Abstract
Numerous research groups are now using analysis of blood-oxygen-level dependent (BOLD) functional magnetic resonance imaging (fMRI) results and relaying back information about regional activity in their brains to participants in the scanner in “real time”. In this study, we explored the feasibility of self-regulation of frontal cortical activation using real time fMRI (rtfMRI) neurofeedback in nicotine-dependent cigarette smokers during exposure to smoking cues. Ten cigarette smokers were shown smoking-related visual cues in a 3 Tesla MRI scanner to induce their nicotine craving. Participants were instructed to modify their craving using rtfMRI feedback with two different approaches. In a “reduce craving” paradigm, participants were instructed to “reduce” their craving, and decrease the anterior cingulate cortex (ACC) activity. In a separate “increase resistance” paradigm, participants were asked to increase their resistance to craving and to increase middle prefrontal cortex (mPFC) activity. We found that participants were able to significantly reduce the BOLD signal in the ACC during the “reduce craving” task (p=0.028). There was a significant correlation between decreased ACC activation and reduced craving ratings during the “reduce craving” session (p=0.011). In contrast, there was no modulation of the BOLD signal in mPFC during the “increase resistance” session. These preliminary results suggest that some smokers may be able to use neurofeedback via rtfMRI to voluntarily regulate ACC activation and temporarily reduce smoking cue-induced craving. Further research is needed to determine the optimal parameters of neurofeedback rtfMRI, and whether it might eventually become a therapeutic tool for nicotine dependence.
Keywords: nicotine dependence, smoking cessation, real-time fMRI, neurofeedback, anterior cingulate cortex, cue-induced craving
Introduction
Technical advancements in real time analysis of functional magnetic resonance imaging (fMRI) results has led to studies exploring the ability of individuals to use neurofeedback signals to modify behavior and regional brain activation (Beauregard and Levesque 2006; Caria et al 2007; Weiskopf et al 2007; deCharms 2008; LaConte 2011). By using real-time fMRI (rtfMRI), human participants can be trained to regulate their brain activity in circumscribed regions in the presence of contingent feedback of blood oxygenation level dependent (BOLD) response (deCharms 2008; Caria et al 2010; Johnson et al 2010). The use of rtfMRI neurofeedback has been explored for therapeutic benefit in a number of diseases including pain (deCharms et al 2005), tinnitus (Haller et al 2010), and anxiety disorders (Caria et al 2010; Johnston et al 2010). Additionally, recent data indicate that modulation of neural activity based on neurofeedback can lead to changes in cognitive and motor performance (Johnson et al 2010; Johnston et al 2010; Hamilton et al 2011), pain perception (deCharms et al 2005), and language processing (Rota et al 2009). These findings have intriguing implications for improved understanding and treatment of addictive disorders. However to date there have been no reports of rtfMRI feedback applied to addictions. For example, rtfMRI could provide insight into the functional role of circumscribed brain regions in addictive behavior via manipulation of brain systems activated during drug cue-induced craving.
A number of investigators have used neuroimaging techniques to explore regional brain activation associated with craving during the presentation of smoking-related cues in nicotine-dependent individuals (Brody et al 2006; Brody et al 2007; Hartwell et al 2011). Exposure to smoking-related cues generally provokes activation in regions subserving attention including the anterior cingulate cortex (ACC) (Brody et al 2004; Brody et al 2007), precuneus, and cuneus (Brody et al 2002; McClernon et al 2005; Wilson et al 2005; Smolka et al 2006) and areas involved in decision making and goal directed behavior such as the prefrontal cortex (PFC) (Zhang et al 2011). In an fMRI study recently conducted by our research group, smoking-related cues were associated with the increased activation of the prefrontal cortex, orbital frontal cortex (OFC) and anterior cingulate cortex (ACC) (Hartwell et al 2011).
Recently, the search for a unifying concept of the functional nature of the anterior cingulate cortex (ACC) has become an important topic of research in cognitive neuroscience (Bush et al 2000; Brassen et al 2011). An important guiding principle about ACC function is that cognitive and emotional information is processed separately. Its two major subdivisions subserve distinct functions. These include a dorsal cognitive division and a rostral-ventral affective division. The cognitive division has been activated by cognitively demanding tasks that involve stimulus response selection in the face of competing streams of information, including color stroop tasks (George et al 1993; George et al 1997). The affective division has been activated by affect-related tasks, including studies of emotional processing in normal healthy volunteers and symptom provocation studies in a number of psychiatric disorders. More recently, Azizian and colleagues demonstrated that smoking reduced conflict-related anterior cingulated activity in abstinent cigarette smokers during performing a stroop task. The study also suggested that exaggerated neural activity in the ACC during nicotine withdrawal may reflect a compensatory mechanism by which cognitive control networks expend excessive energy to support selective attention processes (Azizian et al 2010). Hong and colleague reported that resting-state dorsal anterior cingulate cortex-striatum functional connectivity may serve as a circuit-level biomarker for nicotine addiction, and the development of new therapeutic agents aiming to enhance the d ACC-striatum functional pathways may be effective for nicotine addiction treatment (Hong et al 2010). These studies suggeste that ACC may be a convergent structure pivotal for the diverse central nervous system effects of nicotine.
