SUMMARY
Vaccines are instrumental in controlling the burden of influenza virus infection in humans and animals. Antibodies raised against both major viral surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), can contribute to protective immunity. Vaccine-induced HA antibodies have been characterized extensively, and they generally confer protection by blocking the attachment and fusion of a homologous virus onto host cells. Though not as well characterized, some functions of NA antibodies in influenza vaccine-mediated immunity have been recognized for many years. In this review we summarize the case for NA antibodies in influenza vaccine-mediated immunity. In the absence of well-matched HA antibodies, NA antibodies can provide varying degrees of protection against disease. NA proteins of seasonal influenza vaccines have been shown in some instances to elicit serum antibodies with cross-reactivity to avian- and swine-origin influenza strains, in addition to HA drift variants. NA-mediated immunity has been linked to [i] conserved NA epitopes amongst otherwise antigenically distinct strains, partly attributable to the segmented influenza viral genome; [ii] inhibition of NA enzymatic activity; and [iii] the NA content in vaccine formulations. There is potential to enhance the effectiveness of existing and future influenza vaccines by focusing greater attention on the antigenic characteristics and potency of the NA protein.
Keywords: influenza, neuraminidase, antibody, vaccine
INTRODUCTION
Influenza viruses pose multiple threats to public health, including seasonal epidemics in the human population, disease burdens in agricultural animal species, and global pandemics. Influenza infection typically elicits long-lived strain-specific immunity, and subsequent strains must evade this response by antigenic variation [1]. Antigenic drift is the accumulation of mutations in mainly two major envelope glycoproteins of seasonal influenza viruses, whereas antigenic shift involves introduction of viral antigens completely novel to most of the human population, either by reassortment of the segmented genome with an animal-lineage virus or by the direct transmission of animal strains to humans. The HA glycoprotein, which mediates attachment and fusion with the host cell membrane, is the prime target for neutralizing antibodies. Several defined epitopes surrounding the HA receptor binding domain [2,3] are frequently mutated in the course of antigenic drift variation [4]. HA proteins of type A influenza viruses have been classified into 16 subtypes based on serological cross-reactivity.
The other major envelope protein of influenza viruses is NA, a glycoprotein with sialidase enzymatic activity. Among influenza A viruses there are nine known subtypes of NA, based on serological cross-reactivity. Type B influenza viruses are not classified into multiple HA or NA subtypes. NA-specific antibodies are not known to neutralize viral infectivity, but they can sharply inhibit replication efficiency and reduce the severity of disease upon infection [5,6]. On a related note, the high efficacy of NA inhibitor drugs (e.g. oseltamivir, zanamivir) against many influenza viruses demonstrates the importance of NA to the viral replication cycle [7]. Because well-matched antibodies to HA are sufficient to block infection, whereas NA antibodies exert most of their effects further downstream in the infection process, vaccine efficacy has often been measured and interpreted as a function of HA antibody induction. However, the NA response is potentially quite important in cases of HA mismatch between a vaccine strain and the predominantly circulating seasonal or pandemic viruses.
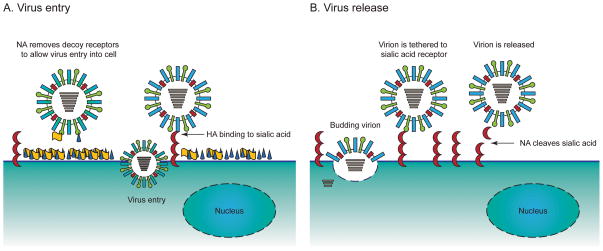
NA protein is a homotetramer composed of monomers typically 470 amino acids in length (reviewed by Air and Laver [8] and Colman [9]). Each monomer contains a short cytoplasmic domain, a transmembrane region, a narrow stalk up to about 80 amino acids in length, and a globular head domain. Structures of NA proteins from subtypes N1, N2, and N9 have been characterized by crystallography, and all share the same general morphology [10–12]. The box-like tetrameric head of NA has sialidase catalytic sites located at four upper vertices (Figure 1). NA normally protrudes a similar length from the viral envelope as does HA; exceptions to this rule, when reduced stalk length makes NA shorter than HA, favor stronger receptor attachment [13]. Epitopes for NA inhibiting antibodies are located predominantly on the globular head of the protein (Figure 1) [14]. A suggested mechanism by which NA facilitates viral entry into host cells (Figure 2A) is by aiding the penetration of respiratory tract mucins or the glycocalyx barrier of respiratory epithelial cells [15]. Functions of NA include mediating detachment of nascent virions from host cells and preventing aggregation of virions (aiding their dispersal). During the course of influenza replication, NA functions to cleave sialic acid carbohydrate residues on the cell surface (Figure 2B), thus liberating nascent influenza virions and helping to facilitate virus spread to naïve cells [8,16].
Figure 1. Structure of the influenza virus neuraminidase protein.
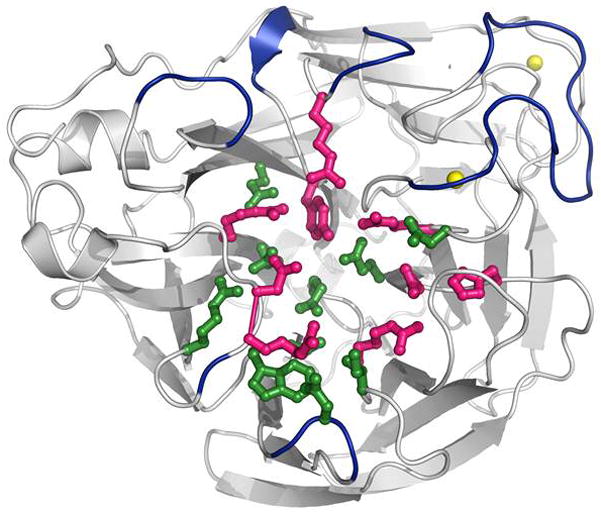
The NA structure shown is from the H1N1 influenza virus strain A/California/04/2009 (PDB code 3NSS). Active site residues are colored magenta, framework residues are colored green, and calcium ions are shown as yellow spheres. Antigenic epitopes are colored blue and modeled after previously determined antigenic sites of N2 influenza viruses [14]. Conversion of antigenic sites to N1 numbering was achieved using previously described method [112].
Figure 2. Functionality of the neuraminidase of influenza viruses.
The NA glycoprotein of influenza viruses is an enzyme sialidase which possesses multiple functions during virus replication. A) Virus entry: In addition to the binding activity of HA, NA contributes to entry of the influenza virus particle by removal of decoy receptors on mucins on the host cell allowing for virus penetration. B) Virus release: NA catalyzes the cleavage of glycosidic linkages: α 2,6 or α 2,6 sialic acid residues on the surface of host cells. Progeny virions are released from infected cells.
