so. A study by Jurdak and Kanarek (2009) found that rats maintained on a high-sucrose diet exhibited impaired NOR memory, whereas mice maintained on a high-fat diet had intact NOR memory (Jurdak & Kanarek, 2009). This would open up the possibility that diets of differing macronutrient compositions could influence hippocampal function to differing degrees.
During this experiment mice maintained on a high-fat diet exhibited intact hippocampus-dependent contextual fear conditioning. On the surface this particular result contradicts a recent finding that mice maintained on a high-fat diet for 9–12 months have impaired contextual fear conditioning (Hwang et al., 2009). Yet, there are key methodological distinctions that can account for these disparities. The mice used in the Hwang et al. (2009) study were not only placed on their diet much earlier (i.e., immediately post-weaning at 3 weeks of age) than our mice, which were placed on the diet at 12 weeks of age, but they were also maintained on the diet for approximately twice as long. Moreover the memory formed during contextual fear conditioning is rather robust, and requires extensive hippocampus impairment before being disrupted. Considering that deficits in contextual fear conditioning are evidence of a profound impairment of normal hippocampus physiology, it is reasonable to think that the DIO mice in the Hwang et al., (2009) study suffered from a high-fat diet induced hippocampus insult that was more profound than that experienced by our DIO mice. Therefore, it appears that our DIO mice presented with subtle, albeit detectable, hippocampus-dependent memory deficit, whereas the Hwang DIO mice exhibited a more extensive hippocampus-dependent memory deficit. These results are consistent with there being a gradation of diet-induced memory impairments that worsen in severity as a function of high-fat diet age of onset and duration. Therefore, we affirm that the OLM paradigm is an optimal paradigm with which to detect subtle diet-induced hippocampus-dependent memory deficits.
In our molecular studies we made the observation that SIRT1 gene expression was reduced within the hippocampus of DIO mice. These findings lend credence to another study that documented decreased SIRT1 protein within the hippocampus of rats maintained on a high-fat diet (Wu, Ying, & Gomez-Pinilla, 2006). The evidence suggests that high-fat diet induced obesity decreases SIRT1 at the transcriptional or post-transcriptional level. Another study has demonstrated that a high-fat diet reduces the expression of SIRT1 mRNA expression within the liver of high-fat diet fed rats (Costa et al., 2010). Cross-experimental comparisons imply that reduced hippocampal SIRT1 may be reflective of systemic aberrations in the regulation of SIRT1 expression within the context of high-fat diet induced obesity.
Moreover, mounting evidence has implicated SIRT1 as a member of the molecular milieu that is permissive for learning and memory (Gao et al., 2010; Michan et al., 2010). Future experiments will be needed to determine whether the high-fat diet induced reductions of SIRT1 within the hippocampus drive the spatial memory impairments exhibited by DIO mice.
The lack of a high-fat diet related reduction in hippocampal BDNF mRNA somewhat contradicts previously published results, and is therefore perplexing (Molteni et al., 2002). One possible explanation for this discrepancy is that the aforementioned studies were conducted in a rat, rather than mouse, model of obesity. Also, it is conceivable that mice need to be maintained on a high-fat diet longer than the time course used in the current experiment before exhibiting reductions in BDNF mRNA expression. Therefore, these results still allow for the possibility that diet-induced obesity affects BDNF gene expression within the mouse hippocampus.
Lastly, we observed a significant reduction in PP1 mRNA expression within the hippocampus of DIO mice. To our knowledge this is the first time this result has been demonstrated. At present, it is not clear what role, if any, reduced PP1 mRNA expression might be having in the DIO spatial memory impairment phenotype. There is evidence suggestive of PP1 being disruptive to hippocampus-dependent memory consolidation (Genoux et al., 2002). Moreover, reduced PP1 gene expression has been characterized as being memory-permissive (Miller & Sweatt, 2007). Bearing this in mind, we did not expect the spatial memory impaired DIO mice to exhibit a decrease in PP1, but rather an increase. Therefore, it is currently unclear as to whether the reduced PP1 expression is contributing to the OLM memory deficit exhibited in the DIO mice. Future experiments need to be conducted in order to elucidate the role that both SIRT1 and PP1 play in the high-fat diet-induced hippocampus-dependent spatial memory dysfunction.
Using a mouse model of diet-induced obesity (DIO) we sought to detect memory impairments.
DIO mice exhibited a selective impairment in hippocampus-dependent object location memory.
DIO mice also exhibited reduced hippocampal SIRT1 and PP1 mRNA expression.
