Abstract
Carcinoid tumors are neuroendocrine neoplasms derived from enterochromaffin cells. Skeletal metastases from carcinoid tumors are considered to be extremely rare. In this study, we present two cases of carcinoid tumors that metastasized to the bone. Furthermore, we review 50 published case reports and reveal the features of skeletal metastasis of carcinoid tumors. The first case involved a 59-year-old man with a history of multiple metastases of a lung carcinoid tumor. The patient complained of back pain and numbness in the lower limbs. Magnetic resonance imaging revealed metastases in the thoracic spine. A spinal decompression was performed and the symptoms were resolved. The second case involved a 74-year-old man had been diagnosed with a lung carcinoid tumor 15 years previously and complained of left thigh pain. A radiograph showed osteolytic lesions in the shaft of the left femur. We repaired the femur using an intramedullary nail following curettage of the tumor. A radiograph of the femur revealed a callus on the pathological fracture. The patient was able to walk using a crutch 3 months after surgery. We reviewed 50 cases that described skeletal metastasis from carcinoid tumors. The average age of the patients was 54.9 years and 33 patients (66%) were male. The most common site of skeletal metastasis was the spine. We also investigated the survival rate of patients who developed skeletal metastasis from carcinoid tumors. The findings showed that survival of patients who developed osteolytic skeletal metastasis was worse than that of patients who developed osteosclerotic skeletal metastasis.
Keywords: carcinoid tumor, skeletal metastasis, prognosis
Introduction
Carcinoid tumors are neuroendocrine neoplasms derived from enterochromaffin cells, which are widely distributed in the body (1–4). Carcinoid tumors usually occur in the gastrointestinal tract (58–75%) and lung (20–31%), although they may occur anywhere in the body (2–4). Carcinoid tumors are rare, occurring in only 1–2 cases per 100,000, and vary with age, gender and ethnicity. Clinical presentation of a carcinoid tumor is dominated by symptoms caused by amines secreted by the tumor. The classical carcinoid syndrome includes diarrhea, flushing, bronchial obstruction and carcinoid heart disease and correlates with the serotonin-secreting activity of the tumor (2,5). Carcinoid tumors usually metastasize to the liver, lymph nodes and lungs (3). In approximately 10% of cases, the primary tumor site remains unknown (4). The mainstay of treatment for carcinoid tumors is surgical resection. Octreotide has become the main therapeutic regimen for carcinoid syndrome-related complaints. Octreotide has often been suggested to prolong survival, but this hypothesis has never been confirmed (6). Chemotherapy is not considered to be a first-line treatment for carcinoid tumors. The overall 5-year survival rate for all carcinoid tumors ranges from 70 to 80% (6,7). As expected, the stage of disease significantly affects the prognosis, with the best 5-year survival rate in patients with localized disease (93%) and a poor 5-year survival rate in patients with distant metastatic disease (20–30%) (4,5).
In the past, skeletal metastases from carcinoid tumors were considered to be extremely rare (1,8). However, skeletal metastases are becoming more prevalent due to longer survival and advances in earlier detection (9). Recent studies have revealed that the incidence of skeletal metastases in carcinoid tumors is approximately 10% (3,5). Some patients with skeletal metastases from carcinoid tumors complain of pain at the site of metastases, and occasionally develop pathological fractures and spinal cord injuries due to metastasis to the spine. Despite the increasing need for precise evaluation and treatment, skeletal metastasis may frequently remain undetected, as some patients with skeletal metastases do not complain of any symptoms. Moreover, the nature of the skeletal metastasis from carcinoid tumors has not been fully elucidated.
In this study, we present two cases of carcinoid tumors that metastasized to the bone. Furthermore, we reviewed 50 published case reports and described the occurrence, site and clinical course of skeletal metastasis in patients with carcinoid tumors.
The study was approved by the Ethics Committee of the hospital, and consent was obtained from the patients involved.