The enzymatic activity of NA can be assayed functionally by several methods, including quantifying temperature-dependent reversal of erythrocyte hemagglutination by the virus or the liberation of sialic acid from a substrate that mimics cellular glycoproteins. Small and large substrates can be used to characterize different properties of the enzyme or enzyme inhibitory antibodies, including specificity/preference for α2–3 versus α2–6 linkages at the receptor’s terminal sialic acid [16]. The classical substrate for assaying NA inhibiting (NI) antibodies is fetuin, a large and complex glycoprotein typically purified from fetal bovine serum. Virus or purified NA incubated in the presence of fetuin liberates N-acetylneuraminic acid molecules. A colorimetric chemical assay for free sialic acid has traditionally been used in NA serology assays [17,18]. However, this “thiobarbituric acid” (TBA) assay has practical drawbacks, including the use of several hazardous compounds. The TBA assay in its classical, “macro” form is an impractical method for high-throughput serology. In recent years, the method was successfully miniaturized to a 96-well format, in which all incubations can be conducted in a standard thermocycler [19]. Another microplate-formatted technique to measure functional NA activity is based on selective binding of the peanut agglutinin lectin (PNA) [20,21]. PNA binds to fetuin only after terminal sialic acid residues are cleaved by NA. In the assay, fetuin is coated on the microplate wells, viral antigen and serially-diluted serum are added, and cleaved carbohydrate moieties are detected with a PNA-HRP (horse radish peroxidase) conjugate. This version of the functional NA assay may prove more amenable to high-throughput serology applications. A summary of the main NA antibody assay methods currently in use is listed in Table 1.
Table 1. Methods for analysis of NA-specific antibodies.
Listed in this table are methods currently in use for serological evaluation of NA-specific antibodies, and practical considerations associated with each.
| Assay format | Main advantage | Main drawback |
|---|---|---|
| NA protein binding (e.g. ELISA) [25,27,88] | Efficient | Does not demonstrate inhibition of NA function |
| Inhibition of NA enzymatic activity on sialic acid substrates | Detection of functional antibodies | In all formats, viral reagent with antigenically matched HA may confound results |
| Specific format: | ||
| Microplate TBA method with fetuin substrate and colorimetric readout [19] | Large molecule mimics natural substrate | Hazardous chemicals |
| Lectin-HRP detection of cleaved sialic acid on plate- bound fetuin [20,21] | Large molecule mimics natural substrate | Long procedure1 |
| Cleavage of muNANA with fluorescence readout [25,26] | Rapid procedure | Small molecule an imperfect mimic of natural substrate2 |
As with the microplate TBA method, an overnight incubation for enzyme digestion is typical.
Some NA inhibitory antibodies may not inhibit cleavage of small sialic acid substrates [23].
There are also small-molecule NA substrates which lend themselves to fluorescence or colorimetric readouts of NA activity. One of these, 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (muNANA), releases a fluorescent product after cleavage by NA. This forms the basis of a very simple and high-throughput assay for NA activity, which is routinely used to test circulating influenza strains for resistance to NA inhibitor drugs [22]. Unfortunately, muNANA may not be a reliable substrate for NI antibody assays because its small size allows it to enter the NA catalytic site even in the presence of some antibodies that would block contact with larger substrates (such as cellular surface glycans or fetuin) [23]. This is presumably less problematic with antibodies that bind in close proximity to the catalytic site, which could explain why some investigators have detected serum NI antibodies with muNANA-based inhibition assays [24,25].
Animal studies have shown protective roles for NA antibodies in vivo. Passive immunization experiments in mice have demonstrated that NA antibodies alone can reduce the severity of challenge infection [5,26,27]. Although it is difficult to make equivalent determinations in human cohorts, clinical data pointing to an inverse correlation between NA antibody titers and the incidence of seasonal human influenza infection have been reported [28,29]. The protective mechanisms of NA-specific antibodies during infection presumably correspond to all functional roles of NA protein (Figure 2). Specifically, NA can interact with antibodies that generally bind near the catalytic sites [30]. Electron microscopy data support the concept that NA antibodies can inhibit elution of nascent virions from infected cells and promote aggregation of the viral particles [31]. Because NA may aid in virus infection of the respiratory epithelium [15], antibodies which inhibit these NA functions might hinder the establishment of infection in vivo. Also of note, there is evidence that influenza viral NA can potentiate secondary bacterial infection by exposing bacterial receptors in the respiratory tract [32]. In this instance, NA-specific antibodies may ultimately protect against the secondary pathogen [33].
One key to understanding protective NA antibodies is to define regions of the protein at which they bind. NA epitopes of the N2 subtype were identified through studies that analyzed escape mutations selected in the presence of N2-specific monoclonal antibodies [30,34]. Most of the amino acid positions susceptible to mutation map to structural loops surrounding the catalytic site. Sequence data from clinical isolates across many years reveal a high frequency of mutation at or near many of the same positions, supporting their relevance to antigenic drift [35–37]. The existence of NA antigenic drift in H1N1 and H3N2 human lineages was demonstrated with functional assays for antibody cross-reactivity [38,39]. According to recent analyses, NA antigens from H3N2 and H1N1 seasonal influenza vaccine strains of the 1990s and 2000s underwent discontinuous antigenic drift with significant periods of stasis sometimes occurring between drift events [40]. In some cases, comparison between one vaccine strain and its successor showed marked variation between HA antigens but little change between NA antigens, and in certain cases NA varied more than HA.
VACCINE-INDUCED NA ANTIBODIES
Currently licensed trivalent inactivated (TIV) and live-attenuated influenza virus (LAIV) vaccines are mainstays in the fight against seasonal human influenza. Both formulations are safe and are proven to elicit robust homologous responses to wild-type influenza viruses [41–44]. The immune response generated to seasonal vaccines is highly efficacious in producing homotypic immunity. Due to the highly variable nature of influenza viruses, each vaccine formulation must be updated annually. Although licensed influenza vaccines are designed to induce primarily homotypic protection, studies indicate that immunization with or natural exposure to seasonal influenza A strains induce serum antibodies with cross-reactivity to antigenically distinct viruses [45–49]. While TIV and LAIV vaccine formulations induce HA-specific serum antibodies, live-attenuated influenza vaccines are reportedly more proficient than TIV in inducing broadly-reactive cellular responses [50,51] and nasal IgA antibodies [52].
Serum neutralizing antibodies to the HA protein are the major correlate of immunity afforded by TIV vaccines, and these functional antibodies are routinely measured by hemagglutination inhibition (HI) or micro-neutralization assays. HA-specific antibodies do often cross-react to some degree with variant influenza virus strains. This paradoxical response was first described as the phenomenon of antigenic sin [53]. In early human studies, antibody responses to vaccines were in higher titer to not the homologous vaccine strain but rather to unrelated influenza viruses as a result of prior exposures [54]. More recently, immunization with influenza vaccines from either the 1976 “swine flu” H1N1 virus or contemporary 2006–2009 seasonal H1N1 virus elicited cross-reactive neutralizing antibodies to the HA of pH1N1 in mainly elderly human recipients [48,55]. However, the continuous antigenic drift of HA from previous and current influenza vaccine compositions poses a serious challenge towards achieving reliable cross-protection against various influenza strains [56,57]. To achieve optimal immunity against influenza-related morbidity, robust antibody responses must be generated against not merely HA but other proteins as well. In light of this, other influenza virus proteins have been investigated for their immunogenic properties in vaccines.
