Abstract
Microinfusions of the nonselective muscarinic antagonist scopolamine into perirhinal cortex impairs performance on visual recognition tasks, indicating that muscarinic receptors in this region play a pivotal role in recognition memory. To assess the mnemonic effects of selective blockade in perirhinal cortex of muscarinic receptor subtypes, we locally infused either the m1-selective antagonist pirenzepine or the m2-selective antagonist methoctramine in animals performing one-trial visual recognition, and compared these scores with those following infusions of equivalent volumes of saline. Compared to these control infusions, injections of pirenzepine, but not of methoctramine, significantly impaired recognition accuracy. Further, similar doses of scopolamine and pirenzepine yielded similar deficits, suggesting that the deficits obtained earlier with scopolamine were due mainly, if not exclusively, to blockade of m1 receptors. The present findings indicate that m1 and m2 receptors have functionally dissociable roles, and that the formation of new visual memories is critically dependent on the cholinergic activation of m1 receptors located on perirhinal cells.
Keywords: pirenzepine, methoctramine, muscarinic, cholinergic, perirhinal cortex
Introduction
Visual recognition memory depends on activation of cholinergic muscarinic receptors in the perirhinal cortex, as evidenced by the memory impairment that is produced in both rodents and monkeys by intraperirhinal injections of the muscarinic receptor antagonist scopolamine (Tang, Mishkin, & Aigner, 1997; Warburton et al., 2003). Furthermore, this drug-induced impairment is known to result from interference with memory storage as opposed to memory retrieval, since scopolamine is effective when administered shortly before stimulus familiarization but not when it is administered in the period between familiarization and test (Aigner, Walker, & Mishkin, 1991). Although the above findings establish muscarinic receptors as critical players in memory formation, these receptors consist of several different subtypes, and, because scopolamine is a nonselective muscarinic antagonist, it is still unknown which subtypes are the essential ones for the storage of visual memories. The present study aimed to address this issue.
Muscarinic receptors are a non-homogeneous class of G-protein-coupled receptors, composed of five discrete subtypes, m1-m5 (Bonner, 1989; Caulfield, 1993; Wess, 1996). The five subtypes divide naturally into two groups on the basis of their cellular and molecular effects. One group, M1, consists of the m1, m3, and m5 subtypes, which couple to Gq/11 and thereby produce such changes as activation of phospholipase C, increased MAPK activity, and mobilization of intracellular Ca2+. The other group, M2, consists of the m2 and m4 subtypes, which couple to Gi/o and so induce changes such as inhibition of adenylyl cyclase activity and inactivation of Ca2+ channels (Eglen, 2006; Ishii & Kurachi, 2006; Lucas-Meunier, Fossier, Baux, & Amar, 2003). Receptor subtypes m1 and m2 are both abundantly expressed in cerebral cortex, but they differ in their laminar distribution as well as in their synaptic location, m1 being expressed mainly on postsynaptic neurons, and m2, mainly on presynaptic terminals (Alcantara et al., 2001; Levey, 1996; Rouse, Marino, Potter, Conn, & Levey, 1999).
Scopolamine does not discriminate between m1 and m2 subtypes as well as some other compounds do. For example, the muscarinic receptor antagonist pirenzepine was found in one in vitro assay to have a 57-fold greater affinity for the m1 than for the m2 subtype (Buckley, Bonner, Buckley, & Brann, 1989; Hammer, Berrie, Birdsall, Burgen, & Hulme, 1980), whereas the reverse was the case for the muscarinic receptor antagonist methoctramine, which had an approximately 4.4-fold greater affinity for the m2 than for the m1 receptor subtype (Buckley, et al., 1989; Giraldo et al., 1988). Tinsley and colleagues (2011) recently reported that intraperirhinal injections of pirenzepine impaired visual recognition memory in the rat, but there has been no report on the visual memory effects of similarly infusing a selective m2 antagonist. Here, in a study conducted with monkeys, we attempted to compare directly m1 and m2 contributions to visual recognition memory by intraperirhinal microinfusions in separate sessions of the m1 blocker pirenzepine and the m2 blocker methoctramine.
2. Materials and Methods
2.1 Subjects
The subjects were three naïve male monkeys (Macaca mulatta) weighing 4-8 kg at the start of the experiment. They were housed individually or in social pairs in rooms with an automatic lighting schedule (light/dark: 12/12 h). They were fed primate chow (No. 5038, PMI Nutrition International, LLC Brendwood, MO) with a variety of supplements, including fruits and nuts, and they had free access to water. The procedures used in this study were approved by the National Institute of Mental Health Animal Care and Use Committee and conducted in accord with the National Research Council Guide for the Care and Use of Laboratory Animals.
2.2 Apparatus
The stimuli were displayed on a 15-inch, flat-screen, touch-sensitive monitor (Microtouch, 3M Center, St. Paul, MN) in an unlit, sound-attenuated chamber (Industrial Acoustics Company, Inc., Bronx, NY). The transport chairs in which the monkeys sat for testing allowed them free arm movements. Each correct response was rewarded with a 190-mg food pellet (equal mixture of banana, fruit punch, and grape flavors; Research Diets, New Brunswick, NJ) automatically dispensed into a plastic cup located centrally beneath the monitor.
2.3 Behavioral procedures
The monkeys were habituated for a few sessions to both the transport chair and test apparatus while given free access to pellets in the cup. They were then trained to touch colored pictures on the monitor for pellet rewards. The visual stimuli, 9 × 9 cm square, appeared against a black background (LabView software; www.ni.com).