Case report
Case 1
A 59-year-old man had a history of multiple previous metastases, with a carcinoid tumor that was originally found in the lung in 2000. The patient underwent a surgical resection of the primary lesion, but developed liver and skeletal metastases in 2004. Although the patient had received chemotherapy after the metastases were detected, he complained of back pain and numbness in the lower limbs, and was admitted to our hospital in August 2009. Magnetic resonance imaging (MRI) revealed extensive metastases at almost all levels of the thoracic spine, with epidural extension of the tumor at the Th9 level (Fig. 1A and B). Computed tomography (CT) revealed a mixture of osteosclerotic and osteolytic changes in the Th9 vertebra (Fig. 1C). Bone scintigraphy using 99mTc-methylene diphosphonate (99mTc-MDP) demonstrated abnormal uptake into the Th9 vertebra. A spinal decompression was performed at Th8–9 with Th7–10 posterior fusion using spinal instrumentation. The pain and numbness were resolved following surgery. A histological examination of the surgical specimen demonstrated that the tumor consisted of cellular nests of small round cells (Fig. 2). The majority of tumor cells were positive for chromogranin A, synaptophysin and CD56 (data not shown). The histological diagnosis was metastases to the spine from a carcinoid tumor. The patient underwent chemotherapy following surgery and remained alive with slight numbness in his leg in August 2010.
Figure 1.
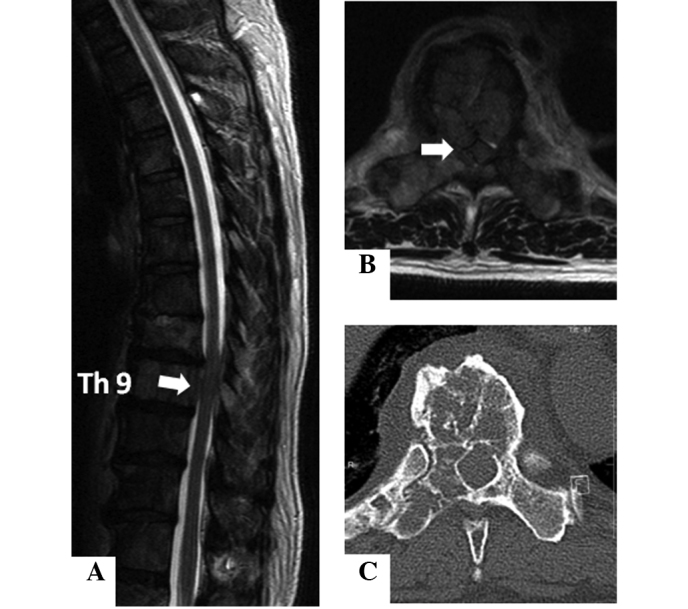
Case 1. (A and B) MRI reveals extensive metastases at nearly all levels of the thoracic spine, with epidural extension of the tumor at the Th9 level. (C) CT shows a mixture of osteosclerotic and osteolytic changes in the Th9 vertebra. MRI, magnetic resonance imaging; CT, computed tomography.
Figure 2.
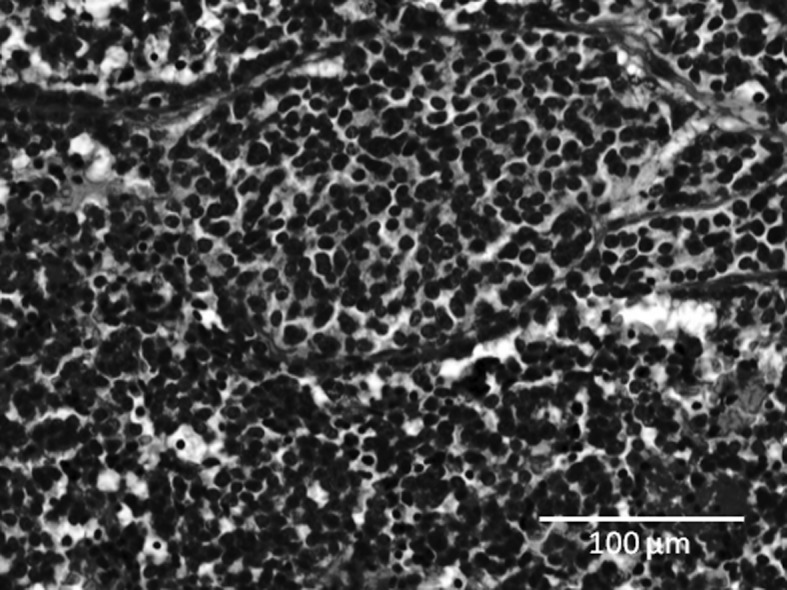
Case 1. Histological examination of the surgical specimen shows that the tumor consisted of cellular nests of small round cells. The histological diagnosis was metastases to the spine from a carcinoid tumor.
