Abstract
In this study, using human tongue squamous carcinoma cells (HSC-4) carcinostatic activity was compared for diverse L-ascorbic acid (Asc) derivatives, including the ‘straight-C16-chain types’, 6-O-palmitoyl-Asc (A6-P) and Asc-2-phosphate-6-O-palmitate sodium salt (APPS), as well as the ‘branched-C16-chain types’, Asc-2-phosphate-6-O-(2′-hexyl)decanoate (APHD), an isomer of APPS, and Asc-2,3,5,6-O-tetra-(2′-hexyl)decanoate (VCIP). The order of magnitude of the carcinostatic effects at 37°C was: APPS>A6-P = APHD>VCIP and at 42°C was APPS = A6-P>APHD>VCIP. Therefore, the two straight-C16-chain derivatives, APPS and A6-P, had a greater effect compared to the two branched-C16-chain Asc derivatives, which are considered to have more difficulty with ‘orientation along cell-membrane-glycerolipid direction’. APPS-treated HCS-4 cells were observed for a decrease in cell number, cell shrinkage, pycnosis indicative of apoptosis and cell deformation. The order of cytotoxicity for the normal human dermal fibroblasts (OUMS-36) at 37°C was: A6-P (50% inhibitory concentration: 150–300 μM)>APHD (450-600 μM)>>Asc = APPS (800–1000 μM). Accordingly, APHD was more cytotoxic than APPS, since the straight-C16-chain type, which was eliminated after the enzymatic esterolysis of APPS, is metabolized via the ‘fatty acid β-oxidation cycle’ more efficiently in normal cells. Thus, APPS had a greater advantage over APHD, A6-P and VCIP in terms of carcinostatic effects at 37°C, carcinostasis promotion at 42°C and a decrease of cytotoxicity to normal cells. This observation suggests a marked potential for aliphatic chain-moiety structures as anticancer agents, due to their cancer-selective carcinostasis and combined efficacy with hyperthermia, without causing side effects.
Keywords: ascorbic acid derivative, carcinostatic effect, hyperthermia, human tongue squamous carcinoma HSC-4 cells
Introduction
Ascorbic acid (Asc) and its oxidized form, dehydroascorbic acid, are important in the inhibitory control of the division and growth of cells in animal tissues (1). Asc has been reported to be a potent antitumor agent (2), but extremely high doses are required for carcinostatic effects. To increase the activity, Asc in combination with supplements (3,4) and the use of its derivatives generate hydrogen peroxide (5,6). Asc acylated with palmitic acid on the 6-O-site suppresses cell growth (7) and DNA synthesis (8). The 6-O-palmitoyl derivative of Asc has been demonstrated to exert cytotoxicity to tumor cells through hydrogen peroxide generation (6). The carcinostatic activity of diverse Asc derivatives consisting of a palmitoyl moiety and phosphatidyl moiety has been demonstrated. Their chemical structures are shown in Table I. In this study, their activity was compared using human tongue squamous carcinoma cells (HSC-4). Hyperthermia, is a potent cancer treatment (9), which inhibits the growth of tumor cells (10–12) and DNA synthesis (13–15), and is in clinical use for cancer therapy. This study aimed to examine whether these derivatives of Asc increase tumor cell death caused by hyperthermia, to further improve cancer treatment.
Table I.
Diverse Asc derivatives examined and their chemical structures.