The monkeys then started training on the rule for delayed nonmatching-to-sample (DNMS) with trial-unique photographs of man-made objects, plants, animals, foods, and nature scenes. For the familiarization phase of each trial, a single sample stimulus appeared centrally on the screen. Touching the sample led to reward and cessation of this stimulus. For the test phase of the trial, presented 10 sec later, the sample and a novel stimulus appeared simultaneously, 9 cm apart and equidistant from the center; touching the novel stimulus led to reward and cessation of both stimuli, whereas touching the familiarized sample led only to cessation of the stimuli. There was no correction for errors. Trials were repeated at a constant intertrial interval, ranging between 20-30 sec depending on the subject, and at the rate of 60 trials per day until the animals met the criterion of 90 percent correct responses on two consecutive days. Throughout training and subsequent testing on the DNMS rule, pairs of trial-unique stimuli were drawn pseudorandomly from a pool of 8000 stimuli until all were used, after which they were recycled.
Once the animals acquired the DNMS rule, list-length was gradually increased from one sample stimulus to five sample stimuli with 10-sec interstimulus intervals (ISIs). In the test phase of each list length, all the sample stimuli were shown again in the same order as before, but now each was paired with a different novel stimulus, with the left-right positions of the sample and novel stimuli changed pseudorandomly. Testing continued at the same rate as before (i.e. 60-trial sessions) until the animals regained the criterion of 90 percent correct responses.
List lengths and ISIs were then increased either in steps of 5 stimuli or 5 sec, until the animal’s performance dropped below a stable level of 90 percent correct responses, at which point the memory demands were reduced one step. This procedure was followed to accommodate individual differences in the animals’ recognition memory ability. In the final version of the task, list-lengths across the three monkeys varied from 15-25 stimuli presented at ISIs of 15-20 sec, resulting in retention intervals between sample and test that ranged from about 4 to 8 min. The monkeys performed 3-5 such lists per session or 75-80 trials per day, 5 days per week. Following training, animals were continued at their final list-length and ISI level until they attained a criterion of 90 percent correct responses for five consecutive days.
2.4. Surgery
For both headpost and chamber attachment procedures, the animal received glycopyrrolate (0.01 mg/kg, i.m.) and ketamine HCl (10-15 mg/kg, i.m.) prior to intubation, after which the anaesthetic isoflurane (1.0-3.0%, inhalation) was given to effect for the duration of the surgery. Using aseptic techniques, the skin was incised and connective and muscle tissues were retracted in anatomical layers to expose the skull. The titanium headpost was custom-shaped during surgery for optimal fit and held in place by titanium screws (Veterinary Orthopedic Implants; www.vetimplants.com). Using MRI brain scans acquired for the purpose, stereotaxic coordinates were calculated for placement of a rectangular plastic chamber (modified electrode holder) through which the injection-target area could be reached. During surgery, the plastic chamber was individually contoured, placed at the appropriate stereotaxic position, and secured to the skull with dental cement anchored by ceramic screws. Once the cement had hardened fully, the soft tissues were sutured in anatomical layers around the chamber edges, and the chamber was closed with a removable plastic cap.
In another aseptic surgical procedure carried out two weeks later, and with the animal anesthetized as before, cranial tissue within the chamber area was removed bilaterally in order to access the target area for microinfusions through a cannula-guide grid inserted in the injection chamber. During each surgical procedure, animals received prophylactic antibiotic treatment (Cefazolin, 25 mg/kg i.m.) and analgesic agents (Ketoprofen, 1 mg/kg i.m. bid). The guide grid was inserted for MRI scanning and for each intraperirhinal injection session, as described below. Before inserting the guide grid and also after removing it following each use, the interior of the plastic chamber and surrounding tissue were thoroughly cleaned with dilute betadine solution followed by sterile saline. Between each use, the chamber was covered with the plastic cap.
2.5 Drug infusions
On completion of surgery, the monkey was given a two-week recovery period, after which daily DNMS training and testing was resumed. On reattaining the performance criterion, each animal received a second MR scan, this one performed to obtain coordinates for the perirhinal infusions. By filling the injection chamber with a sterile solution of gadolinium diluted in saline (1:1000; Magnevist, Berlex Imaging, Wayne, NJ), the holes of the cannula-guide grid could be visualized and individual MRI-based coordinate maps could be constructed for perirhinal targeting (Saunders, Aigner, & Frank, 1990). The estimated coordinates were confirmed by injection of the gadolinium solution (see Fig. 1). Each animal received a series of bilateral-microinfusion sessions. The compounds tested, pirenzepine and methoctramine (each obtained from Sigma-Aldrich, St. Louis, MO), were freshly mixed the day of injection, dissolved in sterile buffered saline (final solution pH 7.0-7.5), and filtered through 0.22 μm sterile filters (Corning Inc., Corning, NY) into sterile vials before use. For each baseline as well as each microinfusion session, the monkey was seated with head fixed while the chamber and surrounding tissue were cleaned. For microinfusion sessions only, the sterile guide grid was inserted in the chamber and sterile cannulae were introduced through the appropriate holes based on that animal’s coordinate map. First, the guide cannulae (0.5 mm OD) were inserted through the appropriate grid holes; next, the infusion cannulae (0.3 mm OD) were inserted into the guide cannulae and lowered to the targets: two perirhinal sites per hemisphere, with the two sites separated 3 mm rostrocaudally. A PHD 2000 infusion pump with microliter rack (Harvard Apparatus, Holliston, MA) was used to drive the infusion from gastight syringes (Hamilton, Reno, NV). A total of 3.5 μl/site was delivered to each of the four sites simultaneously at a rate of 0.25 μl/min for a total infusion time of 14 minutes. The infusion cannulae were left in place for an additional 10 minutes prior to their withdrawal, and testing on the DNMS task began 5 minutes after their withdrawal.