Several pieces of data point to the importance of immune responses to the NA in conferring immunity [24,27,58]. NA serum antibodies have been detected after TIV or live-attenuated seasonal vaccination [21,55,59–61] although the amount of enzymatically active NA in these vaccines can be variable [62]. One study suggests that the magnitude of the antibody response to NA upon vaccination may be partially dependent upon the activity of the NA which correlates with the amount of NA protein in each vaccine preparation [21]. In this study, individuals > 65 years of age who were immunized with a TIV containing H1N1 or H3N2 influenza components developed strong serum anti-NA antibody responses after a high dose of vaccine. The anti-N2 or N1 titer was associated with the enzymatic activity of the NA component in each vaccine preparation suggesting that an increase in the NA content and enzymatic activity in influenza vaccines is a predictive marker for immunogenicity. In some human vaccine trials, rates of NA antibody response were well below rates of HA response, and weak NA responses were associated with low NA antigen content in the vaccine [63] or antigenic competition with HA [62]. However there is no evidence to date to determine the amount of NA antigen required to elicit a protective response.
Studies in animals to assess the antibody responses generated to the NA content of TIV formulations are limited. However, in one approach, competitive enzyme-linked immunosorbent assays (c-ELISA) were successful at screening for NA homologous and heterologous antibodies (subtypes N2, N3 or N9) from chickens immunized with avian influenza inactivated vaccines [64–66]. These studies demonstrate that, at least in chickens, the sub-typing of NA-specific antibodies can be determined using ELISA-based methods. In other studies, modified influenza vaccine formulations or combinatorial platforms containing the NA protein have been evaluated for protective efficacy. Enveloped virus-like particle (VLPs) formulations have been tested in mice and ferrets [67–69]. In one study, a contributing role for NA in homologous protection against influenza was determined in experiments using a vaccine containing three influenza proteins: M1 (matrix), HA and NA [67]. Collectively, these animal studies along with anti-NA antibody responses in humans highlight the important point that the NA component of influenza vaccines is a likely mediator of protective immunity afforded by influenza vaccines.
Varying degrees of immunogenicity to the NA of influenza vaccines are associated with the type of formulation, e.g., inactivated, live-attenuated, DNA or purified protein. To interpret the role of NA with the use of inactivated or live-attenuated influenza vaccines [70], a myriad of single NA protein-based vaccines have been formulated and tested mainly in animal models. DNA-based influenza vaccines containing the NA component have induced homologous immune responses as well as protection against variants of influenza viruses belonging to the same subtype [24,27,58,71–76]. DNA vaccines that encode NA protect mice against lethal challenge infection in terms of reduced replication and disease severity, though they do not block the establishment of infection [24], and a combination of HA and NA DNA vaccines is most effective [76]. This is consistent with long-standing knowledge that NA antibodies do not block viral infectivity but reduce viral replication [5,77]. The contribution of antibodies has been assessed via passive-transfer studies in which serum from Balb/c mice immunized with DNA vaccines encoding the NA segment of human influenza strains, e.g., A/New Caledonia/20/99 (H1N1) or A/Vietnam/1203/04 (H5N1) afforded partial protection against heterologous or homologous weight loss and virus challenge in recipient mice [27].
NA proteins purified from virus preparations or vaccines are also highly immunogenic [6,18,78,79], including in human subjects [79]. Animal studies suggest that supplementation of purified NA to influenza vaccines may broaden the immune response to heterologous challenge. The addition of NA purified from A/Johannesburg/33/94 (H3N2) to monovalent inactivated H3N2 influenza vaccine significantly reduced virus replication in animals after subsequent challenge compared to vaccines used alone [80]. Evidence shows that the magnitude of the antibody response from purified NA is equivalent to that of purified HA [6] but in some cases may be inhibited by HA. On the surface of an influenza viral particle, HA is several-fold more abundant than NA [81]. Antigenic competition has been reported between NA and HA, where HA is dominant over NA in lymphocyte priming in several models [82,83]. However this can be overcome by either injecting purified NA or treating viral particles with an agent (e.g. detergent) that dissociates the two surface antigens from one another; the physical separation of HA from NA eliminates this competitive phenomenon [78,84].
NA ANTIBODY RESPONSES FROM INFECTION WITH PANDEMIC OR ZOONOTIC INFLUENZA VIRUS STRAINS
Antibodies raised against NA upon natural infection with influenza viruses have been long described [85]. Serum antibodies raised against the NA component of these viruses is a proposed correlate of immunity against infection with several influenza strains [18,24,26,28,58,84,86–88]. In some cases, exposure to older influenza strains promotes cross-reactive NA antibody responses to emerging influenza viruses.
The effects of prior exposure to influenza strains on emerging influenza outbreaks have been demonstrated with the H3N2 influenza subtype. In one clinical study, children naturally infected with influenza strain A/Port Chalmers/1/73 (H3N2) or A/England/42/72 (H3N2) during 1972–73 influenza seasons developed serum antibodies that were highly reactive to the 1968 pandemic virus, A/Hong Kong/1/68 [(HK/68) (H3N2)] [89]. Similar results were seen in another study involving young children [90]. Some evidence suggests that pre-existing anti-NA antibodies generated from previous exposure to different viruses may influence the severity of pandemic infection. For example prior H2N2 infections were associated with protection against HK/68 virus. In one finding, adults between the ages of 20–45 who developed pre-existing cross-reactive NA antibodies prior to the outbreak of HK/68, displayed reduced disease severity independent of HA antibodies [91]. Also, the duration of virus replication was proportional to the level of anti-N2 NA antibodies in humans [28]. These studies indicate that pre-existing NA antibodies can contribute to immunity in the absence of homologous HA antibody responses.
Regrettably, there exist limited clinically-licensed vaccine options for highly pathogenic avian H5N1 influenza viruses. However, experimental evidence supports the possibility that antibodies to NA of a seasonal H1N1 virus can supply immunity against H5N1 infection. A DNA vaccine encoding solely the NA of A/Vietnam/1203/04 H5N1 protected against virus lethality upon homologous challenge in mice [27]. In that same study, NA from an H1N1 strain used in seasonal vaccines induced partial protection against the same H5N1 challenge, and a similar level of cross-lineage protection was also observed in naïve mice that were passively immunized with seasonal N1-specific serum. Therefore, antibodies generated against the NA of seasonal H1N1 apparently cross-reacted well enough to reduce the severity of H5N1 disease in a mammalian model.
Growing evidence supports the role of exposures to seasonal H1N1 influenza strains in cross-reactive immunity to the pandemic H1N1 2009 virus (pH1N1). Immunization with or natural exposure to seasonal influenza A strains induced anti-HA serum antibodies with cross-reactivity to antigenically distinct pH1N1 [48,49]. Previously it was shown that seasonal influenza viruses from 2004–09 seasons possess the capacity to boost cross-reactive serum antibodies to the NA of pH1N1, most prominently in elderly individuals [21,55]. In support of this, priming mice with seasonal influenza H1N1 strains from the 2006 and 2007 seasons contributed to immunity against pH1N1 [88].
NA IN RELATION TO CONTEMPORARY VACCINE TECHNOLOGIES
Several factors have fueled heightened interest in new influenza vaccine technologies in the past 10–15 years. These factors include the threat of a pandemic caused by H5N1 or other avian influenza viruses; difficulties manufacturing adequate supplies of seasonal vaccine antigen (due to the poor growth of selected vaccine strains in egg culture); and the 2009 H1N1 pandemic. All of which underscored the importance of rapid vaccine manufacturing in the face of a novel strain adapted to humans. As with conventional vaccines, most of these strategies are justifiably focused on producing and delivering HA antigen. However, across the broad range of approaches being explored, there are important implications with regards to inducing NA-specific immune responses (Table 1).