On the other hand, addiction also entails perturbations in cortically regulated cognitive and emotional processes, which cause the overvaluing of drug reinforcers at the expense of the undervaluing of natural reinforcers, and deficits in inhibitory control of drug responses (Goldstein and Volkow 2002). As a result, an underperforming prefrontal system is widely believed to be crucial to the addiction process. Dysfunction of the circuitry could logically be related to inappropriate behavioral choices, such as drug seeding regardless of the potential negative outcome. Preliminary structural and functional neuroimaging studies have found that nicotine-dependent individuals show a relationship between abnormal neural responses in the PFC, OFC and ACC, nicotine exposure and craving levels (Domino et al 2000a; Domino et al 2000b). In an fMRI study recently conducted by our research group, when participants were instructed to resist the urge to smoke, there was activation in the superior and medial frontal cortex and the right middle prefrontal cortex (mPFC), areas involved in associative learning and reward processing (Hartwell et al 2011). Therefore, these studies provide support for further research into the role of the PFC in nicotine craving and addictive behaviors.
In the present study, we explored the ability of nicotine-dependent cigarette smoking adults to use rtfMRI neurofeedback to improve their ability to reduce their neural and subjective craving response to smoking cues. Based on the study by Hartwell and colleagues described above (Hartwell et al 2011), we hypothesized that using rtfMRI neurofeedback smokers would decrease the BOLD signal in the ACC following instructions to “reduce craving” and increase the BOLD signal in middle prefrontal cortex following instructions to “increase resistance” to craving while viewing smoking-related cues in the scanner. These hypotheses are based on the idea that craving is associated with increased activity in the ACC and that the mPFC belongs to the cortical control system that can be used to resist craving (Brody et al 2007; Hartwell et al 2011). Establishing a relationship between modulation of brain activity in critical brain regions and changes in craving would be helpful in defining the optimal parameters for the study of neurofeedback rtfMRI as a therapeutic tool for nicotine dependence.
Methods and Materials
Participants
Healthy right-handed treatment-seeking, nicotine-dependent smokers (≥10 cigarettes/day) between 21 and 60 year old were recruited through local flyers, newspaper, and internet advertisements. All study procedures were performed in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki with approval from the Medical University of South Carolina Institutional Review Board. Telephone screening included brief medical, substance use, and psychiatric histories completed without personal identifiers prior to scheduling the initial assessment. All participants gave written informed consent prior to any study procedures. The Fagerström Test for Nicotine Dependence (FTND) (Fagerstrom 1978; Heatherton et al 1991), Questionnaire of Smoking Urges-Brief (Cox et al 2001), Minnesota Withdrawal Scale-Revised (Hughes 2007) and Tobacco Use History were administered. Exhaled carbon monoxide levels (≥ 10 ppm) were measured with a MicroSmokelyzer (Bedfont Scientific Ltd., Kent, United Kingdom) to confirm recent smoking. The Mini-International Neuropsychiatric Interview was completed to assess for current psychiatric and substance use disorders (Sheehan et al 1998) and a physical examination assessed current physical health.
Exclusion criteria included: the use of other tobacco products, current use of nicotine replacement therapy, bupropion, or varenicline, medical conditions or medications that could affect brain function, pregnancy, non-nicotine substance dependence or abuse, individuals with a history of psychiatric disorder and current pending charges for a violent crime.
fMRI Procedures
Overview
Participants were instructed not to smoke for 2 hours prior to the MRI scanning visit and thus were expected to have some degree of craving and responsiveness to the cues without the potential confound of a ceiling effect from prolonged abstinence. Subjects underwent four fMRI sessions within 1.5 hours (Fig 1), two without feedback designed to generate the regions to be used in feedback for that subject, and two sessions with feedback. A cue-induced craving region of interest (ROI) within the ACC was generated based on the first no feedback session during which participants were instructed to “allow yourself to crave when you see the smoking related pictures”. Toward the end of this session, a 3-slice ROI was selected for activation in the frontal region (using a t value threshold of 3 and a cluster threshold size of 4 voxels) visually approximated to be the ACC ( Fig. 2). During the second session, participants received feedback via a thermometer driven by activity from the ACC region identified during session 1. Participants were instructed to decrease the feedback-thermometer rating by decreasing their craving. The third session was a no feedback session designed to identify brain regions employed while they resisted the urge to crave. Participants were shown smoking-related cues and were instructed to “resist the urge to smoke when you see the smoking pictures by any means you find helpful”. Toward the end of this session, a 3-slice ROI was selected for activation in the frontal region (using a t value threshold of 3 and a cluster threshold size of 4 voxels) visually approximated to be the right mPFC. During the fourth session, the participants received feedback via a thermometer which was driven by activity from the area identified during session 3, the right middle prefrontal cortex (mPFC). The participants were instructed to increase the thermometer reading by “resisting” the urge to crave. Following the fMRI scan, participants underwent a brief structured interview to determine the strategies that they had employed to assist them in reducing or resisting the urge to smoke.
Fig. 1.