Figure 1.
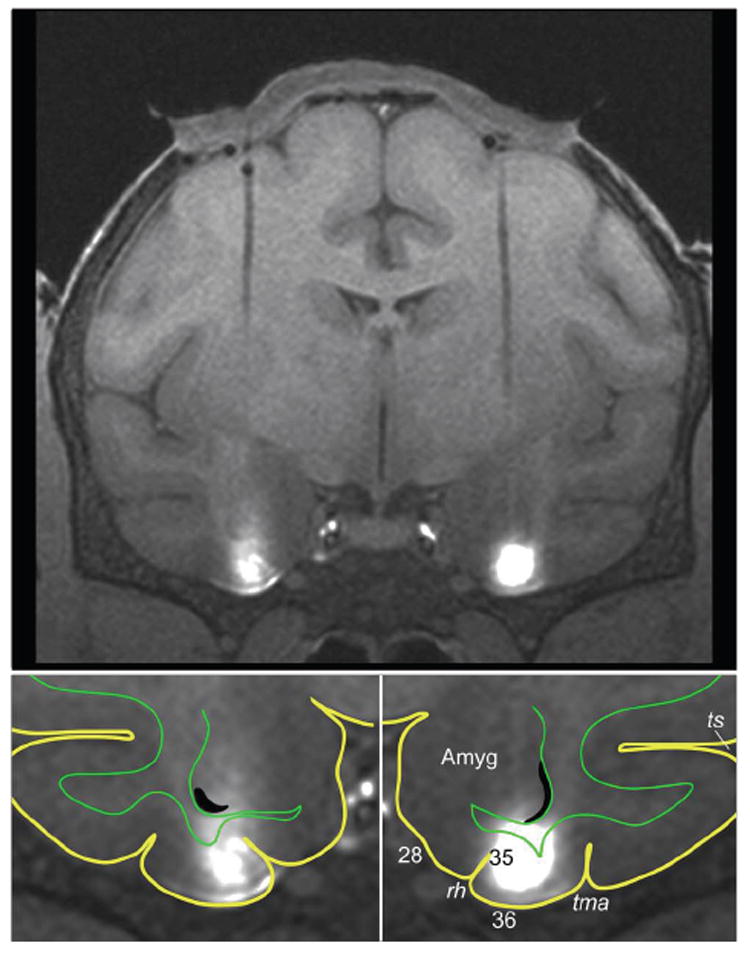
MRI-guided targeting of the monkey’s perirhinal cortex. Upper panel: MR image of a coronal brain section showing (i) bilateral tracks left by guide tubes through which the injection cannulae were lowered and (ii) bilateral infusions of the MR contrast agent gadolinium (white areas). The cap-shaped object above the brain is postoperative granular-tissue growth between the dura mater and the guide-grid chamber (the latter being invisible to MR in this image taken after the gadolinium was removed from the chamber). Lower panels: Enlarged views of the ventral part of the section shown in the upper panel. Yellow lines outline the brain’s pial surface, and green lines, the border between grey and white matter. As shown in these views, the infusions were limited largely to the perirhinal cortex, located in the lateral half of the inferior temporal gyrus (i.e. the gyrus between tma and rh). Abbreviations: 28, Brodmann area (BA) 28 or entorhinal cortex; 35, BA 35 or perirhinal cortex in the lateral bank of the rhinal sulcus; 36, BA 36 or perirhinal cortex on the gyral surface; Amyg, amygdala; rh, rhinal sulcus; tma, anterior middle temporal sulcus; ts, superior temporal sulcus.
2.6 Drug doses
Information from both in vitro assays and in vivo experiments was used to determine dosages for each drug. Briefly, findings from an in vitro study by Buckley et al. (1989) indicated that 90 nM of pirenzepine displaced about 50% of [3H]-labeled N-methylscopolamine ([3H] NMS) bound to m1 receptors, and, under the same conditions, 6 nM methoctramine displaced 50% of [3H] NMS bound to m2 receptors. Extrapolating from these data, a given concentration of pirenzepine and a 15-fold lesser concentration of methoctramine could be expected to block a roughly equivalent percentage of each ligand’s preferred receptor subtype. In vivo data were available from a study by Ohno and colleagues (1994), who found that hippocampal injections of 1.2 mM of pirenzepine in rats produced significant memory deficits, an effect that was attenuated by a low methoctramine dose (0.7 mM) in the same tissue; these investigators also observed a memory impairment after hippocampal injection of a higher, nonselective dose of 2.2 mM of methoctramine, which was countered by injection of an m1 selective agonist. Based on these several findings, as well as on our own perirhinal scopolamine data (Tang et al., 1997), we chose dosages for perirhinal injection of 1.0, 10.0, and 30.0 mM of pirenzepine, and, to compare with these, dosages of 0.5 and 1.0 mM of methoctramine. Saline and these drug doses were infused in separate sessions in pseudorandom order, and each animal received saline and each dose of each drug at least twice. (Higher doses of each compound were also tested, though not systematically; see Results.) Each infusion was preceded by at least 3 noninjection sessions, with mean scores on the latter sessions providing a noninjection baseline of performance.