| Chemical name | Abbreviation | Chemical structure |
|---|---|---|
| Ascorbic acid | Asc |
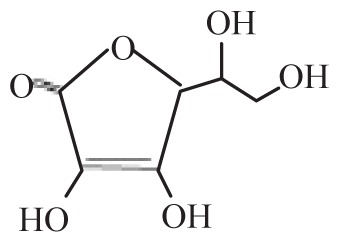
|
| 6-O-Palmitoyl-L-ascorbic acid | A6-P |
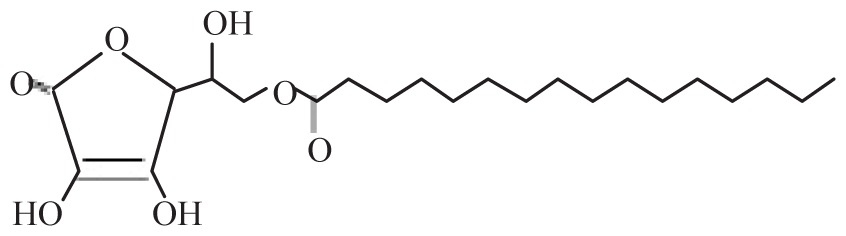
|
| Ascorbic acid-2-phosphate-6-O-palmitate trisodium salt | APPS |
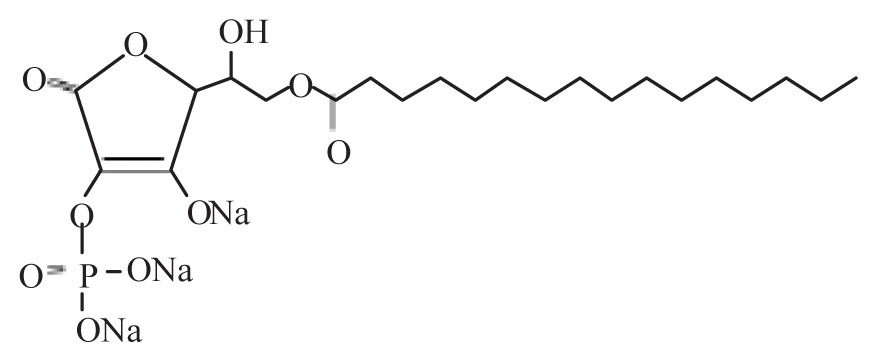
|
| Ascorbic acid-2-phosphate-6-O- (2′-hexyl) decanoate | APHD |
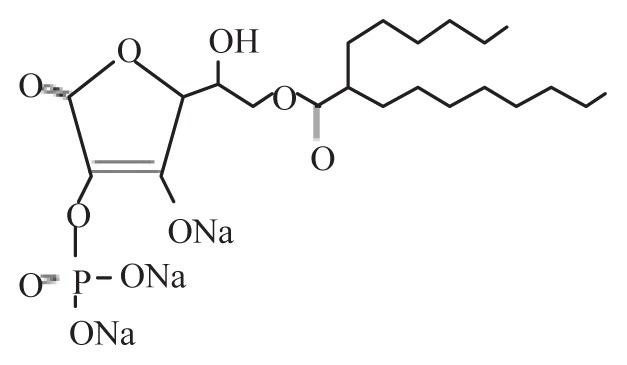
|
| Ascorbic acid 2,3,5,6-O- tetra-(2′-hexyl) decanoate (Ascorbyl tetra-iso-palmitate) | VCIP |
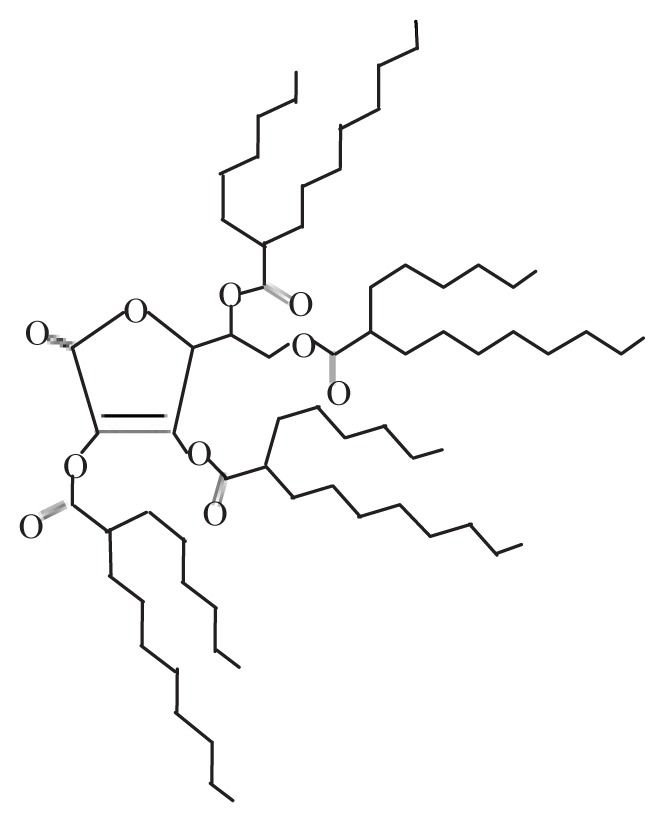
|
Tumor cells treated with Asc derivatives at 37°C or 42°C were examined. Firstly, differences in the carcinostatic ability between two types of Asc derivatives, the straight-chain types with palmitoyl moiety, Asc-2-phosphate-6-O-palmitate sodium salt (APPS) and 6-O-palmitoyl-Asc (A6-P), as well as the branched-chain types, Asc-2-phosphate-6-O-(2′-hexyl)decanoate (APHD) and Asc-2,3,5,6-O-tetra-(2′-hexyl)decanoate (VCIP), were examined. Then, differences in the carcinostatic ability between the isomers, APPS and APHD were assessed. Following that tumor cells administered with APPS and APHD were observed for morphological changes. Finally, the side-effects of the Asc derivatives towards the normal (OUMS-36) cells were examined.
Materials and methods
Cell culture
Human tongue squamous carcinoma (HSC-4) cells were cultivated in Eagle's minimum essential medium (MEM; Nissui Pharmaceutical Co., Ltd., Tokyo) supplemented with 18% fetal bovine serum (FBS; Biological Industries Ltd., Israel) in a humidified atmosphere of 5% CO2 in air at 37°C.
Examination of carcinostatic effects