A novel approach which has advanced to Phase III clinical trials as a seasonal influenza vaccine is recombinant HA (rHA) expression in an insect cell system with a baculovirus vector [92]. This technology should be readily adaptable to handle novel virus strains, and clinical data indicate that the insect cell-derived proteins induce functional antibody responses in humans [93,94]. It is worth noting, however, that the vaccine contains no NA antigen. The HA and NA could potentially both be expressed from the baculovirus vector and blended to formulate a vaccine that contains standardized amounts of each antigen [95]. However, doubling the number of recombinant proteins to express, purify, and formulate during vaccine manufacture may not have a favorable cost-benefit ratio.
The same principles of baculovirus-vectored antigen expression in insect cell culture are utilized in the enveloped virus-like particle (VLP) vaccine approach (reviewed by Kang et al [96]). In this method, the HA, NA, and M1 genes of a given strain are each cloned into the vector (individually or together). The recombinant baculoviruses are used to infect insect cell cultures. VLPs, which mimic the morphology of whole virions, self-assemble from these three protein constituents plus cellular envelope material. Pre-clinical studies have demonstrated immunogenicity and efficacy for VLPs against homologous and some heterologous influenza viruses. Published data indicating the amount of NA incorporated into VLPs, and the degree to which this mirrors the content of whole virions is fairly limited, but the NA quantity appears less than HA [97,98]. However, significant titers of anti-NA antibody were detected by ELISA in sera of mice immunized with VLP antigens representing H5N1 influenza strains [98]. This indicates that NA packaged into a VLP can retain immunogenicity and the VLP vaccine may elicit beneficial NI titers in human recipients as well. One potential concern with VLP antigens is the possibility of intra-virion competition between HA and NA antigens (discussed in a previous section) [84]. Influenza VLPs containing different combinations of HA, NA, and M1 have also been produced successfully in transfected human cells lines [99].
A variation on the baculovirus vectoring strategy to produce VLP expressing influenza antigens is pseudotyped lentiviral particles that consist of HA and NA along with Gag [100]. Antigens constructed in this manner, with HA and NA of either H5N1 avian or H1N1 seasonal influenza viruses, had significant NA and HA activity, and they protected mice and ferrets from virulent experimental infection. A seasonal H1N1 pseudotyped VLP partially protected ferrets against H5N1 challenge, pointing to likely cross-reactivity by N1-specific antibodies or T lymphocytes. It is not clear from published data whether the presence of Gag along with HA and NA improves immunogenicity in comparison with a VLP containing only influenza proteins. The authors reported that batch yields and particle diameters are highly consistent in the pseudotyped VLP system, properties that may aid manufacturing.
Recently, recombinant HA and NA antigens were designed with N-terminal regions [stalk, transmembrane (TM), cytoplasmic] replaced by simpler multimerization domains, and expressed as soluble proteins in mammalian cell culture [101]. The multimerization domains were chosen to maintain the trimeric structure of HA and the tetrameric structure of NA. The soluble nature of these chimeric proteins allowed for one-step chromatography purification. The HA ectodomain and NA globular head of 2009 pandemic H1N1 were expressed in their native form, preserving epitopes for induction of functional antibodies, although both the HA- and NA-specific antibody responses required formulation with an adjuvant: immune stimulating complex-matrix M (ISCOM Matrix M). Higher levels of HI antibodies were induced by a mixture of HA and NA antigens than HA alone. After homologous challenge, animals vaccinated with HA alone had greater protection in terms of viral replication, but NA alone conferred more protection from clinical disease signs. Ferrets vaccinated with both antigens had the greatest all-around protection. These results suggest that benefits of NA immunity can be reaped more effectively if influenza vaccines are produced in recombinant expression systems, partly because it allows easy adjustments to the HA:NA ratio, whereas traditional purification from viral particles typically yields more HA.
Influenza antigens expressed in attenuated viral vectors, including vaccinia and replication-deficient human adenovirus, have been tested in numerous animal models. Vectored NA antigens were included in a limited subset of these studies. Vaccinia-vectored NA of the 2009 pandemic H1N1 virus given to mice in two doses, elicited robust NI antibody titers and primed CD4+ and CD8+ T lymphocytes for antigen-specific responses [69]. These responses were associated with partial protection against homologous challenge, with respect to viral replication in lungs. An alphavirus-based virus-like replicon particle expressing NA of a highly pathogenic avian influenza virus (H5N2) was used to vaccinate chickens [102]. This construct induced significant serum NI titers after the second and third dosages, which were associated with protection from lethal homologous challenge (reduced oropharyngeal shedding and increased survival rate). One recent study yielded insight into the efficacy of virally-vectored NA in a non-human primate [103]. African green monkeys were immunized by the intranasal/intratracheal route with NA of a highly pathogenic avian influenza virus expressed in a Newcastle disease virus vector. This vaccine elicited significant serum NI titers and after a second dose, virus neutralizing antibody titers. This vaccine largely blocked detectable replication of highly pathogenic challenge virus, as measured in nasal swabs, tracheal lavage, and lung tissue. Taken together, the results of these animal studies show that virally vectored NA antigens can elicit protective responses against antigenically similar challenge viruses. As with conventional vaccines, combinations of vectored HA and NA antigens seem likely to provide broader and more protective antibody responses than one antigen alone. In the context of veterinary vaccines or pre-pandemic vaccine stockpiles, where the range of possible variations in HA is greatest, the benefits of including vectored NA antigens may balance more favorably against the extra cost of manufacturing them.
HURDLES TO IMPROVING NA RESPONSES FROM VACCINES
NI antibody responses in humans immunized with seasonal vaccines have rarely been monitored in clinical trials. Often, the rate and magnitude of responses against NA antigens has been less than against corresponding HAs [63,104,105]. This is not surprising in the case of inactivated virus vaccines, since commercial manufacturing processes (e.g. virion disruption, inactivation, purification, storage buffer formulation) are designed to optimize the recovery, potency, and stability of HA. Furthermore, trivalent vaccines are formulated to meet a minimum HA potency of 15 μg per strain per dose, whereas NA content is not standardized. In an ideal situation, NA would also be standardized in vaccine formulations, but achieving this might be next to impossible when the two proteins derive from the same batch of virus. With vaccines made from recombinant antigens, formulating standard amounts of both HA and NA would be a more straight-forward task. However, as mentioned previously, preparing the second recombinant protein could approximately double the expense and time of manufacturing.
One factor that likely affects the strength of NA antibody responses to inactivated and protein vaccines is antigen stability. NA proteins are subject to loss of stability under conditions of insufficient divalent cation concentrations [106,107]. A loss of NA sialidase activity has been closely associated with loss of potency, i.e. much weaker induction of functional NI antibodies [107]. Treatment with a reducing agent was also shown to affect the NA antigenicity of vaccine preparations [108]. Minor adjustments to physical conditions during antigen purification and storage may improve the quality of NA antigens and enhance vaccine efficacy.