3. Results
3.1 Baseline performance and effects of vehicle infusions
Animals performed the DNMS task at high levels of accuracy on the noninjection baseline sessions, with a mean (±SE) error rate of 7.0% (±2.0%). Further, the animals’ performance was not affected significantly by perirhinal infusions of the vehicle (sterile saline, pH 7.5; mean error rate, 9.0% (±2.0%).
3.2 Effects of infusing the selective muscarinic antagonists
Effects on performance of infusing the m1 and m2 antagonists were evaluated with a one-way repeated-measures ANOVA, which compared memory losses (percent errors above noninjection baseline) across the six conditions: saline, the three doses of pirenzepine, and the two doses of methoctramine. The analysis yielded a highly significant main effect of condition (F = 15.96, Greenhouse-Geisser corrected df = 2.38, 11.88, p < 0.0001). Post-hoc t-tests demonstrated that pirenzepine infusions produced a dose-dependent impairment relative to the saline control condition (Fig. 2; Table 1, p-values above the diagonal), revealing a stepwise increase in deficit with increasing doses. Similar results were obtained with nonparametric analyses [Friedman test: χ2(3) = 13.8, p = 0.003, followed by post-hoc analyses with Wilcoxon signed-rank tests (Table 1, p-values below the diagonal)].
Figure 2.
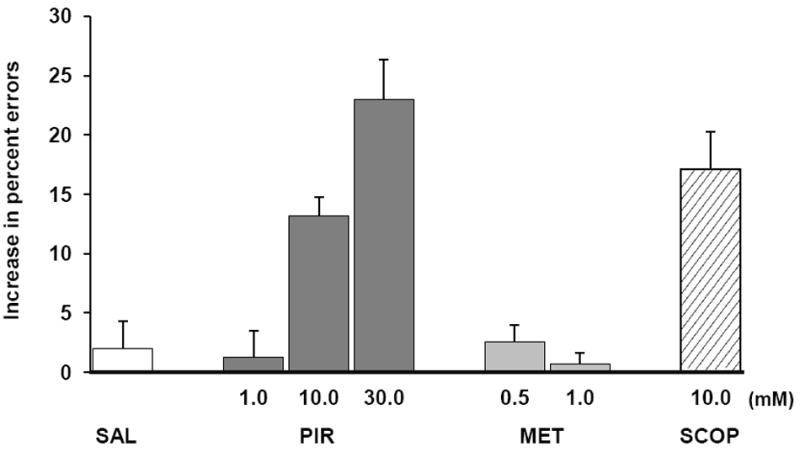
DNMS performance. Percent errors above the mean noninjection baseline score after bilateral perirhinal infusions of saline and the indicated drug concentrations in millimolar (mM). Abbreviations: MET, methoctramine; PIR, pirenzepine; SAL, saline; SCOP, scopolamine.
Table 1.
p-values for dose-dependent differences in effects of pirenzepine on error rates
| Saline | 1 mM | 10 mM | 30 mM | |
|---|---|---|---|---|
| Saline | --- | 0.796 | 0.015 | 0.005 |
| 1 mM | 0.917 | --- | 0.013 | 0.0001 |
| 10 mM | 0.046 | 0.028 | --- | 0.044 |
| 30 mM | 0.028 | 0.028 | 0.046 | --- |
p-values above and below diagonal are based on t-tests and rank-sum tests, respectively (significant differences in bold font). mM of pirenzepine.
In contrast to the m1 antagonist pirenzepine, the m2 antagonist methoctramine failed to produce significant effects. Neither dose of the latter drug reduced memory scores below that of the saline infusions (ANOVA followed by post-hoc t-tests: p-values of 0.21 and 0.76 for the saline vs. 0.5 and 1.0 mM doses, respectively; negative results were also obtained with the nonparametric Friedman test followed by Wilcoxon Signed-Rank tests: p-values of 0.17 and 0.92 for the saline vs. 0.5 and 1.0 mM doses, respectively). Moreover, the animals’ performance on both doses of methoctramine was significantly better than that following infusion of the two higher doses (10.0 and 30.0 mM) of pirenzepine (Fig. 2, all p values < 0.02). (A probe test conducted twice in one animal with a much higher dose of methoctramine was also without effect: a mean error rate of 8% after infusing 10.0 mM of the drug vs. 9% after infusing saline. However, according to the in vitro binding data of Buckley et al. (1989), as well as the in vivo findings noted by Ohno et al. (1994), a concentration of methoctramine this high would be nonselective, and so we did not pursue testing this dose in the other animals.
3.3. Comparative effects of the nonselective muscarinic receptor antagonist, scopolamine
Two studies in rodents showed that insular cortex infusions of similar doses of pirenzepine (100 mM) and scopolamine (136-156 mM) led to similar levels of deficit in a conditioned taste aversion task (Naor & Dudai, 1996; Ramirez-Lugo, Miranda, Escobar, Espinosa, & Bermudez-Rattoni, 2003). The latter study also ruled out a contribution from unintended blockade of m2 receptors by demonstrating that injections of the m2 antagonist AFDX-116 (0.5 mM) had no effect on acquisition of the conditioned taste aversion. If the visual memory impairments found earlier after infusions of scopolamine in the perirhinal cortex of monkeys (Tang, et al., 1997) were likewise caused mainly by blockade of the m1 receptor, then, extrapolating from the rodent-insula results, infusions of similar doses of scopolamine and pirenzepine should yield similar levels of deficit in visual memory as well. We tested this possibility by comparing the effects of bilateral perirhinal infusions of 10.0 mM of scopolamine with those we observed in the present study after infusing 10.0 mM of pirenzepine. As predicted, the increase in percent errors caused by scopolamine relative to the saline baseline was 17.1% (±3.2%), compared to an increase of 13.2% (±1.6%) after pirenzepine (pairwise comparison: p = 0.50, Wilcoxon Signed-Ranks test z = -1.07, p = 0.28; see Fig. 2).