The examination of carcinostatic effects was conducted as previously described (15,16). Cells were previously cultured for 24 h and suspended in culture medium at a density of 2×104 cells/ml. The test solutions of the diverse Asc derivatives were placed into test tubes. After the solvents were evaporated by jet flow of nitrogen gas, culture medium was added to the residue and sonicated to become homogenously emulsified. The cell suspensions and the test substance were mixed in a glass sample bottle (14 mm i.d. × 40 mm). The cells were adjusted and diluted to a cell density of 2×104 cells/ml and then, the bottle was tightly covered with a plastic cap.
Hyperthermic treatment
The suspension was incubated for 60 min at 37°C or 42°C in a water bath (16,17) (BT-23 model, Yamato Scientific Co., Ltd., Tokyo) and maintained by sequential culture in a humidified atmosphere of 5% CO2 in air at 37°C for 24 h.
Cell viability assay
Cell viability was measured using the redox indicator dye WST-8 (16,18) (Cell Counting kit, Dojin Chemicals, Kumamoto, Japan). The assay solution became increasingly chromic according to the mitochondrial dehydrogenase activity. The cultured cell suspension was transferred into a sampling tube and centrifuged. After the supernatant was completely removed from the tube, 110 μl 8% WST-8 was added to the cell precipitate, suspended and transferred into each well of a 96-well microplate. Following 3 h of incubation at 37°C, the resulting Diformazan solution was determined by measuring the absorption at 450 nm using a plate reader (Benchmark, Bio-Rad Laboratories, Hercules, CA, USA).
Crystal violet staining
The carcinostatic activities were evaluated using a crystal violet stain assay followed by cell morphological observation (18,19). The cell suspensions and the test tube substance were mixed in a 24-well culture plate (Becton, Dickinson and Co., Franklin Lakes, NJ, USA). The stain was then removed and the wells were rinsed thoroughly with running water until no additional dye leached from the wells. Cell morphology was observed under a phase-contrast microscope (Olympus IX-70).