Another way to potentially improve NA-specific immune responses to vaccines is to monitor NA antigenic drift in circulating influenza virus strains. The selection of H1N1, H3N2 and influenza B strains for vaccine manufacturing is guided by data from a global surveillance program that monitors evolution of HA and NA genes and the antigenic drift of HA [109]. There have been circumstances when approved seasonal vaccine strains were poorly matched to NA antigen, notably in the case of A/Panama/2007/1999 [(Pan/99) (H3N2)]. The Pan/99 strain was substituted for the recommended H3N2 strain, A/Moscow/10/1999 (Mos/99), because it grew more efficiently in eggs and was antigenically similar in terms of HI. North American vaccines contained this strain for four seasons, even though its NA gene sequence suggested dissimilarity to the NA of Mos/99 and the predominant circulating strains [110]. More recently, analysis of NI cross-reactivity between the strains confirmed there was antigenic mismatch between NAs of Pan/99 and Mos/99 [40]. Carrying out NA drift analysis on a scale similar to what takes place semi-annually for HA antigenic drift would be an imposing task considering that even the newer, microplate-formatted NI assays are more cumbersome than standard HI assays. However, a useful and practical step can be envisioned where the reference strains used in global HAI drift studies would be tested against a panel of strain-specific animal sera to assess their suitability as sources of NA antigen.
Regulatory guidance given to manufacturers by the European Pharmacopoeia sets modest and flexible standards to help ensure that appropriate NA proteins are present in seasonal vaccines [111]. These guidelines instruct manufacturers to test each seed virus to confirm the proper NA origin, confirm the presence of NA in the first three monovalent bulk virus lots of every strain (by NA enzymatic activity or immunological methods), and use processes that preserve NA antigenicity. Although a case might be made for more rigorous steps, this set of guidelines could be a useful template for consideration by other vaccine regulating agencies. In summary, many conventional and next-generation influenza vaccine platforms already include NA proteins, and their effectiveness might be improved by innovative steps to optimize NA quality and quantity.
Table 2. Neuraminidase antigens in various conventional and developmental influenza vaccine platforms.
Summarized here are two conventional and three prospective new vaccine technologies, with references to published reports that have demonstrated the induction of NA-specific antibodies by each class of vaccine.
| Vaccine Platform | NA Protein Content | NA Antibody Reported |
|---|---|---|
| Inactivated (split or subunit) | NA present | Human [21,55,59,61] |
| Amount may vary by strain & process | ||
| Live-attenuated | NA expressed as virus replicates | Human [60] |
| Amount may vary by strain | ||
| Purified protein | NA optional | Animal models [95,101] |
| NA & HA could both be blended to standardized levels | Human [79] | |
| Virus-like particle | NA optional (favorable for VLP yield) | Animal model [97] |
| Amount may vary by strain | ||
| Virally-vectored | NA optional | Animal models [102,103] |
Acknowledgments
This review was supported by the National Institute of Allergy and Infectious Diseases (Contract No. HHSN266200700005C), the United States Department of Agriculture (Specific Cooperative Agreement No. 58-3625-0-612) and the American Lebanese Syrian Associated Charities. We thank Rebecca M. Dubois for producing the three dimensional image of the NA structure.
ABBREVIATIONS
- HI
hemagglutinin inhibition
- HRP
horse radish peroxidase
- ISCOM
immune stimulating complex
- muNANA
2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid
- NI
neuraminidase inhibition
- pH1N1
pandemic H1N1 2009 influenza A virus
- pHA
Hemagglutinin of pandemic H1N1 2009 influenza A virus
- PNA
peanut agglutinin lectin
- rHA
recombinant Hemagglutinin
- TBA
thiobarbituric acid
- TIV
trivalent inactivated vaccine
- TM
transmembrane
- VLPs
virus like particles
Footnotes
CONFLICT OF INTEREST
The authors have no competing interests.
References
- 1.Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 2.Gerhard W, Yewdell J, Frankel ME, Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981;290:713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- 3.Jackson DC, Murray JM, White DO, Gerhard WU. Enumeration of antigenic sites of influenza virus hemagglutinin. Infect Immun. 1982;37:912–918. doi: 10.1128/iai.37.3.912-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang SF, Lee YM, Chan YJ, Liu HF, Yen YF, Liu WT, Huang JC, Chen YM. Influenza A virus in Taiwan, 1980–2006: Phylogenetic and antigenic characteristics of the hemagglutinin gene. J Med Virol. 2009;81:1457–1470. doi: 10.1002/jmv.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulman JL, Khakpour M, Kilbourne ED. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol. 1968;2:778–786. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson BE, Bucher DJ, Kilbourne ED. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol. 1989;63:1239–1246. doi: 10.1128/jvi.63.3.1239-1246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garman E, Laver G. Controlling influenza by inhibiting the virus’s neuraminidase. Curr Drug Targets. 2004;5:119–136. doi: 10.2174/1389450043490604. [DOI] [PubMed] [Google Scholar]
- 8.Air GM, Laver WG. The neuraminidase of influenza virus. Proteins. 1989;6:341–356. doi: 10.1002/prot.340060402. [DOI] [PubMed] [Google Scholar]
- 9.Colman PM. Influenza virus neuraminidase: structure, antibodies, and inhibitors. Protein Sci. 1994;3:1687–1696. doi: 10.1002/pro.5560031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varghese JN, Laver WG, Colman PM. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983;303:35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- 11.Bossart-Whitaker P, Carson M, Babu YS, Smith CD, Laver WG, Air GM. Three-dimensional structure of influenza A N9 neuraminidase and its complex with the inhibitor 2-deoxy 2,3-dehydro-N-acetyl neuraminic acid. J Mol Biol. 1993;232:1069–1083. doi: 10.1006/jmbi.1993.1461. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Zhu X, Dwek RA, Stevens J, Wilson IA. Structural characterization of the 1918 influenza virus H1N1 neuraminidase. J Virol. 2008;82:10493–10501. doi: 10.1128/JVI.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Els MC, Air GM, Murti KG, Webster RG, Laver WG. An 18-amino acid deletion in an influenza neuraminidase. Virology. 1985;142:241–247. doi: 10.1016/0042-6822(85)90332-0. [DOI] [PubMed] [Google Scholar]
- 14.Colman PM, Varghese JN, Laver WG. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983;303:41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- 15.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol. 2004;78:12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobasa D, Kodihalli S, Luo M, Castrucci MR, Donatelli I, Suzuki Y, Suzuki T, Kawaoka Y. Amino acid residues contributing to the substrate specificity of the influenza A virus neuraminidase. J Virol. 1999;73:6743–6751. doi: 10.1128/jvi.73.8.6743-6751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WARREN L. The thiobarbituric acid assay of sialic acids. 1959:1971–1975. 1959/08/01. [PubMed] [Google Scholar]
