Abstract
Introduction
Radiation therapy (RT) for prostate cancer is frequently associated with post-treatment erectile dysfunction (ED).
Aim
To investigate whether injection of adipose-derived stem cells (ADSCs) can ameliorate RT-associated ED.
Methods
Thirty male rats were divided into 3 groups. The Control+PBS group received tail-vein injection of phosphate-buffered saline (PBS). The Radiation+PBS group received radiation over the prostate and tail-vein injection of PBS. The Radiation+ADSC group received radiation over the prostate and tail-vein injection of ADSCs, which were labeled with 5-ethynyl-2-deoxyuridine (EdU). Seventeen weeks later erectile function was evaluated by intracavernous pressure (ICP) in response to electrostimulation of cavernous nerves (CN). Penile tissue and major pelvic ganglia (MPG) were examined by immunofluorescence (IF) and EdU staining.
Main Outcome Measures
Erectile function was measured by ICP. Protein expression was examined by IF, followed by image analysis and quantification.
Results
Radiation over the prostate caused a significant decrease in erectile function and in the expression of neuronal nitric oxide synthase (nNOS) in penis and MPG. Cavernous smooth muscle (CSM) but not endothelial content was also reduced. Injection of ADSCs significantly restored erectile function, nNOS expression, and CSM content in the irradiated rats. EdU-positive cells were visible in MPG.
Conclusions
Radiation appears to cause ED via CN injury. ADSC injection can restore erectile function via CN regeneration.
Introduction
Radiotherapy (RT) is an effective and common treatment for prostate cancer; however, it is frequently associated with post-treatment erectile dysfunction (ED) [1–3]. While the etiology of post-RT ED remains unclear, clinical studies have suggested that radiation might damage blood vessels and nerves that supply the penile tissues [1–3]. In addition, studies using RT animal models have shown that radiation caused a reduction of nNOS-containing erectile nerves [4,5] and fibrosis of penile arteries [5,6]. Thus, similar to radical prostatectomy (RP), RT may cause ED via the destruction of cavernous nerves (CN) [1–3].
RT-induced ED is most commonly treated with phosphodiesterase-5 inhibitors (PDE5Is) [1–3]. However, PDE5Is are ineffective in approximately one-third of patients and can produce adverse side effects such as headache and flushing [1–3]. As such, there is a need to identify alternative treatments for patients who cannot be effectively treated with PDE5Is. Previously we and others have demonstrated in animal models that RP-associated ED could be effectively treated with various types of stem cells (SCs) [7–11]. Specifically, after the induction of ED via CN transection or crush injury, intracavernous (IC) injection of SCs significantly improved erectile function when compared to saline-treated controls. Furthermore, the improved erectile function was accompanied by regeneration of nNOS-containing nerves in the corpus cavernosum. Thus, SC therapy has the potential to restore the natural erectile capability, as opposed to symptomatic relief afforded by agents such as PDE5Is.
In the present study we tested two hypotheses: (1) RT can cause ED via destruction of nNOS-containing nerves, and (2) intravenous (IV) injection of adipose-derived stem cells (ADSCs) can restore erectile function via regeneration of nNOS-containing nerves.
Materials and methods
Animal groups
Thirty-five 12-weeks-old Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA, USA). Five of them were used for the purpose of ADSC isolation while the remaining ones were used for the actual experimentation. The Control+PBS group (n=6) received tail-vein injection of phosphate-buffered saline (PBS). The Radiation+PBS group (n=12) received radiation over the prostate and tail-vein injection of PBS. The Radiation+ADSC group (n=12) received radiation over the prostate and tail-vein injection of ADSCs. All procedures were approved by Institutional Animal Care and Use Committee. Note that in our previous study [12] injection of ADSCs into normal rats (equivalent of a Control+ADSC group) did not have an effect on the erectile function.
ADSC isolation and labeling
ADSCs were isolated from paratesticular fat and cultured as described previously [7,12]. They were labeled with thymidine analog 5-ethynyl-2-deoxyuridine (EdU) (Invitrogen, Carlsbad, CA, USA) for 48 h before injection.