Statistical analysis
Student's t-test was used for statistical analysis, with p (probability) values <0.05 considered as indicative of statistical significance.
Results
Carcinostatic effects of diverse Asc derivatives and hyperthermia
The Asc derivatives were added to the HSC-4 cells. The culture samples were then heated in a water bath for 60 min at 37°C or 42°C, and were maintained by sequential culture for 24 h at 37°C. The carcinostatic effects were measured using a redox reaction-based WST-8 assay (Fig. 1). The cell viability of the control at 37°C was considered to be 100%. At 37°C, A6-P, APPS, APHD and VCIP yielded cell survival rates of 86.8±5.7, 63.0±5.5 (P<0.0001), 88.01±3.70 and 77.8±2.55%, respectively. The cell viability for the control was reduced to 57.3±2.7% at 42°C (P<0.0001). At 42°C, A6-P, APPS, APHD and VCIP decreased cell viability to 42.0±2.1 (P<0.0001), 40.3±2.1 (P<0.0001), 60.6±4.3 and 66.8±7.4%, respectively. The carcinostatic activities of A6-P and APPS were markedly increased with hyperthermia.
Figure 1.
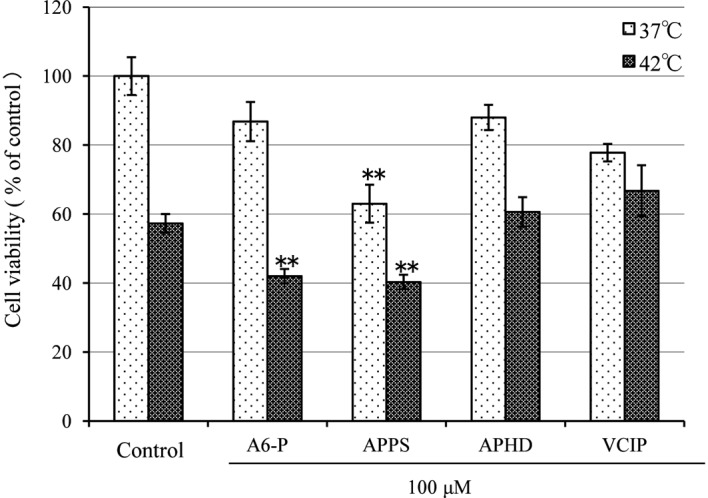
Carcinostatic effects of diverse Asc derivatives and hyperthermia on human tongue carcinoma cells (HSC-4) cultured for 24 h after heat treatment and evaluation by WST-8 assay. Cells were seeded and cell viability was measured as indicated in Materials and methods. The absorbance of cells treated for 24 h in the absence of diverse Asc derivative at 37°C was 0.791±0.035 or 0.715±0.027 (the control value), respectively. Data shown represents the means ± SD for quadruplicate measurements as percentages of the control value. **p<0.001 (vs.control). Asc, ascorbic acid; A6-P, 6-O-palmitoyl-Asc; APPS, Asc-2-phosphate-6-O-palmitate sodium salt; APHD, Asc-2-phosphate-6-O-(2'-hexyl)decanoate.