- 18.Kilbourne ED, Laver WG, Schulman JL, Webster RG. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968;2:281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandbulte MR, Gao J, Straight TM, Eichelberger MC. A miniaturized assay for influenza neuraminidase-inhibiting antibodies utilizing reverse genetics-derived antigens. Influenza and Other Resp Viruses. 2009;3:233–240. doi: 10.1111/j.1750-2659.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambre CR, Terzidis H, Greffard A, Webster RG. Measurement of anti-influenza neuraminidase antibody using a peroxidase-linked lectin and microtitre plates coated with natural substrates. J Immunol Methods. 1990;135:49–57. doi: 10.1016/0022-1759(90)90255-t. [DOI] [PubMed] [Google Scholar]
- 21.Cate TR, Rayford Y, Nino D, Winokur P, Brady R, Belshe R, Chen W, Atmar RL, Couch RB. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine. 2010;28:2076–2079. doi: 10.1016/j.vaccine.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubareva LV, Webster RG, Hayden FG. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob Agents Chemother. 2001;45:3403–3408. doi: 10.1128/AAC.45.12.3403-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster RG, Brown LE, Laver WG. Antigenic and biological characterization of influenza virus neuraminidase (N2) with monoclonal antibodies. Virology. 1984;135:30–42. doi: 10.1016/0042-6822(84)90114-4. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Kadowaki S, Hagiwara Y, Yoshikawa T, Matsuo K, Kurata T, Tamura S. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine. 2000;18:3214–3222. doi: 10.1016/s0264-410x(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 25.Gavrilov V, Orekov T, Alabanza C, Porika U, Jiang H, Connolly K, Pincus S. Influenza virus-like particles as a new tool for vaccine immunogenicity testing: Validation of a neuraminidase neutralizing antibody assay. J Virol Methods. 2011;173:364–373. doi: 10.1016/j.jviromet.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Deroo T, Jou WM, Fiers W. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine. 1996;14:561–569. doi: 10.1016/0264-410x(95)00157-v. [DOI] [PubMed] [Google Scholar]
- 27.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4:e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy BR, Kasel JA, Chanock RM. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med. 1972;286:1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- 29.Naikhin AN, Tsaritsina IM, Oleinikova EV, Syrodoeva LG, Korchanova NL, Denisov GM, Shvartsman Ya S. The importance of antineuraminidase antibodies in resistance to influenza A and immunologic memory for their synthesis. J Hyg (Lond) 1983;91:131–138. doi: 10.1017/s0022172400060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Air GM, Els MC, Brown LE, Laver WG, Webster RG. Location of antigenic sites on the three-dimensional structure of the influenza N2 virus neuraminidase. Virology. 1985;145:237–248. doi: 10.1016/0042-6822(85)90157-6. [DOI] [PubMed] [Google Scholar]
- 31.Seto JT, Chang FS. Functional significance of sialidase during influenza virus multiplication: an electron microscope study. J Virol. 1969;4:58–66. doi: 10.1128/jvi.4.1.58-66.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187:1000–1009. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 33.Huber VC, Peltola V, Iverson AR, McCullers JA. Contribution of vaccine-induced immunity toward either the HA or the NA component of influenza viruses limits secondary bacterial complications. J Virol. 2010;84:4105–4108. doi: 10.1128/JVI.02621-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laver WG, Air GM, Webster RG, Markoff LJ. Amino acid sequence changes in antigenic variants of type A influenza virus N2 neuraminidase. Virology. 1982;122:450–460. doi: 10.1016/0042-6822(82)90244-6. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Cox NJ, Bender CA, Regnery HL, Shaw MW. Genetic variation in neuraminidase genes of influenza A (H3N2) viruses. Virology. 1996;224:175–183. doi: 10.1006/viro.1996.0519. [DOI] [PubMed] [Google Scholar]
- 36.Fanning TG, Reid AH, Taubenberger JK. Influenza A virus neuraminidase: regions of the protein potentially involved in virus-host interactions. Virology. 2000;276:417–423. doi: 10.1006/viro.2000.0578. [DOI] [PubMed] [Google Scholar]
- 37.Bragstad K, Nielsen LP, Fomsgaard A. The evolution of human influenza A viruses from 1999 to 2006: a complete genome study. Virol J. 2008;7:40. doi: 10.1186/1743-422X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luther P, Bergmann KC, Oxford JS. An investigation of antigenic drift of neuraminidases of influenza A (H1N1) viruses. J Hyg (Lond) 1984;92:223–229. doi: 10.1017/s002217240006424x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilbourne ED, Johansson BE, Grajower B. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc Natl Acad Sci U S A. 1990;87:786–790. doi: 10.1073/pnas.87.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandbulte MR, Westgeest KB, Gao J, Xu X, Klimov AI, Russell CA, Burke DF, Smith DJ, Fouchier RA, Eichelberger MC. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1113801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, Gao Q, Zhang Z, Liu Y, Wang Z, Yang M, Sun R, Li C, Lin S, Ji M, Liu Y, Wang X, Wood J, Feng Z, Wang Y, Yin W. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368:991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 42.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Hoschler K, Zambon MC. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 43.Keitel WA, Couch RB, Quarles JM, Cate TR, Baxter B, Maassab HF. Trivalent attenuated cold-adapted influenza virus vaccine: reduced viral shedding and serum antibody responses in susceptible adults. J Infect Dis. 1993;167:305–311. doi: 10.1093/infdis/167.2.305. [DOI] [PubMed] [Google Scholar]
- 44.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 45.van Maurik A, Sabarth N, Dacho H, Bruhl P, Schwendinger M, Crowe B, Barrett N, Kistner O, Howard M. Seasonal influenza vaccine elicits heterosubtypic immunity against H5N1 that can be further boosted by H5N1 vaccination. Vaccine. 2010;28:1778–1785. doi: 10.1016/j.vaccine.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Gioia C, Castilletti C, Tempestilli M, Piacentini P, Bordi L, Chiappini R, Agrati C, Squarcione S, Ippolito G, Puro V, Capobianchi MR, Poccia F. Cross-subtype immunity against avian influenza in persons recently vaccinated for influenza. Emerg Infect Dis. 2008;14:121–128. doi: 10.3201/eid1401.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis. 2008;198:1016–1018. doi: 10.1086/591465. [DOI] [PubMed] [Google Scholar]
- 48.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz JM. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 49.Katz J, Hancock K, Veguilla V, Zhong W, Lu XH, Sun H, Butler E, Dong L, Liu F, Li ZN, Devos J, Gargiullo P, Cox N. Serum Cross-Reactive Antibody Response to a Novel Influenza A (H1N1) Virus After Vaccination With Seasonal Influenza Vaccine (Reprinted from MMWR, vol 58, pg 521–524, 2009) JAMA. 2009;302:249–250. [PubMed] [Google Scholar]
- 50.He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mbawuike IN, Piedra PA, Cate TR, Couch RB. Cytotoxic T lymphocyte responses of infants after natural infection or immunization with live cold-recombinant or inactivated influenza A virus vaccine. J Med Virol. 1996;50:105–111. doi: 10.1002/(SICI)1096-9071(199610)50:2<105::AID-JMV1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 52.Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002;20:1340–1353. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- 53.Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104:572–578. [Google Scholar]