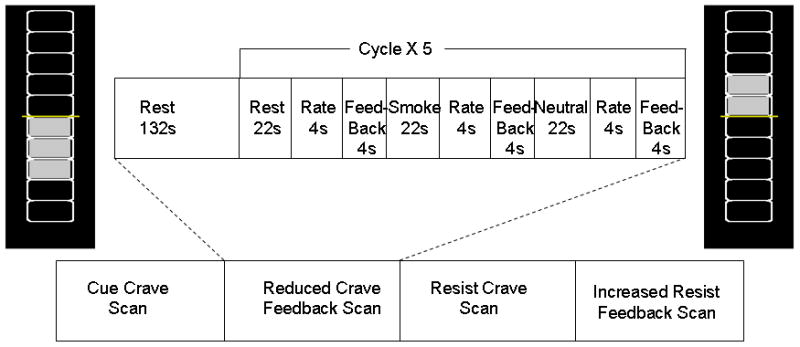
Scans and tasks diagram
Fig. 2.
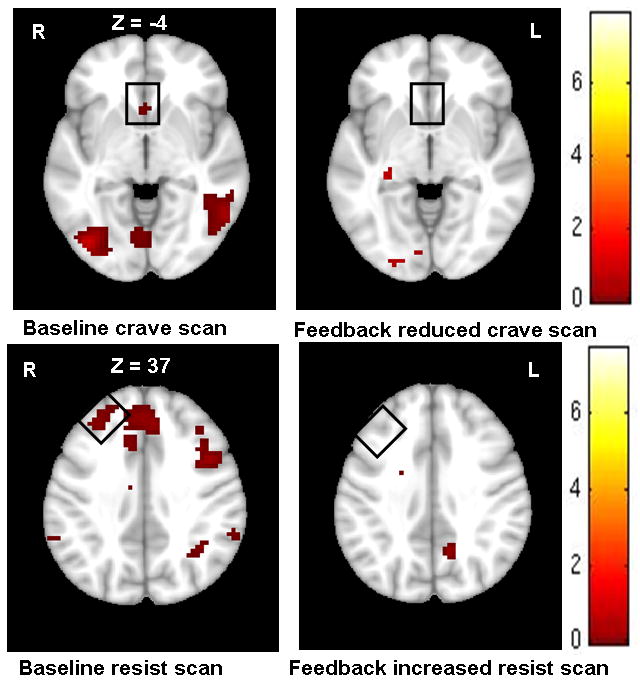
Random effects analysis on the experimental group confirmed a changed BOLD in ROIs. Top: craving and reduced craving scan-ROI (anterior cingulate cortex); Bottom: resisting and increasing resisting scan-ROI (right middle prefrontal cortex). A significance activation (p <0.005 uncorrected at cluster level with extent threshold of 10 voxels)
Cue Presentation and Craving Measurements
Stimuli were adapted from previous smoking cue fMRI studies conducted by our group (Hartwell et al 2011) and were presented in a block design using standardized pictures of people smoking or engaged in matched neutral activities. Objects related to smoking (packages of cigarettes, ashtrays, etc.) and matched neutral objects (pencils, dishes, etc) were included. Pictures were obtained from Elliot Stein’s lab at NIDA (Geier et al 2000) along with updated images for contemporary salience. A 10-minute sequence for stimuli presentation consisting of a 132-second “REST” period (cross-hair fixation) followed by five-90-second epochs was presented after participants briefly handled and smelled a preferred brand cigarette. Each epoch contained three, 22 second blocks (1 block of smoking related and 1 block of neutral images) and one block of rest (static crosshair) followed by a 4 second screen with a self-rating of craving using a hand-pad fitted on to the right hand and a 4 second with a biofeedback thermometer. Each 22- second block contained 5 individual pictures each displayed for 4.4 seconds. In order to control for time and order effects across participants, the order of individual pictures, blocks, and epochs were randomly presented (see Fig. 1).
fMRI data acquisition
Functional scanning was performed using a 3 Tesla MRI Trio (Siemens Medical, Erlangen, Germany). Each fMRI scan was acquired using a standard multislice single-shot gradient echo planar imaging (EPI) sequence with the following parameters: repetition time (TR) = 2.2 secs, echo time (TE) = 35 ms, 64 × 64 matrix, parallel imaging factor of 2, 3 × 3 × 3 mm voxels, 271 volumes, 36 ascending transverse slices with approximate anterior commissure-posterior commissure (AC-PC) alignment. After each volume was acquired, it was automatically exported in DICOM format from the MRI scanner computer to a separate computer for in-scan processing.