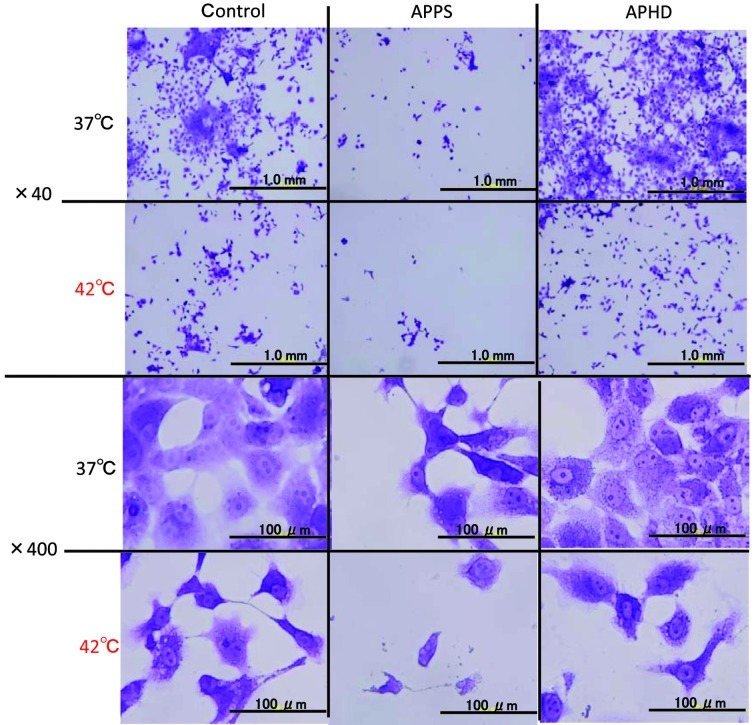
Morphological changes in tumor cells observed by crystal violet stain assay
Morphological observations in the tumor cells was performed by crystal violet staining, while the carcinostatic effects were assayed. The morphological changes occurred in the HSC-4 cells treated with APPS or APHD, which exhibited the greatest carcinostatic activities (Fig. 2). The morphological observations in the HCS-4 cells treated with APPS, were decreased cell numbers, cell shrinkage and pycnosis (nuclear condensation) indicative of apoptosis and cell deformation. At 42°C, the morphological changes of the cells were increased and fragmentation of the cells was also observed.
Figure 2.
Morphological changes in HSC-4 cells treated with the control, APPS and APHD at body temperature and in combination with hypothermia using crystal violet stain assay. The cells were cultured for 24 h after the treatment at 37°C or 42°C, stained with the crystal violet and photographed under the microscope. APPS, Asc-2-phosphate-6-O-palmitate sodium salt; APHD, Asc-2-phosphate-6-O-(2′-hexyl)decanoate.
Cytotoxicities of diverse Asc derivatives on OUMS-36 cells
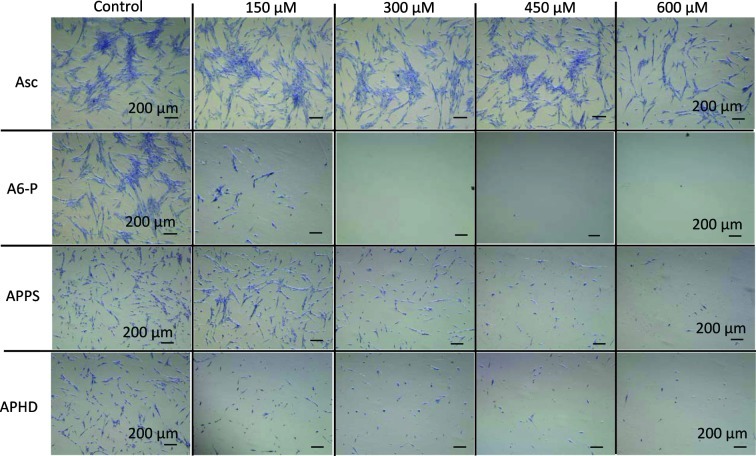
The diverse Asc derivatives were added to OUMS-36 cells and the culture samples were maintained by sequential culture for 24 h at 37°C. The cytotoxicities were measured by a WST-8 assay (Figs. 3 and 4). The cell viability of the controls in the absence of the Asc derivative was considered to be 100%. The order of magnitude of cytotoxicity at a dose of 600 μM was as follows: A6-P>APHD>Asc>APPS. APPS did not injure the OUMS-36 cells, even at 600 μM (Fig. 3). This tendency was also exhibited in the morphological observation (Fig. 5)
Figure 3.