Radiation and ADSC injection
Initially, rats in the Radiation+PBS and Radiation+ADSC groups were irradiated according to van der Wielen et al [6]. However, half of the rats died within two weeks. Thus, we reduced both the radiation dosage and window size, as described in the following procedure. The rats were anesthetized with ketamine (100mg/kg) and midazolam (5mg/kg). A 3-mm-thick lead shield with a 25mm×30mm window was used to limit the radiation to the prostate. Radiation was delivered with a J.L. Shepherd & Associates Mark I radiator (Model: 68-A, San Fernando, CA, USA) operating at 662 keV with a dose rate of 1.65 Gy/min. For the first radiation session, 4 Gy/day was delivered for 5 consecutive days. One week later, the rats were anesthetized with isoflurane and injected through the tail vein with one million ADSCs in 400μl PBS or with 400μl PBS alone. Eleven weeks later, the rats were irradiated with 2 Gy/day for 5 consecutive days. Another 6 weeks later, the rats were evaluated for erectile function.
Erectile function evaluation
Intracavernous pressure (ICP) in response to electrostimulation of the CN was used to evaluate erectile function. Briefly, under ketamine (100mg/kg) and midazolam (5mg/kg) anesthesia, the major pelvic ganglion (MPG) and CN were exposed via midline laparotomy. The corpus cavernosum was cannulated with a heparinized (200U/ml) 25G needle connected to a pressure transducer (Utah Medical Products, Midvale, UT, USA). The stimulus parameters were 20Hz, pulse width of 0.2 ms, and duration of 50s with three different current settings: 0.5 mA, 1.0 mA and 1.5 mA. The maximum increase of ICP of three stimuli per side was selected for statistical analysis in each animal. ICP was normalized to mean arterial pressure (MAP), which was recorded using a 25G needle inserted into the aortic bifurcation.
Histology
At the conclusion of erectile function evaluation, the rats were sacrificed and their penis and MPG harvested for histology. The mid-shaft of the penis and MPG were fixed with 2% formaldehyde and 0.002% picric acid in 0.1M PBS for 4 hours, followed by immersion in 30% sucrose in PBS overnight at 4°C. The fixed tissues were then embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA), cut into 5 μm-thick sections, mounted on glass slides (3 sections per slide), and subjected to immunofluorescent (IF) and EdU staining. For IF staining, the slides were placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining this solution from the tissue section, the slides were incubated at room temperature with rabbit anti-nNOS (1:100, Santa Cruz Biotechnologies, Santa Cruz, CA, USA), mouse anti-α smooth muscle actin (α-SMA) (1:1000, Sigma-Aldrich, St. Louis, MO, USA), mouse anti-S100 (1:500, Chemicon, Temecula, CA, USA), or mouse anti-von Willebrand (vWF) (1:400, Abcam Inc., Cambridge, MA, USA) overnight. Control tissue sections were similarly prepared except no primary antibody was added. After rinses with PBS, the sections were incubated with Alexa-488- or Alexa-594-conjugated secondary antibody (Invitrogen). After rinses with PBS, the slides were incubated with freshly made Click-iT reaction cocktail containing Alexa-594 (Invitrogen) for 30 min at room temperature without light followed by staining with 4',6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 μg/ml, Sigma-Aldrich).
Image analysis and quantification
The stained tissues were examined with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera using the ACT-1 software (Nikon Instruments Inc., Melville, NY). For evaluation of cavernous smooth muscle and endothelium contents, two fields (both sides of the corpus cavernosum at 100X magnification) on each tissue section were photographed. For evaluation of arterial endothelium content, two fields (both dorsal arteries at 400X magnification) on each tissue section were photographed. For evaluation of dorsal nerve nNOS expression, two fields (the two largest dorsal nerve branches at 400X magnification) on each tissue section were photographed. For evaluation of MPG nNOS expression, two random fields at 400X magnification on each tissue section were photographed. In each of these photographic recordings the images were generated in green, red, and blue channels, and these single-color images were then superimposed to generate the multi-color figures. For quantification, the single-color images were analyzed with Image-Plus 5.1 software (Media Cybernetics, Bethesda, MD, USA). To quantify cavernous smooth muscle, α-SMA-stained area (in green) was measured and expressed as a percentage of the entire corpus cavernosa. To quantify cavernous endothelium, vWF-stained area (in red) was measured and expressed as a percentage of the entire corpus cavernosa. To quantify arterial endothelium, vWF-stained area (in red) was measured and expressed as a ratio (in percentage) to the α-SMA-stained area. To quantify nNOS in dorsal nerves, nNOS-stained area (in red) was measured and expressed as a percentage of the entire dorsal nerve. To quantify nNOS in MPG, nNOS-positive cells (in red) per 400× field were counted.