Case 2
A 74-year-old man was referred to our hospital with complaints of left thigh pain and impaired gait. The patient had been diagnosed with a lung carcinoid tumor 15 years previously, and had developed liver metastases 1 year before admission to our hospital. A radiograph showed osteolytic lesions in the shaft of the left femur (Fig. 3A). CT revealed destruction of the cancellous bone and thinning of the cortex in the shaft of the left femur (Fig. 3B). Bone scintigraphy using 99mTc-MDP demonstrated increased uptake into multiple bones including the cervical, thoracic and lumbar vertebrae, ribs, humerus, femurs and pelvis (Fig. 3C). According to these images, we diagnosed a pathological fracture of the left femur due to skeletal metastasis from a carcinoid tumor and surgery was performed to remove the lesion. We repaired the femur using intramedullary nails following curettage of the tumor. A histological examination of the surgical specimen demonstrated that the tumor consisted of cellular nests of small round cells (Fig. 4). The majority of tumor cells were positive for chromogranin A, synaptophysin and CD56 (data not shown). The histological diagnosis was metastases to the femur from a carcinoid tumor. A radiograph of the femur showed a callus on the pathological fracture. The patient was able to walk using a crutch 3 months after surgery.
Figure 3.
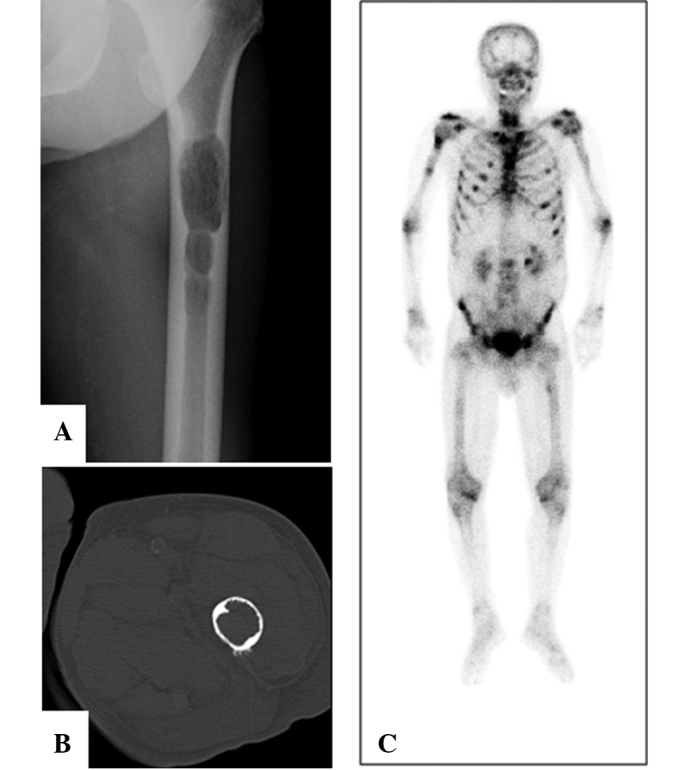
Case 2. (A) A radiograph shows osteolytic lesions in the shaft of the left femur. (B) CT reveals destruction of the cancellous bone and thinning of the cortex in the shaft of the left femur. (C) Bone scintigraphy shows increased uptake into multiple bones including cervical, thoracic and lumbar vertebrae and ribs, humerus, femurs and pelvis. CT, computed tomography.
Figure 4.