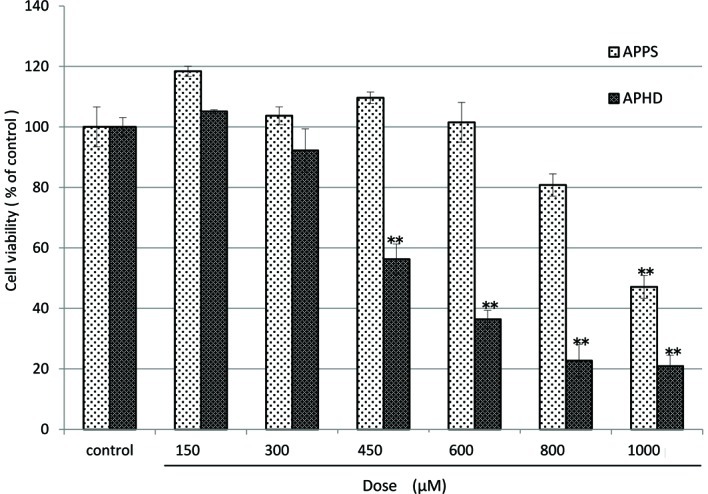
Cytotoxicities of APPS or APHD on normal human dermal fibroblast (OUMS-36) cells cultured for 24 h. APPS or APHD was added to OUMS-36 cells. The culture samples were maintained by sequential culture for 24 h at 37°C. Cell viability was measured by the absorption at 450 nm in the WST-8 assay. Data shown are the means ± SD for quadruplicate measurements as percentages of the control value, **p<0.001 (vs. control). APPS, Asc-2-phosphate-6-O-palmitate sodium salt; APHD, Asc-2-phosphate-6-O-(2'-hexyl)decanoate.
Figure 4.
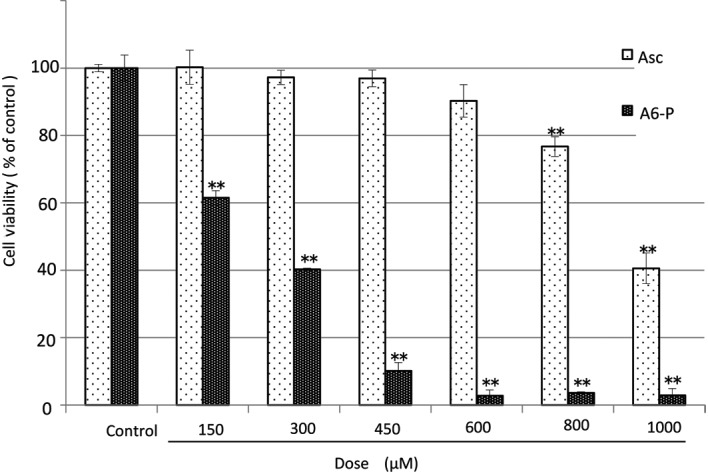
Cytotoxicities of Asc or A6-P on normal human dermal fibroblast (OUMS-36) cells cultured for 24 h. Asc or A6-P was added to OUMS-36 cells. The culture samples were maintained by sequential culture for 24 h at 37°C. Cell viability was measured by the absorption at 450 nm in the WST-8 assay. Data shown are the means ± SD for quadruplicate measurements as percentages of the control value, **p<0.001 (vs. control). Asc, ascorbic acid; A6-P, 6-O-palmitoyl-Asc.
Figure 5.
Morphological changes in tumor cells of Asc derivatives in OUMS-36 cells observed by crystal violet stain assay under the microscope. Asc, ascorbic acid; A6-P, 6-O-palmitoyl-Asc; APPS, Asc-2-phosphate-6-O-palmitate sodium salt; APHD, Asc-2-phosphate-6-O-(2′-hexyl)decanoate.