Statistical analysis
Data were analyzed using Prism 4 (GraphPad Software, San Diego, CA, USA) and expressed as mean ± standard error of mean (SEM). Multiple groups were compared using one-way analysis of variance followed by the Tukey-Kramer test for post-hoc comparisons. Statistical significance was set at p < 0.05.
Results
ADSCs restore radiation-impaired erectile function
Radiation over the prostate significantly impaired erectile function as seen in the sharp decline of the ICP/MAP value in radiated rats versus normal control (Fig. 1). Injection of ADSCs significantly restored erectile function to levels similar to normal control.
Figure 1. Evaluation of erectile function.
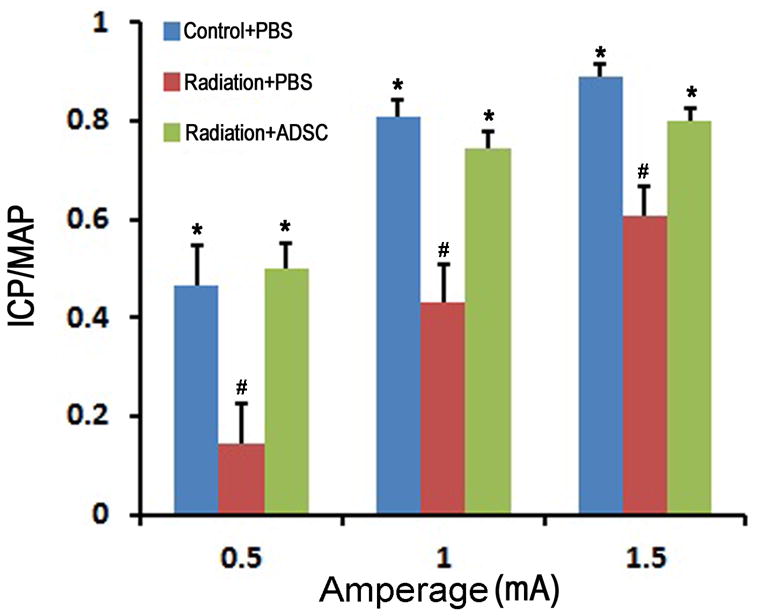
Rats in Control+PBS group (n=6) received tail-vein injection of PBS. Rats in Radiation+PBS group (n=12) received radiation over the prostate and tail-vein injection of PBS. Rats in Radiation+ADSC group (n=12) received radiation over the prostate and tail-vein injection of ADSCs. Their erectile function was evaluated as response in ICP to electrostimulation of CN at three different amperages (0.5, 1.0, and 1.5). ICP was normalized with MAP. # and * denote p<0.05 compared to the Control+PBS and Radiation+PBS groups, respectively.
ADSCs promote nerve regeneration
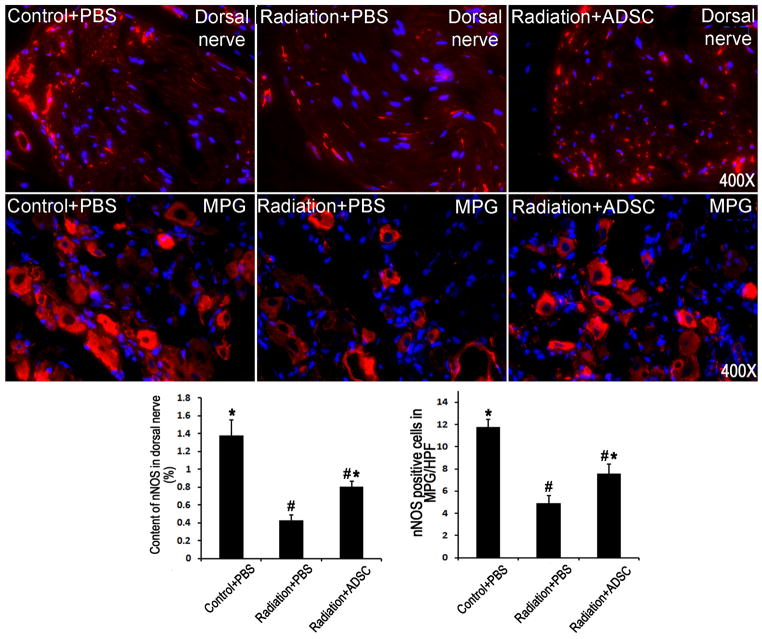
Radiation over the prostate caused significant decreases of nNOS-containing nerves in the penis and nNOS-expressing neurons in the MPG (Fig. 2). Injection of ADSCs partially but significantly restored these nNOS-positive nerves and neurons.