- 54.Fazekas de SG, Webster RG. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966;124:331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marcelin G, Bland HM, Negovetich NJ, Sandbulte MR, Ellebedy AH, Webb AD, Griffin YS, DeBeauchamp JL, McElhaney JE, Webby RJ. Inactivated seasonal influenza vaccines increase serum antibodies to the neuraminidase of pandemic influenza A(H1N1) 2009 virus in an age-dependent manner. J Infect Dis. 2010;202:1634–1638. doi: 10.1086/657084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 57.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25:6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 58.Bragstad K, Martel CJ, Thomsen JS, Jensen KL, Nielsen LP, Aasted B, Fomsgaard A. Pandemic influenza 1918 H1N1 and 1968 H3N2 DNA vaccines induce cross-reactive immunity in ferrets against infection with viruses drifted for decades. Influenza Other Respi Viruses. 2011;5:13–23. doi: 10.1111/j.1750-2659.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Powers DC, Kilbourne ED, Johansson BE. Neuraminidase-specific antibody responses to inactivated influenza virus vaccine in young and elderly adults. Clin Diagn Lab Immunol. 1996;3:511–516. doi: 10.1128/cdli.3.5.511-516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexandrova GI, Polezhaev FI, Budilovsky GN, Garmashova LM, Topuria NA, Egorov AY, Romejko-Gurko YR, Koval TA, Lisovskaya KV, Klimov AI. Recombinant cold-adapted attenuated influenza A vaccines for use in children: reactogenicity and antigenic activity of cold-adapted recombinants and analysis of isolates from the vaccinees. Infect Immun. 1984;44:734–739. doi: 10.1128/iai.44.3.734-739.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogra PL, Chow T, Beutner KR, Rubi E, Strussenberg J, DeMello S, Rizzone C. Clinical and immunologic evaluation of neuraminidase-specific influenza A virus vaccine in humans. J Infect Dis. 1977;135:499–506. doi: 10.1093/infdis/135.4.499. [DOI] [PubMed] [Google Scholar]
- 62.Kendal AP, Noble GR, Dowdle WR. Neuraminidase content of influenza vaccines and neuraminidase antibody responses after vaccination of immunologically primed and unprimed populations. J Infect Dis. 1977;136 (Suppl):S415–S424. doi: 10.1093/infdis/136.supplement_3.s415. [DOI] [PubMed] [Google Scholar]
- 63.Kendal AP, Bozeman FM, Ennis FA. Further studies of the neuraminidase content of inactivated influenza vaccines and the neuraminidase antibody responses after vaccination of immunologically primed and unprimed populations. Infect Immun. 1980;29:966–971. doi: 10.1128/iai.29.3.966-971.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, Mundt E, Mundt A, Sylte M, Suarez DL, Swayne DE, Garcia M. Development and evaluation of an avian influenza, neuraminidase subtype 1, indirect enzyme-linked immunosorbent assay for poultry using the differentiation of infected from vaccinated animals control strategy. Avian Dis. 2010;54:613–621. doi: 10.1637/8844-040409-Reg.1. [DOI] [PubMed] [Google Scholar]
- 65.Avellaneda G, Sylte MJ, Lee CW, Suarez DL. A heterologous neuraminidase subtype strategy for the differentiation of infected and vaccinated animals (DIVA) for avian influenza virus using an alternative neuraminidase inhibition test. Avian Dis. 2010;54:272–277. doi: 10.1637/8677-030409-Reg.1. [DOI] [PubMed] [Google Scholar]
- 66.Kim JN, Byun SH, Kang SY, Mo IP. Evaluation of neuraminidase antigen based competitive enzyme-linked immunosorbent assay in chickens vaccinated with avian influenza inactivated vaccine. Avian Dis. 2010;54:682–685. doi: 10.1637/8747-033009-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 67.Ross TM, Mahmood K, Crevar CJ, Schneider-Ohrum K, Heaton PM, Bright RA. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One. 2009;4:e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ding H, Tsai C, Zhou F, Buchy P, Deubel V, Zhou P. Heterosubtypic Antibody Response Elicited with Seasonal Influenza Vaccine Correlates Partial Protection against Highly Pathogenic H5N1 Virus. PLoS One. 2011;6:e17821. doi: 10.1371/journal.pone.0017821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hessel A, Schwendinger M, Fritz D, Coulibaly S, Holzer GW, Sabarth N, Kistner O, Wodal W, Kerschbaum A, Savidis-Dacho H, Crowe BA, Kreil TR, Barrett PN, Falkner FG. A pandemic influenza H1N1 live vaccine based on modified vaccinia Ankara is highly immunogenic and protects mice in active and passive immunizations. PLoS One. 2010;5:e12217. doi: 10.1371/journal.pone.0012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 71.Torrieri-Dramard L, Lambrecht B, Ferreira HL, Van den BT, Klatzmann D, Bellier B. Intranasal DNA vaccination induces potent mucosal and systemic immune responses and cross-protective immunity against influenza viruses. Mol Ther. 2011;19:602–611. doi: 10.1038/mt.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W, Li W, Li Y, Li H, Wang B, Wang F, Zhu Y, Jiang Z, Zhong L, Li M. Immune effects against influenza A virus and a novel DNA vaccine with co-expression of haemagglutinin- and neuraminidase-encoding genes. J Med Microbiol. 2009;58:845–854. doi: 10.1099/jmm.0.006825-0. [DOI] [PubMed] [Google Scholar]
- 73.Li X, Fang F, Song Y, Yan H, Chang H, Sun S, Chen Z. Essential sequence of influenza neuraminidase DNA to provide protection against lethal viral infection. DNA Cell Biol. 2006;25:197–205. doi: 10.1089/dna.2006.25.197. [DOI] [PubMed] [Google Scholar]
- 74.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu M, Fang F, Chen Y, Wang H, Chen Q, Chang H, Wang F, Wang H, Zhang R, Chen Z. Protection against avian influenza H9N2 virus challenge by immunization with hemagglutinin- or neuraminidase-expressing DNA in BALB/c mice. Biochem Biophys Res Commun. 2006;343:1124–1131. doi: 10.1016/j.bbrc.2006.03.088. [DOI] [PubMed] [Google Scholar]
- 76.Chen Z, Yoshikawa T, Kadowaki S, Hagiwara Y, Matsuo K, Asanuma H, Aizawa C, Kurata T, Tamura S. Protection and antibody responses in different strains of mouse immunized with plasmid DNAs encoding influenza virus haemagglutinin, neuraminidase and nucleoprotein. J Gen Virol. 1999;80 ( Pt 10):2559–2564. doi: 10.1099/0022-1317-80-10-2559. [DOI] [PubMed] [Google Scholar]
- 77.Couch RB, Kasel JA, Gerin JL, Schulman JL, Kilbourne ED. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis. 1974;129:411–420. doi: 10.1093/infdis/129.4.411. [DOI] [PubMed] [Google Scholar]
- 78.Johansson BE, Kilbourne ED. Immunization with purified N1 and N2 influenza virus neuraminidases demonstrates cross-reactivity without antigenic competition. Proc Natl Acad Sci U S A. 1994;91:2358–2361. doi: 10.1073/pnas.91.6.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kilbourne ED, Couch RB, Kasel JA, Keitel WA, Cate TR, Quarles JH, Grajower B, Pokorny BA, Johansson BE. Purified influenza A virus N2 neuraminidase vaccine is immunogenic and non-toxic in humans. Vaccine. 1995;13:1799–1803. doi: 10.1016/0264-410x(95)00127-m. [DOI] [PubMed] [Google Scholar]
- 80.Johansson BE, Matthews JT, Kilbourne ED. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine. 1998;16:1009–1015. doi: 10.1016/s0264-410x(97)00279-x. [DOI] [PubMed] [Google Scholar]
- 81.Murti KG, Webster RG. Distribution of hemagglutinin and neuraminidase on influenza virions as revealed by immunoelectron microscopy. Virology. 1986;149:36–43. doi: 10.1016/0042-6822(86)90084-x. [DOI] [PubMed] [Google Scholar]
- 82.Johansson BE, Moran TM, Bona CA, Kilbourne ED. Immunologic response to influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. III. Reduced generation of neuraminidase-specific helper T cells in hemagglutinin-primed mice. J Immunol. 1987;139:2015–2019. [PubMed] [Google Scholar]