Discussion
Among the studied diverse ascorbic acid (Asc) derivatives, the straight chain type palmitic acid-phosphorus acid derivative, APPS, demonstrated the greatest antitumor effect, whereas, the branched chain type, APHD, did not demonstrate any antitumor effects. This finding may be due to the fact that the straight chain type is considered to be more permeable through the cell membrane than the branched type. Asc radicals, which are produced by the enzyme-catalyzed esterolysis of APPS, are absorbed into the cells where they injure DNA leading to cell death. By contrast, the branched types, APHD and VCIP, are less permeable or not permeable through the cell membranes, and therefore not effective for carcinostasis. From the morphological observations of the HCS-4 cells treated with APPS, a decrease in cell number, cell shrinkage and pycnosis (nuclear condensation) indicative of apoptosis and cell deformation were observed. These observed morphological changes were increased upon combination with hyperthermia treatment where further fragmentation of the cells was also observed. Whether the Asc derivatives were cytotoxic to the normal cells was then examined. A6-P was found to be cytotoxic to the tumor cells and normal (OUMS-36) cells, APHD was cytotoxic to the normal cells, but scarcely to the tumor cells, and APPS was cytotoxic to the tumor cells but not the normal cells at a dose of 600 μM. The results demonstrate that APPS has a marked carcinostatic advantage over A6-P. This benefit may be due to the addition of the of 2-O-phosphatidyl moiety, which adjusts the molecular LHB (lipophilicity-hydrophilicity balance) to be more hydrophilic. By contrast, the branched chain type APHD, an isomer of APPS, was almost ineffective, even at 100 μM. This finding may be due to the difference in molecular structure, which is related to surface activity and cell membrane permeability.
In conclusion, APPS exhibited a marked carcinostatic effect, and therefore may be developed as a potent antitumor agent with limited side-effects towards normal cells, and as a promoter by combination with hyperthermia. APPS has also been demonstrated to inhibit invasion of human fibrosarcoma cells (HT-1080) through the constituted basement membrane Matrigel and metastasis of mouse melanoma cells (B16-BL6)transplanted from the tail vein in mice (20).
Acknowledgements
The authors thank Dr Shinya Kato for his technical assistance.
References
- 1.Edgar JA. Dehydroascorbic acid and cell division. Nature. 1970;2273:24–26. doi: 10.1038/227024a0. [DOI] [PubMed] [Google Scholar]
- 2.Cameron E, Pauling L, Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res. 1779;39:663–681. [PubMed] [Google Scholar]
- 3.Poydock ME, Reikert D, Rice J. Influence of vitamins C and B12 on the survival rate of mice bearing ascites tumor. Exp Cell Biol. 1982;50:88–91. doi: 10.1159/000163132. [DOI] [PubMed] [Google Scholar]
- 4.Pierson HF, Fisher JM, Rabinovitz M. Depletion of extracellular cysteine with hydroxocobalamin and ascorbate in experimental murine cancer chemotherapy. Cancer Res. 1985;45:4727–4731. [PubMed] [Google Scholar]
- 5.Hacker MP, Khokhar AR, Brown DB, McCormack JJ, Krakoff IH. Ascorbato(1,2-diaminocyclohexane): platinum (II) complexes, a new series of water-soluble antitumor drugs. Cancer Res. 1985;45:4748–4753. [PubMed] [Google Scholar]