Figure 2. Evaluation of nNOS expression in penile dorsal nerves and MPG.
Rats in Control+PBS group (n=6) received tail-vein injection of PBS. Rats in Radiation+PBS group (n=12) received radiation over the prostate and tail-vein injection of PBS. Rats in Radiation+ADSC group (n=12) received radiation over the prostate and tail-vein injection of ADSCs. Their penile tissues and MPG were examined by immunofluorescence staining for nNOS expression. The results are shown in the representative histological images with red and blue stains indicating nNOS expression and cell nuclei, respectively. Quantification of nNOS expression in penile dorsal nerves is shown in the left bar chart. Numbers of nNOS-positive cells in MPG are shown in the right bar chart. # and * denote p<0.05 compared to the Control+PBS and Radiation+PBS groups, respectively.
ADSCs improve cavernous smooth muscle content
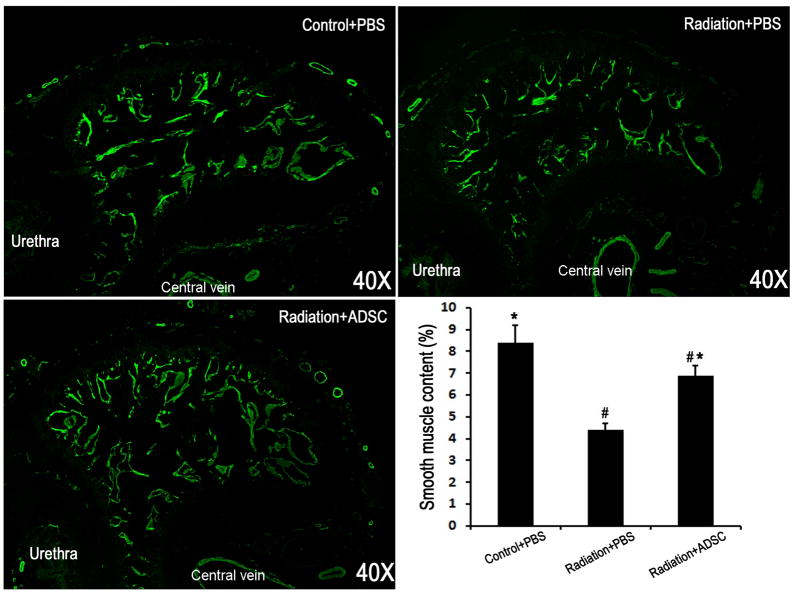
Radiation over the prostate caused a significant decrease of CSM (Fig. 3). Injection of ADSCs partially but significantly restored the CSM content.
Figure 3. Evaluation of smooth muscle content in corpus cavernosum.
Rats in Control+PBS group (n=6) received tail-vein injection of PBS. Rats in Radiation+PBS group (n=12) received radiation over the prostate and tail-vein injection of PBS. Rats in Radiation+ADSC group (n=12) received radiation over the prostate and tail-vein injection of ADSCs. Their penile tissues were examined by immunofluorescence staining for α-SMA expression. The results are shown in the representative histological images with green stain indicating α-SMA expression. Quantification of α-SMA expression in corpus cavernosa is shown in the bar chart. # and * denote p<0.05 compared to the Control+PBS and Radiation+PBS groups, respectively.