Real-time fMRI data processing
Online rtfMRI was made possible via a fast connection between the MRI scanner and the analysis/display computer. After acquisition and reconstruction, data from the scanner were sent to the analysis computer. Turbo-Brain Voyager (TBV) 2.0 software (Maastricht, The Netherlands) was used to perform real-time in-scan processing. Real-time prestatistical processing included motion correction and spatial smoothing using a Gaussian kernel of 8.0 mm full width half maximum (FWHM). No feedback craving and resist baseline fMRI sessions were acquired to guide ROI placement (described above). Toward the end of craving baseline scan session, 3-slice ROIs were selected for activation in the anterior cingulate cortex (using a t value threshold of 3 and a cluster threshold size of 4, see Fig. 2) as a target region. Similarly, toward the end of resist baseline scan session, 3-slice ROIs were selected for activation in the right middle prefrontal cortex (Fig. 2). The following settings were used for generating neurofeedback: average values to calculate feedback value = 6 timepoint for intermittent feedback paradigm, maximum percent signal change (PSC) of feedback bar = 5 (Fig. 1), general linear model (ROI-GLM) baseline enabled for stable baseline estimation, dynamic ROI enabled (using best voxel selection of top 33%); dynamic ROI effectively creates a sub-ROI to give better signal extraction from a coarse anatomical ROI selection and with small alignment errors with and between scan sessions. The resultant signal estimate for each incoming functional imaging volume within the selected region of interest was “fed back” to the participant using a “thermometer” display by exporting the ROI from the analysis computer (running TBV software) to the presentation computer. The thermometer value was a statistical measure of the difference between smoking and rest state in the ROI, which was calculated as follows: Feedback = (smoke − rest)/rest*100. The experimental paradigm and feedback were presented with a mirrored-projector system, with EPrime 2.0 software (Psychology Software Tools, Pittsburgh, PA).
Offline data analysis
All offline fMRI data analysis was performed using Statistical Parametric Mapping software 8 (SPM8, The Wellcome Department of Cognitive Neurology, London, http://www.fil.ion.ucl.ac.uk/spm). MR scans were transferred into ANALYZE format with MRIcron (http://www.sph.sc.edu/comd/rorden/mircro.html) and then further processed by SPM in Matlab 7.3 (Mathworks, Sherborn, MA). Default settings were used unless indicated otherwise. All volumes were realigned to the first volume. After realignment, for all participants, movement across the 271 volume scan was less than 0.5 mm in 3 axes and less than 0.5 degree in 3 orientations. The images were normalized stereotactically into a standard space with a resolution of 3 mm3 voxels using the averaged functional EPI image – the Montreal Neurological Institute (MNI) EPI template. Subsequently, the data were smoothed with an anisotropic 8 mm3 Gaussian kernel and high-pass filtered (cut-off period=128s). At the individual subject level, the data were modeled with three conditions (smoke, neutral, rest), each modeled by a boxcar convolved with a synthetic hemodynamic response function. The estimates of the participants’ movement during the scan were also entered as confounds. Contrasts were constructedto examine smoke and neutral conditions versus rest.
Individual-specific contrasts were entered into a second-level analysis to obtain a random effect analysis of activation effects across the entire group (one-sample t tests). The combined group t maps were thresholded at p ≤ 0.005 uncorrected for multiple comparisons and cluster analyses were performed with a spatial extent threshold of 10 voxels.
ROI Post-hoc Analysis
As TBV is an operational software with limited capacity for post-hoc analysis, time series extraction was performed using SPM 8. Two approaches were used to extract time series: 1) using parameters to approximate the TBV setting and 2) characterizing data from all unfiltered voxels in the ROI. “Easy ROI” (SPM extension tool) was used with spatially smoothed data to characterize data from all voxels in an ROI without temporal filtering. Brain volumes were then masked by the individual ROI created in TBV, and a time course of mean intensities from all voxels in the ROI was extracted. For time series extraction approaches, intensity values were converted to percent signal change (PSC) with baseline defined as the average of volumes 51–60 (end of first REST period). The hemodynamic response to the “SMOKE” period was temporally defined by the average time series of the no feedback ROI localizer scans.
Results
General Subject Characteristics
Twelve treatment seeking participants (8 women and 4 men) signed informed consent and 10 participants completed the study. One of them was terminated from the study for missing her appointments. The other was excluded after we learned after enrollment that he was involved in multiple other studies at the same time. The average age was 28.7 years (SD=10.9) and participants smoked an average of 14.5 cigarettes per day (SD=4.69). The FTND scores were moderate with an average of 4.7 (SD=2.49). Random exhaled CO levels were 15.3 ppm (SD=6.1) at the time of screening and 14.5 (SD=8.3) at the time of scanning. Commonly reported strategies employed during biofeedback periods included distraction (n=6), self-talk (n=2), social support (n=1) and contemplating adverse effects of smoking (n=1).
Craving vs Reducing Craving with Biofeedback from the ACC
ROI Analysis
Through the reducing crave biofeedback training, the subjects were able to control the fMRI signal in the anterior cingulate cortex (ACC). The primary index of controlling was the reduction in the fMRI signal between the baseline cue craving scan session and biofeedback scan session. Compared to baseline cue craving scan session, the mean PSC during the feedback scan session for all voxels was significantly reduced in the ACC (0.514 ± 0.35 vs −0.941 ± 0.57, t = 2.19; df =9, p = 0.028; Fig. 3a). Within biofeedback scan session, through five biofeedback blocks, the subjects did consistently reduce the fMRI signal in ACC. Repeated measures analysis of variance (ANOVA) demonstrated that blocks two, three and four were significantly decreased compared to the first block [F(4,44) = 3.725, p = 0.012)].
Fig. 3.