3.4. Response latency and position bias
Bilateral perirhinal administrations of muscarinic antagonists had no effect on response latencies compared with the effect of saline, either in the sample phase of DNMS (F = 0.36, Greenhouse-Geisser corrected df = 1.98, 9.90, p = 0.70) or in the test phase (F = 1.07, Greenhouse-Geisser corrected df = 2.13, 10.64, p = 0.38; see Table 2). Neither did the drugs affect spatial bias (i.e. L-R/L+R errors: F = 0.70, Greenhouse-Geisser corrected df = 1.12, 5.58, p = 0.45).
Table 2.
Increase in errors above baseline following local intraperirhinal microinfusions
| Infusion material in mM | Percent errors above baseline | Response latency in seconds | |
|---|---|---|---|
| sample phase | test phase | ||
| Saline | 2.0 (2.3) | 1.2 (0.1) | 2.1 (0.2) |
| 1.0 PIR | 1.3 (2.3) | 1.3 (0.3) | 2.0 (0.3) |
| 10.0 PIR | 13.2 (1.6)* | 1.3 (0.2) | 2.2 (0.2) |
| 30.0 PIR | 23.2 (3.5)* | 1.3 (0.2) | 2.2 (0.3) |
| 0.5 MET | 2.6 (1.4) | 1.2 (0.3) | 2.0 (0.3) |
| 1.0 MET | 0.7 (1.1) | 1.4 (0.3) | 2.2 (0.4) |
| 10.0 SCOP | 17.1 (3.2)* | 1.4 (0.3) | 2.5 (0.6) |
Means (and SEMs) for each dose tested.
significant deficit (see Table 1 for p-values).
4. Discussion
Bilateral infusions of the m1 selective antagonist pirenzepine into the monkey’s perirhinal cortex produced a dose-dependent impairment in visual recognition memory. By contrast, infusions of the m2 selective antagonist methoctramine into this same region failed to yield significant deficits. The memory impairment caused by local administration of the nonselective muscarinic receptor antagonist scopolamine did not differ from the deficit caused by the same dose of the m1 selective antagonist pirenzepine, suggesting that scopolamine-induced impairments described in previous studies were due primarily, if not exclusively, to blockade of the m1 receptor subtype.
As indicated in the Methods, information from both in vitro and in vivo assays suggests that the drug dosages we used were appropriate ones. Given a recent finding in the rat (Tinsley et al., 2011) that a concentration of 165 nM pirenzepine infused into perirhinal cortex was sufficient to produce a recognition memory impairment, it is somewhat surprising that a concentration of 1.0 mM was insufficient to do the same in the monkey. The explanation, however, may simply be that the effective concentrations of the same drugs infused in the same anatomical area will vary depending on the percent of the area infused, and these percentages may well differ across different studies and, especially, across different species. Additional support comes from findings in the taste-aversion study referred to in Results. In that rodent conditioning study (Ramirez-Lugo, et al., 2003), insular cortex injections of 0.5 mM AF-DX 116, an m2 antagonist, increased release of acetylcholine through blockade of m2 autoreceptors, yet had no effect on the animals’ acquisition of taste aversion. By contrast, 100 mM of pirenzepine injected into insular cortex had no effect on acetylcholine release, yet did impair the acquisition of taste aversion. As the affinity of methoctramine is much higher than the affinity of AF-DX 116 for m2 receptors (Ki values of 3.6 nM and 186 nM, respectively (Buckley, et al., 1989)), 1.0 mM of methoctramine should have been more than adequate to block m2 receptors in our preparation and therefore sufficient to impair visual memory if such were the result of excessive release of acetylcholine.
One caveat regarding the foregoing claim is that in vitro studies have also shown that, at sufficiently high concentrations, both pirenzepine and methoctramine can become nonselective, with each antagonist affecting both m1 and m2 receptors (Birdsall et al., 1988; Buckley, et al., 1989; Dorje et al., 1991). We believe our claim is tenable, however, based on the fact that the affinity of pirenzepine for m2 is 250 times less than that of methoctramine (Ki values of 906 nM and 3.6 nM, respectively (Buckley, et al., 1989)); therefore, if the memory impairment we found after perirhinal infusion of 10 mM pirenzepine were through blockade of m2 receptors, infusion of 1.0 mM methoctramine should have been more than sufficient to cause an impairment.
The converse possibility, namely, that the infusions of 1.0 mM of methoctramine may have acted on m1 as well as on m2 receptors, raises a larger issue. High concentrations of methoctramine have been documented to cause nonspecific effects through blockade of m1 both in vitro and in vivo (Buckley, et al., 1989; Ohno, Yamamoto, & Watanabe, 1994). Although an in vitro study (Gulledge & Kawaguchi, 2007) did show that 500 nM of methoctramine binds selectively to m2 receptors (just as the same 500-nM dose of pirenzepine binds selectively to m1), the relative affinity of methoctramine for m2 and m1 receptors is not ideal: Ki values of 3.6 nM and 16 nM, respectively (Buckley, et al., 1989), an affinity ratio of only 4.4 to 1. It is therefore likely that, at mM concentrations, methoctramine does exert some effect on m1 receptors (Tinsley, et al., 2011). However, our behavioral data clearly indicate that any such nonspecific binding to m1 was not sufficient to affect recognition memory in our task, and probe data gathered after infusions of methoctramine concentrations above 1.0 mM (see Results) likewise failed to reveal any loss of recognition accuracy. Yet we cannot exclude the possibility that the facilitation of acetylcholine release offset an impairment caused by M2 receptor blockade.