Radiation does not affect endothelial content
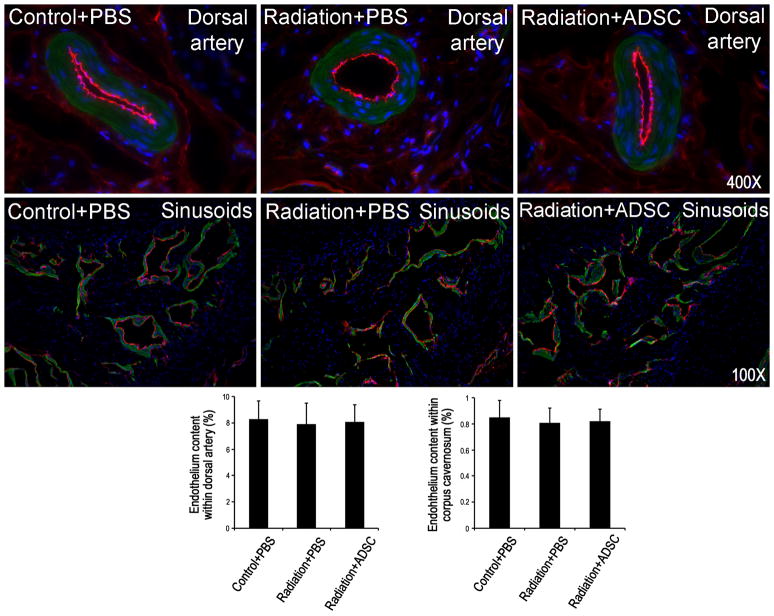
Radiation over the prostate had no effect on the endothelial content in either the dorsal arteries or cavernous sinusoids (Fig. 4). Injection of ADSCs did not affect these endothelial contents either.
Figure 4. Evaluation of endothelial content in dorsal arteries and corpus cavernosa.
Rats in Control+PBS group (n=6) received tail-vein injection of PBS. Rats in Radiation+PBS group (n=12) received radiation over the prostate and tail-vein injection of PBS. Rats in Radiation+ADSC group (n=12) received radiation over the prostate and tail-vein injection of ADSCs. Their penile tissues were examined by immunofluorescence staining for vWF and α-SMA expression. The results are shown in the representative histological images with red, green, and blue stains indicating vWF,α-SMA, and cell nuclei, respectively. Quantification of vWF expression in dorsal arteries and corpus cavernosa is shown in the left and right bar charts, respectively.
IV-injected ADSCs migrate to MPG
Seventeen weeks after their IV injection, ADSCs were undetectable in the penis but were scantly visible in the MPG (Fig. 5).
Figure 5. Identification of transplanted ADSCs in MPG.
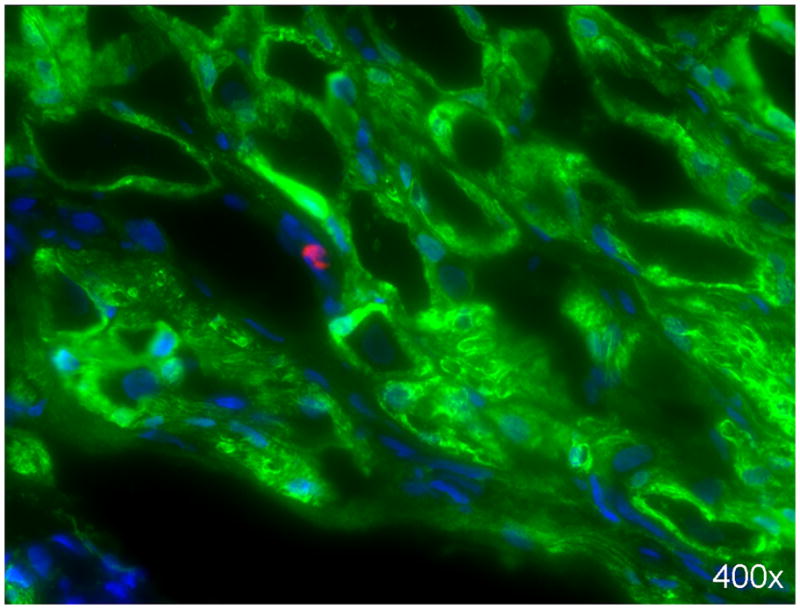
ADSCs were labeled with EdU prior to their injection into rats in the Radiation+ADSC group. Seventeen weeks later, a few ADSCs (red stain) were visible in the MPG, in juxtaposition to S100-stained (green) nerves. Cell nuclei are stained blue.