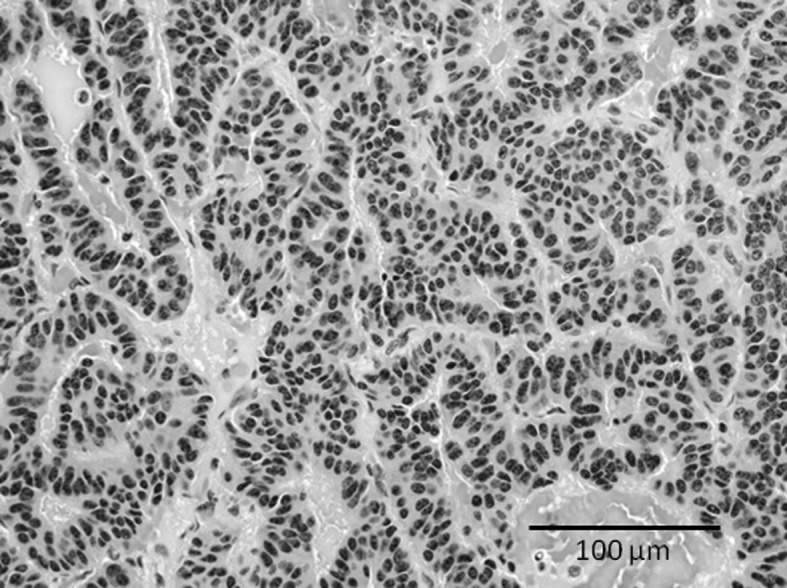
Case 2. Histological examination of the surgical specimen shows that the tumor consisted of cellular nests of small round cells. The histological diagnosis was metastases to the femur from a carcinoid tumor.
Discussion
Skeletal metastases from carcinoid tumors have been considered extremely rare in the past (8). In most cases, examination for the presence of skeletal metastases was initiated in patients with clinical symptoms suggestive of skeletal metastases. However, patients with carcinoid tumors with skeletal metastasis do not always complain of pain at the site of skeletal metastasis, as demonstrated by case 2 and previous reports (5,9). Previous studies evaluating the use of octreotide scintigraphy in carcinoid tumors describe a rate of skeletal metastases of between 7 and 20% (5,9–11). Ross and Roberts reported that autopsy revealed a higher rate (42%) of skeletal metastases in 36 patients with carcinoid tumors (12). Thus, we considered that skeletal metastasis from carcinoid tumors is not as rare as previously thought. Meijer et al reported that bone scintigraphy and octreotide scintigraphy have acceptable sensitivity and that MRI has high sensitivity for detecting bone metastases (5). Fluoro-2-deoxy-D-glucose positron emission computerized tomography is also useful for detecting multiple bone metastases from carcinoid tumors (13). Even if patients do not complain of any symptoms, patients with carcinoid tumors should be followed up carefully using effective inspection methods.
We reviewed 50 cases that described skeletal metastasis from carcinoid tumors (3,13–28). The characteristics of the reported patients are shown in Table I. The average age of the patients was 54.9 years (range, 19–82) and 33 patients (66%) were male. The primary lesion site was the gastrointestinal tract in 17 cases, the pulmonary region in 13 cases and other regions in 20 cases. The rate of skeletal metastasis from pulmonary carcinoid tumors appears higher than that from gastrointestinal carcinoid tumors, as carcinoid tumors usually occur in the gastrointestinal tract (58–75%) (2–4). The most common site of skeletal metastasis is the spine, and 40% of cases revealed metastases to thoracic vertebrae, 34% to the lumbar vertebrae and 32% to the cervical vertebrae. Other common sites were the ribs (28%) and the pelvis (26%). The average period between the initial diagnosis of carcinoid tumor and skeletal metastasis was 22 months (range, 0–144; n=46). Regarding treatment, surgery was performed in 15 out of 50 cases (30%). Spinal decompression was indicated in 11 cases and extirpation was performed in 4 cases. We also used the Kaplan-Meier method to investigate the survival rate of patients who developed skeletal metastasis from carcinoid tumors (n=35). Fig. 5A shows the survival rate of the 35 cases following skeletal metastasis. The survival rate gradually decreased, and 50% of patients died 24 months following skeletal metastasis. We noted that 5 of 9 patients with osteolytic skeletal metastasis died within 1 year of skeletal metastasis, whereas none of the 9 patients with osteosclerotic metastasis died. We compared the survival of patients with osteolytic metastasis to the survival of those with osteosclerotic metastasis. Fig. 5B shows that the survival of patients who developed osteolytic skeletal metastasis is worse than that of patients who developed osteosclerotic skeletal metastasis. We investigated gender, age, site of primary lesion of the carcinoid tumor, secretion and pathology in osteosclerotic and osteolytic cases to identify differences between osteosclerotic and osteolytic metastases, but we did not determine a significant factor leading to osteolytic changes at metastatic sites. The average period between diagnosis of carcinoid tumor and skeletal metastasis in osteolytic patients (n=13) was 2.0 months (0–24), whereas in osteosclerotic patients (n=15) this period was 27.9 months (0–144). Eleven of 13 cases had already developed skeletal metastasis when the initial diagnosis of carcinoid tumors showed osteolytic changes. These results demonstrate that the majority of patients who developed osteolytic skeletal metastasis presented skeletal metastasis at the initial visit, and osteolytic bone metastasis may be an adverse prognostic factor in patients with carcinoid tumors.