Although we did not observe impaired memory formation following blockade of m2 receptors, this does not rule out a role for these receptors in recognition memory. Cortical m2 receptors are located mainly, though not exclusively, on cholinergic terminals (Lazareno & Roberts, 1989; Smiley, Levey, & Mesulam, 1999; Vilaro, Wiederhold, Palacios, & Mengod, 1992). Several studies have shown that whereas antagonism of m2 receptors facilitates acetylcholine release, activation of m2 receptors reduces it (Allen & Brown, 1993; Lachowicz et al., 2001; Ramirez-Lugo, et al., 2003; Vannucchi & Pepeu, 1995). It has also been shown that performance on memory tasks can be improved by increasing the availability of acetylcholine through blockade of its degradation with systemic administration of the cholinesterase inhibitor physostigmine (Aigner & Mishkin, 1986; Lachowicz, et al., 2001; Ohnuki & Nomura, 1996; Packard, Regenold, Quirion, & White, 1990; Rowe et al., 2003). Although facilitating acetylcholine release through methoctramine blockade of m2 autoreceptors could also have served to improve memory performance, such a finding may have been precluded in our study because of the high baseline level of performance (a minimum of 90 percent accuracy) the monkeys were required to maintain. The potential role of the m2 receptor in improving memory thus clearly warrants further investigation.
The precise cellular and molecular processes that underlie perirhinal-dependent memory formation have yet to be determined definitively. However, studies in both monkeys and rats have proposed that synaptic long-term depression in perirhinal neurons is one of the critical mechanisms (Xiang & Brown, 1998; Fahy, Riches, & Brown, 1993; Griffiths et al., 2008; Warburton, et al., 2003; Zhu, Brown, & Aggleton, 1995; Zhu, McCabe, Aggleton, & Brown, 1996), and cholinergic modulation of muscarinic receptors has been shown to play an important role in synaptic depression in rodent perirhinal cortex (Cho et al., 2000; Warburton, et al., 2003). In an in vitro preparation of rat perirhinal cortex, activation of acetylcholine receptors by carbachol produced long-lasting depression of synaptic transmission that was dependent on m1 receptor activation (Massey, Bhabra, Cho, Brown, & Bashir, 2001), suggesting links between muscarinic effects on behavior and plasticity through m1 receptors, specifically. Additional in vitro studies have shown that m1 is also critical for synaptic long-term depression in other areas of rodent cortex (Kirkwood, Rozas, Kirkwood, Perez, & Bear, 1999; McCoy & McMahon, 2007; Scheiderer et al., 2008). The behavioral results of the present study provide further support for this supposition.
Synaptic long-term depression, however, is not the only memory-related physiological process in which m1 receptors could play a critical role. Indeed, activation of muscarinic receptors can induce persistent spiking in entorhinal and perirhinal cortex through activation of TRPC channels (Egorov, Hamam, Fransen, Hasselmo, & Alonso, 2002; Navaroli, Zhao, Boguszewski, & Brown, 2011). Moreover, human fMRI studies have uncovered a muscarinic-dependent correlation between persistent activity in the rhinal cortices during the delay interval of a DNMS task and successful encoding of the visual stimulus (Schon et al., 2005; Schon, Hasselmo, Lopresti, Tricarico, & Stern, 2004). Partly on the basis of these findings, persistent spiking activity has been proposed as an attractive mechanism for encoding new stimuli (Hasselmo & Stern, 2006), and the m1 receptor has been suggested as the subtype responsible for inducing this form of activity in cortical pyramidal cells (Yan, Villalobos, & Andrade, 2009). In short, m1-dependent persistent spiking is a plausible cellular mechanism for m1-receptor mediation of recognition memory.
Other potential m1-related mechanisms include the inhibition of potassium channels (Lanzafame, Christopoulos, & Mitchelson, 2003; Marrion, 1997) and potentiation of NMDA receptors (Calabresi, Centonze, Gubellini, Pisani, & Bernardi, 1998; Marino, Rouse, Levey, Potter, & Conn, 1998). Systematic examination of the various m1-dependent pathways need to be undertaken to explore these numerous possibilities.
Highlights.
Visual memory formation depends on cholinergic activation of muscarinic receptors.
Intra-perirhinal blockade of m1 but not of m2 receptors impairs recognition memory.
The m1 and m2 receptor subtypes have functionally dissociable roles.
Acknowledgments
We are grateful to Aaron Jenkins, Wilma Bainbridge, and Bhavishya Surapaneni for assistance with behavioral training, Rachel Reoli and David Yu for assistance with MRI and surgery, George Dold for computer programming, and Kadharbatcha Saleem for help with drafting the MR figure. This study was supported by the Intramural Research Program of the National Institute of Mental Health, NIH/DHHS.