Discussion
Although highly effective in treating prostate cancer, RT and RP are both frequently associated with post-treatment ED. However, while RP-associated ED has been studied extensively, RT-associated ED has received little attention in terms of animal model-based studies. Likewise, while there is a large body of literature in the experimental treatment of RP-associated ED, no such studies for RT-associated ED exist. As such, the present study was undertaken to address these deficiencies in ED research.
Despite using different radiation protocols, all animal studies, including the present study, have confirmed clinical findings that radiation over the prostate can cause ED [4,5,13,14]. However, among those studies that have conducted histological examination of the penis, their focuses differ and outcomes vary. First, Carrier et al found decreased nNOS-containing nerves in the proximal penises of rats 5 months after a single irradiation of 10 or 20 Gy [4]. Second, the study by van der Wielen et al, which did not measure erectile function, described reduced smooth muscle and increased collagen in dorsal arteries and cavernosal arteries in the proximal penises of rats 2 to 9 weeks after fractionated irradiation of 7.4 Gy/day for 5 consecutive days [6]. Whether there were changes in the nerves, cavernous smooth muscle (CSM), or endothelium was not mentioned. Third, in a recent study by Kimura et al, a single fraction of 20 Gy was delivered to rat prostate [5]. Four weeks later reduced nNOS expression was noted in CN, and 9 weeks later reduced expression of smooth muscle actin and eNOS was noted in the corpus cavernosum while CD31 expression was unaffected. In the present study, we also noted reduced expression of nNOS and smooth muscle actin as well as unchanged endothelial vWF expression. Thus, it appears that radiation on the prostate causes CN injury, and which leads to CSM atrophy. This hypothesis, while requiring further investigation, suggests that both RP- and RT-associated ED are mediated by similar mechanisms, namely, initial CN injury and subsequent CSM atrophy.
RP-associated ED has been the subject of experimental SC therapy in recent years, and the overall outcome appears promising [15]. However, the mechanism responsible for such therapeutic efficacy remains poorly understood. In particular we have raised the question on how the commonly employed intracavernous (IC) injection of SC can lead to regeneration of CN when the nerve cell bodies are located some distance away in the major pelvic ganglia (MPG) [16]. By tracking fluorescently labeled SCs we discovered that IC injected SCs traveled to bone marrow and MPG [12,16]. We thus suggested that, due to similarities between cavernous sinusoids and blood vessels, IC injection is similar to IV injection, and IC injected SCs therefore can travel through bloodstream to various organs and tissues [16]. We also discovered that the MPG of CN injury rats expressed SC homing factor SDF-1, suggesting its role in attracting IC injected SCs to the MPG [12]. In another study we showed that ADSCs exerted neurotrophic effects on CN by secreting CXCL5 cytokine at a high level [17]. Together, these studies establish that CN injury induces SDF-1 expression in MPG, thereby attracting SC migration, and SCs secrete neurotrophic factors, thereby promoting CN regeneration.
In the present study we first confirmed previous findings [4,5] that RT can cause CN injury. We then demonstrated for the first time that IV-injected ADSCs could travel to MPG, repair CN, rebuild CSM, and restore erectile function. Because ADSCs can be easily and safely isolated in large quantity, their transplantation through IC or IV injection should represent a promising therapy for RT-associated ED. However, it should be cautioned that the present study is still preliminary and requires further validation. For example, to better simulate clinical situations, we need to test whether SC injection can prevent the progression of CN degeneration by conducting a single-course radiation (i.e., without the post-ADSC radiation, which was designed to test ADSC’s “protective” effects). We also need to test various cell dosages and time points in order to validate the present study and identify the optimal therapeutic regimen.
Conclusions
Radiation over the prostate can cause ED via CN injury. IV injection of ADSCs can restore erectile function via CN regeneration.
Acknowledgments
This work was funded by NIDDK/NIH R37 DK045370 and by California Urology Foundation. MA is a fellow of the Research Foundation – Flanders (FWO) and a scholar of the Federico Foundation.