Acknowledgments
We would like to thank Erin Johnson, Alicia Hall who were instrumental in our optimizing the novel object paradigms, and Dr. Erik Roberson for allowing us to use his behavioral apparatus. This work was supported by a grant award from the National Heart, Lung, and Blood Institute (T32HL105349) to FDH, by the NIH (MH57014 to JDS; DK038765 and DK083562 to WTG) and the Merit Review program of the Department of Veterans Affairs (to WTG). We also acknowledge support from the UAB Diabetes and Research Training Center (P60DK079626) and the Nutrition Obesity Research Center (P30DK56336).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learning & Memory. 2008;15(9):618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. When Is the Hippocampus Involved in Recognition Memory? Journal of Neuroscience. 2011;31(29):10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA. Obesity-associated cognitive decline: excess weight affects more than the waistline. Neuroepidemiology. 2010;34(4):230–231. doi: 10.1159/000297745. [DOI] [PubMed] [Google Scholar]
- Costa CDS, Hammes TO, Rohden F, Margis R, Bortolotto JW, Padoin AV, Mottin CC, et al. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obesity surgery. 2010;20(5):633–639. doi: 10.1007/s11695-009-0052-z. [DOI] [PubMed] [Google Scholar]
- Cournot M, Marquié JC, Ansiau D, Martinaud C, Fonds H, Ferrières J, Ruidavets JB. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67(7):1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behavioural Brain Research. 1999;99(2):191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11(16):3461–3465. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466(7310):1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418(6901):970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34(4):222–229. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behavioural Brain Research. 2009;198(1):247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, et al. Sex Differences in High-fat Diet-induced Obesity, Metabolic Alterations and Learning, and Synaptic Plasticity Deficits in Mice. Obesity. 2009;18(3):463–469. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- Jurdak N, Kanarek RB. Physiology & Behavior. 1. Vol. 96. Elsevier Inc; 2009. Sucrose-induced obesity impairs novel object recognition learning in young rats; pp. 1–5. [DOI] [PubMed] [Google Scholar]
- Lavin DN, Joesting JJ, Chiu GS, Moon ML, Meng J, Dilger RN, Freund GG. Fasting induces an anti-inflammatory effect on the neuroimmune system which a high-fat diet prevents. Obesity (Silver Spring, Md) 2011;19(8):1586–1594. doi: 10.1038/oby.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307(5708):373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. European Journal of Neurology. 2006;13(12):1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Neurobiology of Learning and Memory. 4. Vol. 93. Elsevier Inc; 2010. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance; pp. 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MMH, Parrella E, Ge H, Long JM, Allard JS, et al. SIRT1 Is Essential for Normal Cognitive Function and Synaptic Plasticity. Journal of Neuroscience. 2010;30(29):9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke JG, Nicolitch K, Avellaneda V, Earlam K, Ahuja T, Mealing G, Messier C. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behavioural Brain Research. 2006;175(2):374–382. doi: 10.1016/j.bbr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent Modification of DNA Regulates Memory Formation. Neuron. 2007;53(6):857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112(4):803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Morton NM. Obesity and corticosteroids: 11beta-hydroxysteroid type 1 as a cause and therapeutic target in metabolic disease. Molecular and cellular endocrinology. 2010;316(2):154–164. doi: 10.1016/j.mce.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Mumby DG. Hippocampal Damage and Exploratory Preferences in Rats: Memory for Objects, Places, and Contexts. Learning & Memory. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai T, Okuda S, Tanaka T, Ohta H. Characteristics of object location memory in mice: Behavioral and pharmacological studies. Physiology & Behavior. 2007;90(1):116–124. doi: 10.1016/j.physbeh.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Bidirectional metabolic regulation of neurocognitive function. Neurobiology of Learning and Memory. 2011;96(4):507–516. doi: 10.1016/j.nlm.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nature Neuroscience. 2008a;11(3):309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008b;18(11):1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol Endocrinol Metab. 1997;273:1168–1177. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- Valladolid-Acebes I, Stucchi P, Cano V, Fernández-Alfonso MS, Merino B, Gil-Ortega M, Fole A, Morales L, Ruiz-Gayo M, Del Olmo N. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiology of Learning and Memory. 2011;95(1):80–85. doi: 10.1016/j.nlm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–9. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. The European journal of neuroscience. 2004;19(7):1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Oxidative stress modulates Sir2α in rat hippocampus and cerebral cortex. European Journal of Neuroscience. 2006;23(10):2573–2580. doi: 10.1111/j.1460-9568.2006.04807.x. [DOI] [PubMed] [Google Scholar]