Footnotes
Conflict of interest
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigner TG, Mishkin M. The effects of physostigmine and scopolamine on recognition memory in monkeys. Behav Neural Biol. 1986;45(1):81–87. doi: 10.1016/s0163-1047(86)80008-5. [DOI] [PubMed] [Google Scholar]
- Aigner TG, Walker DL, Mishkin M. Comparison of the effects of scopolamine administered before and after acquisition in a test of visual recognition memory in monkeys. Behav Neural Biol. 1991;55(1):61–67. doi: 10.1016/0163-1047(91)80127-z. [DOI] [PubMed] [Google Scholar]
- Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM, Goldman-Rakic PS. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 2001;434(4):445–460. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- Allen TG, Brown DA. M2 muscarinic receptor-mediated inhibition of the Ca2+ current in rat magnocellular cholinergic basal forebrain neurones. J Physiol. 1993;466:173–189. [PMC free article] [PubMed] [Google Scholar]
- Birdsall NJ, Curtis CA, Eveleigh P, Hulme EC, Pedder EK, Poyner D, et al. Muscarinic receptor subtypes and the selectivity of agonists and antagonists. Pharmacology. 1988;37(Suppl 1):22–31. doi: 10.1159/000138503. [DOI] [PubMed] [Google Scholar]
- Bonner TI. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989;12(4):148–151. doi: 10.1016/0166-2236(89)90054-4.0166-2236(89)90054-4 [DOI] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Buckley CM, Brann MR. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989;35(4):469–476. [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Endogenous ACh enhances striatal NMDA-responses via M1-like muscarinic receptors and PKC activation. Eur J Neurosci. 1998;10(9):2887–2895. doi: 10.1111/j.1460-9568.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors--characterization, coupling and function. Pharmacol Ther. 1993;58(3):319–379. doi: 10.1016/0163-7258(93)90027-b.0163-7258(93)90027-B [DOI] [PubMed] [Google Scholar]
- Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000;3(2):150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- Dorje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther. 1991;256(2):727–733. [PubMed] [Google Scholar]
- Eglen RM. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol. 2006;26(3):219–233. doi: 10.1111/j.1474-8673.2006.00368.x.AAP368 [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420(6912):173–178. doi: 10.1038/nature01171.nature01171 [DOI] [PubMed] [Google Scholar]
- Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp Brain Res. 1993;96(3):457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- Giraldo E, Micheletti R, Montagna E, Giachetti A, Vigano MA, Ladinsky H, et al. Binding and functional characterization of the cardioselective muscarinic antagonist methoctramine. J Pharmacol Exp Ther. 1988;244(3):1016–1020. [PubMed] [Google Scholar]
- Griffiths S, Scott H, Glover C, Bienemann A, Ghorbel MT, Uney J, et al. Expression of long-term depression underlies visual recognition memory. Neuron. 2008;58(2):186–194. doi: 10.1016/j.neuron.2008.02.022. doi: S0896-6273(08)00176-1 [pii] 10.1016/j.neuron.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Kawaguchi Y. Phasic cholinergic signaling in the hippocampus: functional homology with the neocortex? Hippocampus. 2007;17(5):327–332. doi: 10.1002/hipo.20279. [DOI] [PubMed] [Google Scholar]
- Hammer R, Berrie CP, Birdsall NJ, Burgen AS, Hulme EC. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends Cogn Sci. 2006;10(11):487–493. doi: 10.1016/j.tics.2006.09.005.S1364-6613(06)00242-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Kurachi Y. Muscarinic acetylcholine receptors. Curr Pharm Des. 2006;12(28):3573–3581. doi: 10.2174/138161206778522056. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19(5):1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz JE, Duffy RA, Ruperto V, Kozlowski J, Zhou G, Clader J, et al. Facilitation of acetylcholine release and improvement in cognition by a selective M2 muscarinic antagonist, SCH 72788. Life Sci. 2001;68(22-23):2585–2592. doi: 10.1016/s0024-3205(01)01056-6. [DOI] [PubMed] [Google Scholar]
- Lanzafame AA, Christopoulos A, Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors Channels. 2003;9(4):241–260.LYP6J6KVM46G1R35 [PubMed] [Google Scholar]
- Lazareno S, Roberts FF. Functional and binding studies with muscarinic M2-subtype selective antagonists. Br J Pharmacol. 1989;98(1):309–317. doi: 10.1111/j.1476-5381.1989.tb16896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI. Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc Natl Acad Sci U S A. 1996;93(24):13541–13546. doi: 10.1073/pnas.93.24.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Meunier E, Fossier P, Baux G, Amar M. Cholinergic modulation of the cortical neuronal network. Pflugers Arch. 2003;446(1):17–29. doi: 10.1007/s00424-002-0999-2. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1998;95(19):11465–11470. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Massey PV, Bhabra G, Cho K, Brown MW, Bashir ZI. Activation of muscarinic receptors induces protein synthesis-dependent long-lasting depression in the perirhinal cortex. Eur J Neurosci. 2001;14(1):145–152. doi: 10.1046/j.0953-816x.2001.01631.x.ejn1631 [DOI] [PubMed] [Google Scholar]
- McCoy PA, McMahon LL. Muscarinic receptor dependent long-term depression in rat visual cortex is PKC independent but requires ERK1/2 activation and protein synthesis. J Neurophysiol. 2007;98(4):1862–1870. doi: 10.1152/jn.00510.2007.00510.2007 [DOI] [PubMed] [Google Scholar]