Table I.
Characteristics of the reported patients.
| Characteristics | No. of patients |
|---|---|
| Male/female | 33/17 |
| Mean age (range) | 54.9 (19–82) |
| Primary site | |
| Rectum | 12 |
| Duodenum | 2 |
| Lung | 13 |
| Thymus | 6 |
| Others | 14 |
| Metastasis site | |
| Spine | |
| Cervical | 6 (32.0%) |
| Thoracic | 20 (40.0%) |
| Lumbar | 17 (34.0%) |
| Sacrum | 9 (22.5%) |
| Extremity | |
| Humerus | 3 (6.0%) |
| Femur | 10 (20.0%) |
| Others | |
| Rib | 14 (28.0%) |
| Pelvis | 13 (26.0%) |
Figure 5.
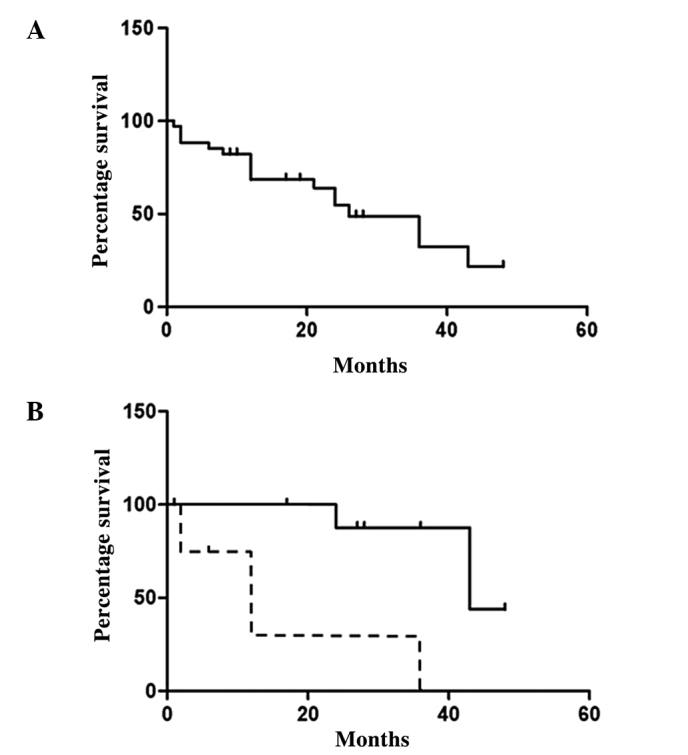
(A) The Kaplan-Meier curve demonstrates the survival rate of patients with carcinoid tumors after skeletal metastasis (n=35). (B) The Kaplan-Meier curve shows that the survival of patients who developed osteolytic skeletal metastasis (dotted line) is worse than that of patients who developed osteosclerotic skeletal metastasis (solid line).
In this study, we reported two cases of skeletal metastasis from carcinoid tumors. The patients had osteolytic skeletal metastatic lesions, and surgery was effective for treating the lesion. However, these patients should be followed up carefully as osteolytic skeletal metastasis is an adverse prognostic factor in patients with carcinoid tumors.