- 6.Miwa N, Yamazaki H, Nagaoka Y, Kageyama K, Onoyama Y, Matsui-Yuasa I, Otani S, Morisawa S. Altered production of the active oxygen species is involved in enhanced cytotoxic action of acylated derivatives of ascorbate to tumor cells. Biochim Biophys Acta. 1988;18:144–151. doi: 10.1016/0167-4889(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 7.Miwa N, Yamazaki H. Potentiated susceptibility of ascites tumor to acyl derivatives of ascorbate caused by balanced hydrophobicity in the molecule. Exp Cell Biol. 1986;54:245–249. doi: 10.1159/000163363. [DOI] [PubMed] [Google Scholar]
- 8.Kageyama K, Onoyama Y, Kimura M, Yamazaki H, Miwa N. Enhanced inhibition of DNA synthesis and release of membrane phospholipids in tumour cells treated with a combination of acylated ascorbate and hyperthermia. Int J Hyperthermia. 1991;7:85–91. doi: 10.3109/02656739109004979. [DOI] [PubMed] [Google Scholar]
- 9.Pajonk F, Ophoven A, McBride WH. Hyperthermia-induced proteasome inhibition and loss of androgen receptor expression in human prostate cancer cells. Cancer Res. 2005;65:4836–4843. doi: 10.1158/0008-5472.CAN-03-2749. [DOI] [PubMed] [Google Scholar]
- 10.Harris M. Criteria of viability in heat-treated cells. Exp Cell Res. 1966;44:658–661. doi: 10.1016/0014-4827(66)90479-4. [DOI] [PubMed] [Google Scholar]
- 11.Palzer RJ, Heidelberger C. Studies on the quantitative biology of hyperthermic killing of HeLa cells. Cancer Res. 1973;33:415–421. [PubMed] [Google Scholar]
- 12.Gerner EW, Boone R, Connor WG, Hicks JA, Boone ML. A transient thermotolerant survival response produced by single thermal doses in HeLa cells. Cancer Res. 1976;36:1035–1040. [PubMed] [Google Scholar]
- 13.Mondovì B, Finazzi Agrò A, Rotilio G, Strom R, Moricca G, Rossi Fanelli A. The biochemical mechanism of selective heat sensitivity of cancer cells. II Studies on nucleic acids and protein synthesis. Eur J Cancer. 1969;5:137–146. doi: 10.1016/0014-2964(69)90060-7. [DOI] [PubMed] [Google Scholar]
- 14.Henle KJ, Leeper DB. Effects of hyperthermia (45 degrees) on macromolecular synthesis in Chinese hamster ovary cells. Cancer Res. 1979;39:2665–2674. [PubMed] [Google Scholar]
- 15.Kageyama K, Onoyama Y, Nakanishi M, Ito K. New culture tube with an inside wall devised for studies of short-term hyperthermia. Int J Hyperthermia. 1988;4:567–570. doi: 10.3109/02656738809027700. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka H, Kageyama K, Kusumoto K, Asada R, Miwa N. Antitumor and antiinvasive effects of diverse new macrocyclic lactones, alkylolides and alkenylolides, and their enhancement by hyperthermia. Oncol Rep. 2007;18:1257–1262. [PubMed] [Google Scholar]
- 17.Asada R, Kageyama K, Tanaka H, Mimura H, Miwa N. The antitumor activities of the structurally-similar two-species aromatics Tonalide and Pearlide and the enhancement of their effects by hyperthermia. Mol Med Rep. 2009;2:33–37. doi: 10.3892/mmr_00000058. [DOI] [PubMed] [Google Scholar]
- 18.Asada R, Kageyama K, Tanaka H, Matsui H, Kimura M, Saitoh Y, Miwa N. Antitumor effects of nano-bubble hydrogen-dissolved water are enhanced by coexistent platinum colloid and the combined hyperthermia with apoptosis-like cell death. Oncol Rep. 2010;24:1463–1470. doi: 10.3892/or_00001006. [DOI] [PubMed] [Google Scholar]
- 19.Saito K, Oku T, Ata N, Miyasiro H, Saiki I. A modified and convenient method for assessing tumor cell invasion and migration and its application to screening for inhibitors. Biol Pharm Bull. 1997;20:345–348. doi: 10.1248/bpb.20.345. [DOI] [PubMed] [Google Scholar]
- 20.Liu JW, Kayasuga A, Nagao N, Masatsuji-Kato E, Tuzuki T, Miwa N. Repressions of actin assembly and RhoA localization are involved in inhibition of tumor cell motility by lipophilic ascorbyl phosphate. Int J Oncol. 2003;23:1561–1567. [PubMed] [Google Scholar]