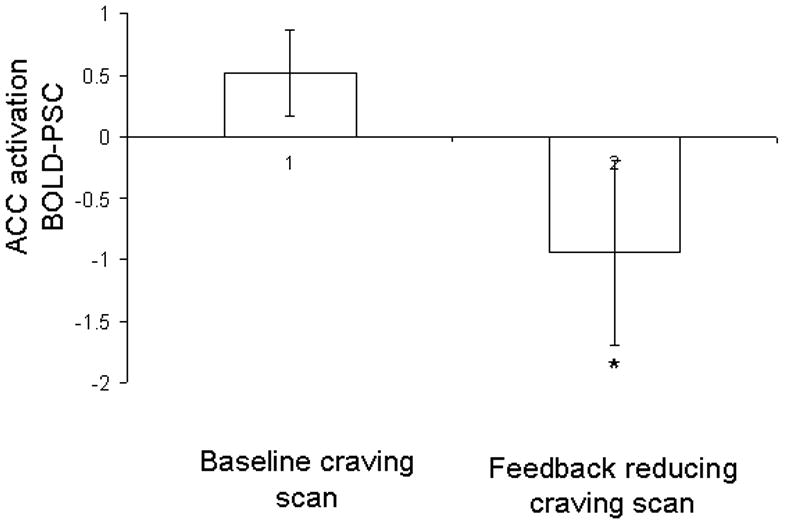
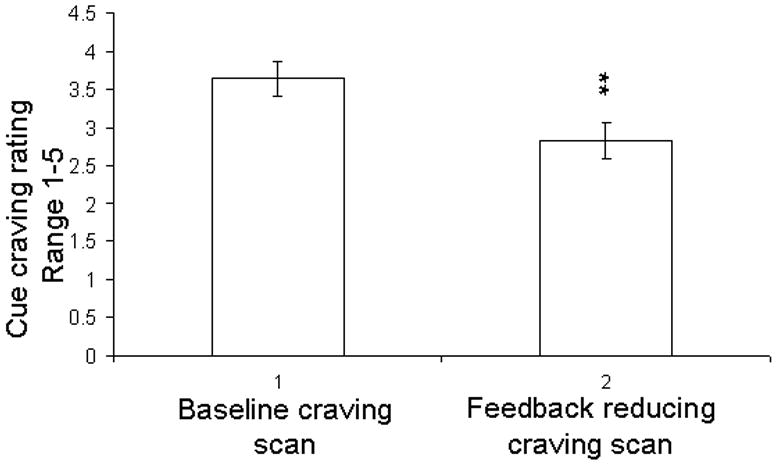
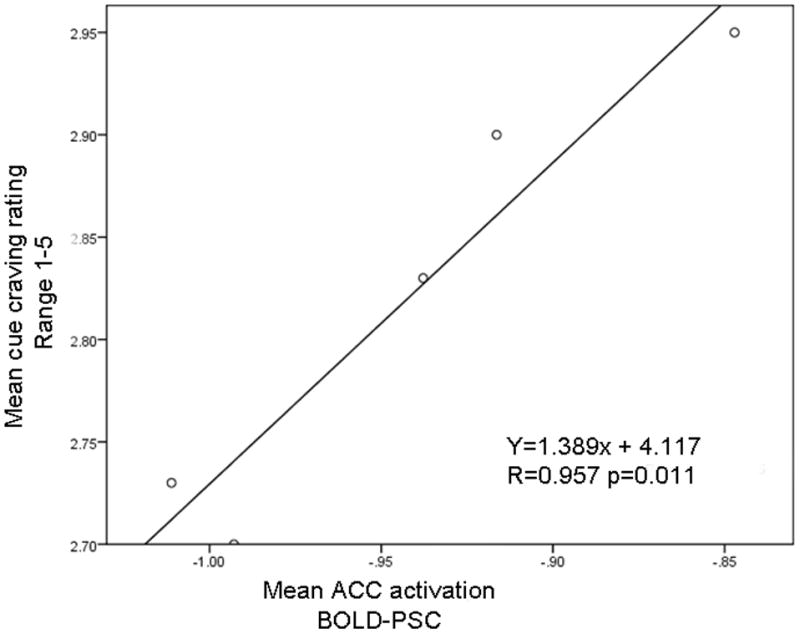
Reduced BOLD activation in ACC and decreased cigarette cue craving. (a) The fMRI BOLD signal in the ACC decreased significantly during the biofeedback scan (right side of figure) compared to during the baseline scan (left) (t = 2.19, df = 9, p = 0.028). (b) Subjective cue-induced craving ratings in the scanner also decreased significantly during the biofeedback scan (t = 4.37, df = 9, p=0.002) compared to during the baseline scan. (c) During each reduce craving feedback session there were 5 blocks (total 150s) of cue-induced craving (with feedback). This figure shows that within each of the 5 blocks, during the actual smoking exposure and feedback (30s) the measured BOLD signal within the ACC correlated with the subjective craving rating. (r = 0.957, p = 0.011)
Real-time cue craving rating comparison between baseline craving scan and reducing craving neurofeedback scan
Compared to baseline cue craving scan session, the mean subjective cue craving rating score during the biofeedback scan session was significantly reduced (3.64 ± 0.23 vs 2.82 ± 0.23 ; t = 4.37, df = 9, p = 0.002; Fig. 3b). Furthermore, repeated measures ANOVA results showed that the cue craving rating score following the feedback scan was significantly less than the baseline cue craving rating in each block [(F(1,19)=19.07, p=0.002)].