- 83.Johansson BE, Moran TM, Kilbourne ED. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc Natl Acad Sci U S A. 1987;84:6869–6873. doi: 10.1073/pnas.84.19.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johansson BE, Kilbourne ED. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competition. J Virol. 1993;67:5721–5723. doi: 10.1128/jvi.67.10.5721-5723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kilbourne ED, Christenson WN, Sande M. Antibody response in man to influenza virus neuraminidase following influenza. J Virol. 1968;2:761–762. doi: 10.1128/jvi.2.7.761-762.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mozdzanowska K, Maiese K, Furchner M, Gerhard W. Treatment of influenza virus-infected SCID mice with nonneutralizing antibodies specific for the transmembrane proteins matrix 2 and neuraminidase reduces the pulmonary virus titer but fails to clear the infection. Virology. 1999;254:138–146. doi: 10.1006/viro.1998.9534. [DOI] [PubMed] [Google Scholar]
- 87.Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase-specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. J Infect Dis. 1979;140:844–850. doi: 10.1093/infdis/140.6.844. [DOI] [PubMed] [Google Scholar]
- 88.Marcelin G, DuBois R, Rubrum A, Russell CJ, McElhaney JE, Webby RJ. A contributing role for anti-neuraminidase antibodies on immunity to pandemic H1N1 2009 influenza A virus. PLoS One. 2011;6:e26335. doi: 10.1371/journal.pone.0026335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith AJ, Davies JR. Natural infection with influenza A (H3N2). The development, persistance and effect of antibodies to the surface antigens. J Hyg (Lond) 1976;77:271–282. doi: 10.1017/s0022172400024712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wright PF, Ross KB, Thompson J, Karzon DT. Influenza A infections in young children. Primary natural infection and protective efficacy of live-vaccine-induced or naturally acquired immunity. N Engl J Med. 1977;296:829–834. doi: 10.1056/NEJM197704142961501. [DOI] [PubMed] [Google Scholar]
- 91.Monto AS, Kendal AP. Effect of neuraminidase antibody on Hong Kong influenza. Lancet. 1973;1:623–625. doi: 10.1016/s0140-6736(73)92196-x. [DOI] [PubMed] [Google Scholar]
- 92.Cox MM, Patriarca PA, Treanor J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respi Viruses. 2008;2:211–219. doi: 10.1111/j.1750-2659.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.King JC, Jr, Cox MM, Reisinger K, Hedrick J, Graham I, Patriarca P. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy children aged 6–59 months. Vaccine. 2009;27:6589–6594. doi: 10.1016/j.vaccine.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 94.Baxter R, Patriarca PA, Ensor K, Izikson R, Goldenthal KL, Cox MM. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok(R) trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50–64 years of age. Vaccine. 2011;29:2272–2278. doi: 10.1016/j.vaccine.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 95.Johansson BE. Immunization with influenza A virus hemagglutinin and neuraminidase produced in recombinant baculovirus results in a balanced and broadened immune response superior to conventional vaccine. Vaccine. 1999;17:2073–2080. doi: 10.1016/s0264-410x(98)00413-7. [DOI] [PubMed] [Google Scholar]
- 96.Kang SM, Song JM, Quan FS, Compans RW. Influenza vaccines based on virus-like particles. Virus Res. 2009;143:140–146. doi: 10.1016/j.virusres.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, Massare M, Pushko P, Mytle N, Rowe T, Smith G, Ross TM. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 98.Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, Kumar NM, Pushko P, Smith G, Tumpey TM, Ross TM. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLos One. 2008;3:e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu CY, Yeh YC, Yang YC, Chou C, Liu MT, Wu HS, Chan JT, Hsiao PW. Mammalian expression of virus-like particles for advanced mimicry of authentic influenza virus. PLos One. 2010;5:e9784. doi: 10.1371/journal.pone.0009784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haynes JR, Dokken L, Wiley JA, Cawthon AG, Bigger J, Harmsen AG, Richardson C. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine. 2009;27:530–541. doi: 10.1016/j.vaccine.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 101.Bosch BJ, Bodewes R, de Vries RP, Kreijtz JH, Bartelink W, van Amerongen G, Rimmelzwaan GF, de Haan CA, Osterhaus AD, Rottier PJ. Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets. J Virol. 2010;84:10366–10374. doi: 10.1128/JVI.01035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sylte MJ, Hubby B, Suarez DL. Influenza neuraminidase antibodies provide partial protection for chickens against high pathogenic avian influenza infection. Vaccine. 2007;25:3763–3772. doi: 10.1016/j.vaccine.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 103.DiNapoli JM, Nayak B, Yang L, Finneyfrock BW, Cook A, Andersen H, Torres-Velez F, Murphy BR, Samal SK, Collins PL, Bukreyev A. Newcastle disease virus-vectored vaccines expressing the hemagglutinin or neuraminidase protein of H5N1 highly pathogenic avian influenza virus protect against virus challenge in monkeys. J Virol. 2010;84:1489–1503. doi: 10.1128/JVI.01946-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hassantoufighi A, Zhang H, Sandbulte M, Gao J, Manischewitz J, King L, Golding H, Straight TM, Eichelberger MC. A practical influenza neutralization assay to simultaneously quantify hemagglutinin and neuraminidase-inhibiting antibody responses. Vaccine. 2010;28:790–797. doi: 10.1016/j.vaccine.2009.10.066. [DOI] [PubMed] [Google Scholar]
- 105.Kendal AP, Noble GR, Dowdle WR. Neuraminidase content of influenza vaccines and neuraminidase antibody responses after vaccination of immunologically primed and unprimed populations. J Infect Dis. 1977;136 (Suppl):S415–S424. doi: 10.1093/infdis/136.supplement_3.s415. [DOI] [PubMed] [Google Scholar]
- 106.Johansson BE, Brett IC. Variation in the divalent cation requirements of influenza a virus N2 neuraminidases. J Biochem. 2003;134:345–352. doi: 10.1093/jb/mvg151. [DOI] [PubMed] [Google Scholar]
- 107.Brett IC, Johansson BE. Variation in the divalent cation requirements of influenza A virus N1 neuraminidases. J Biochem. 2006;139:439–447. doi: 10.1093/jb/mvj051. [DOI] [PubMed] [Google Scholar]
- 108.Desselberger U. Preparation-conditioned changes of the antigenicity of influenza virus neuraminidases. Arch Virol. 1977;53:335–349. doi: 10.1007/BF01315632. [DOI] [PubMed] [Google Scholar]
- 109.Barr IG, McCauley J, Cox N, Daniels R, Engelhardt OG, Fukuda K, Grohmann G, Hay A, Kelso A, Klimov A, Odagiri T, Smith D, Russell C, Tashiro M, Webby R, Wood J, Ye Z, Zhang W. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 Northern Hemisphere season. Vaccine. 2010;28:1156–1167. doi: 10.1016/j.vaccine.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 110.Xu X, Shaw MW, Smith CB, Cox NJ, Klimov AI. Multiple lineages co-circulation and genetic reassortment of the neuraminidase and hemagglutinin genes within influenza viruses of the same type/subtype. 2001;1219:383–397. [Google Scholar]
- 111.Bright RA, Neuzil KM, Pervikov Y, Palkonyay L. WHO meeting on the role of neuraminidase in inducing protective immunity against influenza infection, Vilamoura, Portugal, September 14, 2008. Vaccine. 2009;27:6366–6369. doi: 10.1016/j.vaccine.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 112.Colman PM, Hoyne PA, Lawrence MC. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J Virol. 1993;67:2972–2980. doi: 10.1128/jvi.67.6.2972-2980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]