- Naor C, Dudai Y. Transient impairment of cholinergic function in the rat insular cortex disrupts the encoding of taste in conditioned taste aversion. Behav Brain Res. 1996;79(1-2):61–67. doi: 10.1016/0166-4328(95)00262-6. [DOI] [PubMed] [Google Scholar]
- Navaroli VL, Zhao Y, Boguszewski P, Brown TH. Muscarinic receptor activation enables persistent firing in pyramidal neurons from superficial layers of dorsal perirhinal cortex. Hippocampus. 2011 doi: 10.1002/hipo.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, Watanabe S. Blockade of hippocampal M1 muscarinic receptors impairs working memory performance of rats. Brain Res. 1994;650(2):260–266. doi: 10.1016/0006-8993(94)91790-6.0006-8993(94)91790-6 [DOI] [PubMed] [Google Scholar]
- Ohnuki T, Nomura Y. Effects of selective muscarinic antagonists, pirenzepine and AF-DX 116, on passive avoidance tasks in mice. Biol Pharm Bull. 1996;19(6):814–818. doi: 10.1248/bpb.19.814. [DOI] [PubMed] [Google Scholar]
- Packard MG, Regenold W, Quirion R, White NM. Post-training injection of the acetylcholine M2 receptor antagonist AF-DX 116 improves memory. Brain Res. 1990;524(1):72–76. doi: 10.1016/0006-8993(90)90493-u.0006-8993(90)90493-U [DOI] [PubMed] [Google Scholar]
- Ramirez-Lugo L, Miranda MI, Escobar ML, Espinosa E, Bermudez-Rattoni F. The role of cortical cholinergic pre- and post-synaptic receptors in taste memory formation. Neurobiol Learn Mem. 2003;79(2):184–193. doi: 10.1016/s1074-7427(02)00038-2.S1074742702000382 [DOI] [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Potter LT, Conn PJ, Levey AI. Muscarinic receptor subtypes involved in hippocampal circuits. Life Sci. 1999;64(6-7):501–509. doi: 10.1016/s0024-3205(98)00594-3. [DOI] [PubMed] [Google Scholar]
- Rowe WB, O’Donnell JP, Pearson D, Rose GM, Meaney MJ, Quirion R. Long-term effects of BIBN-99, a selective muscarinic M2 receptor antagonist, on improving spatial memory performance in aged cognitively impaired rats. Behav Brain Res. 2003;145(1-2):171–178. doi: 10.1016/s0166-4328(03)00116-5.S0166432803001165 [DOI] [PubMed] [Google Scholar]
- Saunders RC, Aigner TG, Frank JA. Magnetic resonance imaging of the rhesus monkey brain: use for stereotactic neurosurgery. Exp Brain Res. 1990;81(2):443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Smith CC, McCutchen E, McCoy PA, Thacker EE, Kolasa K, et al. Coactivation of M(1) muscarinic and alpha1 adrenergic receptors stimulates extracellular signal-regulated protein kinase and induces long-term depression at CA3-CA1 synapses in rat hippocampus. J Neurosci. 2008;28(20):5350–5358. doi: 10.1523/JNEUROSCI.5058-06.2008.28/20/5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25(40):9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005.25/40/9112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24(49):11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004.24/49/11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Mesulam MM. m2 muscarinic receptor immunolocalization in cholinergic cells of the monkey basal forebrain and striatum. Neuroscience. 1999;90(3):803–814. doi: 10.1016/s0306-4522(98)00527-2.S0306-4522(98)00527-2 [DOI] [PubMed] [Google Scholar]
- Tang Y, Mishkin M, Aigner TG. Effects of muscarinic blockade in perirhinal cortex during visual recognition. Proc Natl Acad Sci U S A. 1997;94(23):12667–12669. doi: 10.1073/pnas.94.23.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley CJ, Fontaine-Palmer NS, Vincent M, Endean EP, Aggleton JP, Brown MW, et al. Differing time dependencies of object recognition memory impairments produced by nicotinic and muscarinic cholinergic antagonism in perirhinal cortex. Learn Mem. 2011;18(7):484–492. doi: 10.1101/lm.2274911.18/7/484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi MG, Pepeu G. Muscarinic receptor modulation of acetylcholine release from rat cerebral cortex and hippocampus. Neurosci Lett. 1995;190(1):53–56. doi: 10.1016/0304-3940(95)11498-l.030439409511498L [DOI] [PubMed] [Google Scholar]
- Vilaro MT, Wiederhold KH, Palacios JM, Mengod G. Muscarinic M2 receptor mRNA expression and receptor binding in cholinergic and non-cholinergic cells in the rat brain: a correlative study using in situ hybridization histochemistry and receptor autoradiography. Neuroscience. 1992;47(2):367–393. doi: 10.1016/0306-4522(92)90253-x.0306-4522(92)90253-X [DOI] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, et al. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38(6):987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10(1):69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37(4-5):657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Yan HD, Villalobos C, Andrade R. TRPC Channels Mediate a Muscarinic Receptor-Induced Afterdepolarization in Cerebral Cortex. J Neurosci. 2009;29(32):10038–10046. doi: 10.1523/JNEUROSCI.1042-09.2009.29/32/10038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, McCabe BJ, Aggleton JP. Effects of the novelty or familiarity of visual stimuli on the expression of the immediate early gene c-fos in rat brain. Neuroscience. 1995;69(3):821–829. doi: 10.1016/0306-4522(95)00320-i. doi:0306-4522(95)00320-I [pii] [DOI] [PubMed] [Google Scholar]
- Zhu XO, McCabe BJ, Aggleton JP, Brown MW. Mapping visual recognition memory through expression of the immediate early gene c-fos. Neuroreport. 1996;7(11):1871–1875. doi: 10.1097/00001756-199607290-00037. [DOI] [PubMed] [Google Scholar]