Correlation of reduced craving rating and decreased PSC of ACC
A linear regression analysis examined the relation between the change of PSC in ACC and the resultant smoke cue crating in the scanner. The mean PSC of ACC during the feedback scans was significantly correlated to the mean cue craving rating within those 5 blocks (r = 0.957, p = 0.011, Fig. 3c). That is, within the 5 blocks within the session, larger decreases in ACC activation were associated with greater reductions in subjective craving levels.
Whole Brain Analysis
Whole-brain random effect analysis on the contrast of smoke cue vs rest showed that cue-induced craving was associated with increased BOLD activation in several brain regions including bilateral occipital, ACC, nucleus accumbens, frontal cortex, parietal cortex and hippocampus, whereas there were no activated regions during the “reduce craving” neurofeedback scan (p <0.005, cluster size of 10 voxels) (Fig. 2. supplement).
Increasing Craving Resistance with Biofeedback from the mPFC
ROI Analysis
“Resist craving” ROIs were individually created for each participant in the right middle frontal cortex (mPFC). Through the “resisting craving” biofeedback training, the subjects did not control the fMRI signal in the right middle frontal cortex (mPFC). Compared to the baseline resistance scan, the mean PSC during the feedback scan for all voxels feedback did not change in mPFC (0.47 ± 0.32 vs 0.49 ± 0.40, t = −0.212, df =9, p = 0.837).
Real-time cue craving rating comparison between baseline resistance scan and biofeedback scan
Repeated measures AVNOVA did not show any significant difference either between the resist baseline scan and the resist biofeedback scan or among the blocks [F(1,19)=0.584, p=0.676)].
Correlation of increasing resistance cue craving and changed PSC of mPFC
Unlike with the “reduce craving” scans, we failed to find a significant correlation between the subjective craving rating and middle frontal cortex PSC data during the “resist” craving run (r = 0.316, p = 0.604).
Whole Brain Analysis
Whole-brain random effects analysis on the contrast of smoke cue vs rest showed that resisting craving was associated with regional brain activation including the bilateral occipital, bilateral middle frontal cortex, bilateral insula, thalamus, medial frontal cortex. The biofeedback based on the increasing resistance signal did not produce significantly increased BOLD signal in target regions. The “increase resistance” biofeedback scan led to activation in areas including the occipital, middle cingulate and parietal cortex (Fig. 2. supplement).
Discussion
This study builds on a growing body of work suggesting that it may be possible to train individuals to control localized brain activation using real-time neuroimaging feedback (deCharms et al 2005; Johnson et al 2010; Johnston et al 2011). To our knowledge, this is the first study to apply this technology to nicotine-dependent individuals. Specifically, the present study suggests that cigarette smokers may be able to learn to exert deliberate, voluntary control over ACC activation using rtfMRI feedback with instructions to reduce their subjective craving to smoke. Interestingly, subjectively reduced cue-induced craving ratings during rtfMRI feedback significantly correlated with decreased BOLD signal in the ACC. In contrast, participants were not successful in increasing their mPFC activity with an “increase resistance” command. This “resist” instruction also failed to achieve a significant craving reduction.
This study suggests that smokers may be able to use neurofeedback to decrease activity in the ACC. This finding has potential clinical significance. Given that the ACC has been shown to be part of the reward system (Brody et al 2002; Due et al 2002; McClernon et al 2005; McBride et al 2006; Hartwell et al 2011), the current results are consistent with the formulation that through real-time neurofeedback training in smokers, ACC activity may become decoupled from activity in the reward network. These data, considered together with the finding that the therapeutic benefit of bupropion in smokers is associated with exogenous down-modulation of the ACC (Brody et al 2004; Loughead et al 2011), suggest but certainly do not prove that ACC neurofeedback training may be useful in the treatment of nicotine dependence. The results are also consistent with the fact that surgical lesioning of the ACC can decrease drug consumption in morphine and alcohol-dependent individuals (Kanaka and Balasubramaniam 1978). While this study is critical in suggesting that individual smokers can modulate activation in the ACC with the aid of a neurofeedback signal, replication of this finding in a larger group of nicotine-dependent individuals is critical.
A linear regression analysis examined the relation between the deactivation level produced by neurofeedback in the ACC (percent fMRI signal change-PSC) and the subjective cue craving ratings. Interestingly, there was a significant correlation between the induced changes in ACC activation and the corresponding difference in cue craving ratings (Fig. 2C; P = 0.011). Previous studies have reported that reduced smoking cue-elicited craving correlated with brain activation change after treatment (Culbertson et al 2011; Franklin et al 2011). Franklin and colleagues found that varenicline diminished smoking cue-elicited ventral striatum and medial orbitofrontal cortex responses and reduced self-reported smoking cue-elicited craving (Franklin et al). In another study, Culbertson and colleagues reported that treatment with bupropion was associated with improved ability to resist cue-induced craving and reduced limbic and prefrontal brain activity (Culbertson et al 2011). Consistent with the results of Culbertson’s study, the current study results suggest that decreased smoking-related ACC activity significantly correlates with reduced cue craving ratings. The consistency of findings related to ACC activation in these two studies investigating different modalities of treatment for nicotine dependence strengthen the evidence supporting the importance of the ACC in nicotine dependence. As such, the ACC may be an important area to target in future studies of rtfMRI feedback in nicotine dependence.