References
- 1.Akbal C, Tinay I, Simsek F, Turkeri LN. Erectile dysfunction following radiotherapy and brachytherapy for prostate cancer: pathophysiology, prevention and treatment. Int Urol Nephrol. 2008;40:355–363. doi: 10.1007/s11255-007-9247-1. [DOI] [PubMed] [Google Scholar]
- 2.Candy B, Jones L, Williams R, Tookman A, King M. Phosphodiesterase type 5 inhibitors in the management of erectile dysfunction secondary to treatments for prostate cancer: findings from a Cochrane systematic review. BJU Int. 2008;102:426–431. doi: 10.1111/j.1464-410X.2008.07668.x. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Henderson RH, Indelicato DJ, Keole SR, Mendenhall NP. Erectile dysfunction after radiotherapy for prostate cancer. Am J Clin Oncol. 2009;32:443–447. doi: 10.1097/COC.0b013e318173a563. [DOI] [PubMed] [Google Scholar]
- 4.Carrier S, Hricak H, Lee SS, Baba K, Morgan DM, Nunes L, Ross GY, Phillips TL, Lue TF. Radiation-induced decrease in nitric oxide synthase--containing nerves in the rat penis. Radiology. 1995;195:95–99. doi: 10.1148/radiology.195.1.7534430. [DOI] [PubMed] [Google Scholar]
- 5.Kimura M, Yan H, Rabbani Z, Satoh T, Baba S, Yin FF, Polascik TJ, Donatucci CF, Vujaskovic Z, Koontz BF. Radiation-induced erectile dysfunction using prostate-confined modern radiotherapy in a rat model. J Sex Med. 2011;8:2215–2226. doi: 10.1111/j.1743-6109.2011.02351.x. [DOI] [PubMed] [Google Scholar]
- 6.van der Wielen GJ, Vermeij M, de Jong BW, Schuit M, Marijnissen J, Kok DJ, van Weerden WM, Incrocci L. Changes in the penile arteries of the rat after fractionated irradiation of the prostate: a pilot study. J Sex Med. 2009;6:1908–1913. doi: 10.1111/j.1743-6109.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 7.Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, Lue TF. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, Lue TF. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904–909. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- 9.Fall PA, Izikki M, Tu L, Swieb S, Giuliano F, Bernabe J, Souktani R, Abbou C, Adnot S, Eddahibi S, Yiou R. Apoptosis and effects of intracavernous bone marrow cell injection in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2009;56:716–725. doi: 10.1016/j.eururo.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 10.Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJ, Spees JL. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010;184:1560–1566. doi: 10.1016/j.juro.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo JC, Bae WJ, Kim SJ, Kim SD, Sohn DW, Hong SH, Lee JY, Hwang TK, Sung YC, Kim SW. Transplantation of muscle-derived stem cells into the corpus cavernosum restores erectile function in a rat model of cavernous nerve injury. Korean J Urol. 2011;52:359–363. doi: 10.4111/kju.2011.52.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, Lue TF, Lin CS. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61:201–210. doi: 10.1016/j.eururo.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koontz BF, Yan H, Kimura M, Vujaskovic Z, Donatucci C, Yin FF. Feasibility study of an intensity-modulated radiation model for the study of erectile dysfunction. J Sex Med. 2011;8:411–418. doi: 10.1111/j.1743-6109.2010.02125.x. [DOI] [PubMed] [Google Scholar]
- 14.Merlin SL, Brock GB, Begin LR, Hiou Tim FF, Macramalla AN, Seyam RM, Shenouda G, Dion SB. New insights into the role of endothelin-1 in radiation-associated impotence. Int J Impot Res. 2001;13:104–109. doi: 10.1038/sj.ijir.3900652. [DOI] [PubMed] [Google Scholar]
- 15.Lin CS, Xin ZC, Wang Z, Deng C, Huang YC, Lin G, Lue TF. Stem Cell Therapy for Erectile Dysfunction: A Critical Review. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin G, Qiu X, Fandel T, Banie L, Wang G, Lue TF, Lin CS. Tracking intracavernously injected adipose-derived stem cells to bone marrow. Int J Impot Res. 2011;23:268–275. doi: 10.1038/ijir.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin CS. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J Sex Med. 2011;8:437–446. doi: 10.1111/j.1743-6109.2010.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]