The potential therapeutic uses of rtfMRI neurofeedback are just beginning to be explored, and optimal paradigms for use are not well delineated. The mechanisms by which rtfMRI neurofeedback is translated into learned regulation of focal brain activation are as yet unknown. Cue-induced cigarette craving is associated with brain activation in the ACC, orbital frontal and medial frontal cortex, while resisting cue craving is associated with activity in the middle prefrontal cortex (Hartwell et al 2011). One important aim of this study was to explore different paradigms for rtfMRI neurofeedback by comparing neurofeedback from brain areas activated by craving, with the instruction to decrease activity, to feedback from brain areas activated during attempts to resist the urge to crave, with the instruction to increase activation. In contrast to the success of ACC neurofeedback accompanying the instruction to “reduce craving”, participants were not successful in increasing mPFC activity with the command to “increase resistance”. In addition, the participants did not reduce their subjective ratings of cue-induced craving with the “increase resistance” instruction and mPFC neurofeedback. This may be a result of the fact that middle frontal cortex activity is more closely related to higher cognitive function as compared to reward circuitry and cue-induced craving may be more closely related to reward circuitry (Koechlin et al 1999; Crone et al 2006; Cutini et al 2008). Alternatively, it may simply be more difficult to increase activity in a cortical region than it is to reduce activity in the ACC and the different outcomes may result from the fact that these regions belong to different networks. A broader comparison of rtfMRI from other brain regions might be useful in clarifying some of these issues. In designing an rtfMRI study in craving, there are many plausible regions to feedback, either alone or combined. Unfortunately, in this study we only examined two brain regions, with opposite BOLD signal directions, and two ‘cognitive’ commands. In addition, the “increase resistance” sessions always followed the “reduce craving” sessions in this study. Since the entire session was approximately 50 minutes long, subjects’ inability to manipulate neural activity in the mPFC may have been a result of fatigue or anticipation of the end of the session and an opportunity to smoke. Clearly, future studies which control for order effects will be important in clarifying the best brain regions and instructions to be used in rtfMRI for nicotine dependence. Although the clinical implications are not entirely clear, the present results highlight an important point about ROI selection and training paradigm.
There are several important issues that limit interpretation of the study results. The sample size for this study was small, so replication with larger groups of participants will be important. In addition, there is the absence of a sham feedback control group. However, the appropriate control group for a study of this type is unclear, and our group has demonstrated that false feedback is frustrating to participants and can produce neural activation that complicates data interpretation (Johnson et al 2010). As mentioned above, the order of scan sessions was not randomized and the “increase resistance” paradigm was always performed at the end of the series scans, so participants inability to modulate mPFC activity in response to this instruction may have been a result of fatigue or a “ceiling” effect of craving. Additionally, we focused on two brain regions and two sets of instructions, so the results may have been different with a broader range of brain regions and participant instructions.
Additionally, the ACC region that we selected for feedback may be part of the ‘default mode’ network and subjects may be deactivating the ACC simply by engaging the brain in any task that is not ‘default’. Further studies using resting bold and craving and feedback would be necessary to address this point.
In summary, the current pilot study is the first therapeutic application of neurofeedback rtfMRI to an addictive disorder. Using neurofeedback rtfMRI, nicotine-dependent cigarette smokers were able to decrease the BOLD signal in their ACC and temporarily reduce subjective cue craving ratings. There was a significant and positive relationship between the decrease in craving ratings and the decrease in ACC activation. The present preliminary study is consistent with other studies in highlighting the role of the ACC in nicotine dependence and suggests a potential application of self-regulation of ACC activity in smoking cessation (Brody et al 2002; Due et al 2002; McClernon et al 2005; McBride et al 2006; Hartwell et al 2011). While much work remains to be done, this study shows promise for the development of rtfMRI as an innovative treatment strategy for nicotine dependence.
Supplementary Material
Acknowledgments
This study is supported by National Institute of Health (NIH) Grant No. 5R21DA026085 (KTB, MSG)
Footnotes
Author Contributions
MSG, KTB and KJH were responsible for study concept and design. XL and TL acquired study and fMRI data. XL and KJH contributed to data analysis. All authors contributed to interpretation of the findings. XL drafted the manuscript. KAJ, KJH, MSG, and KTB provided critical review and revision. All authors critically reviewed the manuscript and approved final version for publication.
Financial disclosures
Mark George has served as a paid consultant to or received research support from Brainsway, Cyberonics, Force Protection, GlaxoSmithKline, Jazz Pharmaceuticals, MECTA, Neurospace, and PureTech Ventures and as an unpaid consultant to Brainsonix, Brainsway, Cephos, MECTA, NeoSync, and Neuronetics. The Medical University of South Carolina has two patent applications in Dr. George’s name on combining TMS with MRI imaging.
All other authors have no conflicts of interest to disclose.
References
- Azizian A, Nestor LJ, Payer D, Monterosso JR, Brody AL, London ED. Smoking reduces conflict-related anterior cingulate activity in abstinent cigarette smokers performing a Stroop task. Neuropsychopharmacology. 2010;35:775–82. doi: 10.1038/npp.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Levesque J. Functional magnetic resonance imaging investigation of the effects of neurofeedback training on the neural bases of selective attention and response inhibition in children with attention-deficit/hyperactivity disorder. Appl PsychophysiolBiofeedback. 2006;31:3–20. doi: 10.1007/s10484-006-9001-y. [DOI] [PubMed] [Google Scholar]
- Brassen S, Gamera M, Büchela C. Anterior cingulate activation is related to a positivity bias and emotional stability in successful aging. Biol Psychiatry. 2011;70:131–137. doi: 10.1016/j.biopsych.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res. 2004;130:269–81. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, Zamora-Paja E, Farahi J, Saxena S, London ED, McCracken JT. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry. 2006;63:808–16. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiatry. 2010;68:425–32. doi: 10.1016/j.biopsych.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, Birbaumer N. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage. 2007;35:1238–46. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A. 2006;103:9315–20. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson CS, Bramen J, Cohen MS, London ED, Olmstead RE, Gan JJ, Costello MR, Shulenberger S, Mandelkern MA, Brody AL. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry. 2011;68:505–15. doi: 10.1001/archgenpsychiatry.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutini S, Scatturin P, Menon E, Bisiacchi PS, Gamberini L, Zorzi M, Dell’Acqua R. Selective activation of the superior frontal gyrus in task-switching: an event-related fNIRS study. Neuroimage. 2008;42:945–55. doi: 10.1016/j.neuroimage.2008.05.013. [DOI] [PubMed] [Google Scholar]
- deCharms RC. Applications of real-time fMRI. Nat Rev Neurosci. 2008;9:720–9. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102:18626–31. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta JK. Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse. 2000a;38:313–21. doi: 10.1002/1098-2396(20001201)38:3<313::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie SK, Ohl L, Ni L, Koeppe RA, Cross DJ, Zubieta J. Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience. 2000b;101:277–82. doi: 10.1016/s0306-4522(00)00357-2. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–60. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–41. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68:516–26. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology (Berl) 2000;150:283–91. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Horwitz B, Herscovitch P, Post RM. Regional brain activity when selecting a response despite interference: An H215O PET study of the stroop and an emotional stroop. Hum Brain Mapp. 1993;1:194–209. doi: 10.1002/hbm.460010305. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Marangell LB, Callahan AM, Post RM. Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop) J Neuropsychiatry Clin Neurosci. 1997;9:55–63. doi: 10.1176/jnp.9.1.55. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Birbaumer N, Veit R. Real-time fMRI feedback training may improve chronic tinnitus. Eur Radiol. 2010;20:696–703. doi: 10.1007/s00330-009-1595-z. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum Brain Mapp. 2011;32:22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell K, Johnson K, Li X, Myrick H, Lematty T, George M, Brady K. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011:1369–1600. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, Salmeron BJ, Srivastava V, Thaker GK, Goldman D, Stein EA. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107:13509–14. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Hartwell K, Lematty T, Borckardt J, Morgan PS, Govindarajan K, Brady K, George MS. Intermittent “Real-time” fMRI Feedback Is Superior to Continuous Presentation for a Motor Imagery Task: A Pilot Study. J Neuroimaging. 2010 doi: 10.1111/j.1552-6569.2010.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S, Linden DE, Healy D, Goebel R, Habes I, Boehm SG. Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cogn Affect Behav Neurosci. 2011;11:44–51. doi: 10.3758/s13415-010-0010-1. [DOI] [PubMed] [Google Scholar]
- Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DE. Neurofeedback: A promising tool for the self-regulation of emotion networks. Neuroimage. 2010;49:1066–72. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Kanaka TS, Balasubramaniam V. Stereotactic cingulumotomy for drug addiction. Appl Neurophysiol. 1978;41:86–92. doi: 10.1159/000102404. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–51. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- LaConte SM. Decoding fMRI brain states in real-time. Neuroimage. 2011;56:440–54. doi: 10.1016/j.neuroimage.2010.06.052. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, O’Donnell GP, Senecal N, Siegel S, Gur RC, Lerman C. Brain activity and emotional processing in smokers treated with varenicline. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00324.x. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–38. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–7. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota G, Sitaram R, Veit R, Erb M, Weiskopf N, Dogil G, Birbaumer N. Self-regulation of regional cortical activity using real-time fMRI: the right inferior frontal gyrus and linguistic processing. Hum Brain Mapp. 2009;30:1605–14. doi: 10.1002/hbm.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–88. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Sitaram R, Josephs O, Veit R, Scharnowski F, Goebel R, Birbaumer N, Deichmann R, Mathiak K. Real-time functional magnetic resonance imaging: methods and applications. Magn Reson Imaging. 2007;25:989–1003. doi: 10.1016/j.mri.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res. 2005;7:637–45. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–8. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