Acknowledgements
We thank Takashi Kondo, Department of Radiological Science, University of Toyama, for statistical analysis.
References
- 1.Powell JM. Metastatic carcinoid of bone. Report of two cases and review of the literature. Clin Orthop Relat Res. 1988;230:266–272. [PubMed] [Google Scholar]
- 2.Janmohamed S, Bloom SR. Carcinoid tumours. Postgrad Med J. 1997;73:194–200. doi: 10.1136/pgmj.73.858.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold PM, Floyd HE, Anderson KK, Newell KL. Surgical management of carcinoid tumors metastatic to the spine: report of three cases. Clin Neurol Neurosurg. 2010;112:443–445. doi: 10.1016/j.clineuro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10:123–131. doi: 10.1634/theoncologist.10-2-123. [DOI] [PubMed] [Google Scholar]
- 5.Meijer WG, van der Veer E, Jager PL, van der Jagt EJ, Piers BA, Kema IP, de Vries EG, Willemse PH. Bone metastases in carcinoid tumors: clinical features, imaging characteristics, and markers of bone metabolism. J Nucl Med. 2003;44:184–191. [PubMed] [Google Scholar]
- 6.Quaedvlieg PF, Visser O, Lamers CB, Janssen-Heijen ML, Taal BG. Epidemiology and survival in patients with carcinoid disease in The Netherlands. An epidemiological study with 2391 patients. Ann Oncol. 2001;12:1295–1300. doi: 10.1023/a:1012272314550. [DOI] [PubMed] [Google Scholar]
- 7.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick DB, Dawson E, Haskell CM, Batzdorf U. Metastatic carcinoid presenting as a spinal tumor. Surg Neurol. 1975;4:283–287. [PubMed] [Google Scholar]
- 9.Zuetenhorst JM, Hoefnageli CA, Boot H, Valdés Olmos RA, Taal BG. Evaluation of (111)In-pentetreotide, (131)I-MIBG and bone scintigraphy in the detection and clinical management of bone metastases in carcinoid disease. Nucl Med Commun. 2002;23:735–741. doi: 10.1097/00006231-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Westlin JE, Janson ET, Arnberg H, Ahlström H, Oberg K, Nilsson S. Somatostatin receptor scintigraphy of carcinoid tumours using the [111In-DTPA-D-Phe1]-octreotide. Acta Oncol. 1993;32:783–786. doi: 10.3109/02841869309096136. [DOI] [PubMed] [Google Scholar]
- 11.Kwekkeboom DJ, Krenning EP, Bakker WH, Oei HY, Kooij PP. Somatostatin analogue scintigraphy in carcinoid tumours. Eur J Nucl Med. 1993;20:283–292. doi: 10.1007/BF00169802. [DOI] [PubMed] [Google Scholar]
- 12.Ross EM, Roberts WC. The carcinoid syndrome: comparison of 21 necropsy subjects with carcinoid heart disease to 15 necropsy subjects without carcinoid heart disease. Am J Med. 1985;79:339–354. doi: 10.1016/0002-9343(85)90313-4. [DOI] [PubMed] [Google Scholar]
- 13.Kobashi Y, Shimizu H, Mouri K, Irei T, Oka M. Clinical usefulness of fluoro-2-deoxy-D-glucose PET in a case with multiple bone metastases of carcinoid tumor after ten years. Intern Med. 2009;48:1919–1923. doi: 10.2169/internalmedicine.48.2407. [DOI] [PubMed] [Google Scholar]
- 14.Katai M, Sakurai A, Inaba H, Ikeo Y, Yamauchi K, Hashizume K. Octreotide as a rapid and effective painkiller for metastatic carcinoid tumor. Endocr J. 2005;52:277–280. doi: 10.1507/endocrj.52.277. [DOI] [PubMed] [Google Scholar]
- 15.Gray JA, Nishikawa H, Jamous MA, Grahame-Smith DG. Spinal cord compression due to carcinoid metastasis. Postgrad Med J. 1988;64:703–705. doi: 10.1136/pgmj.64.755.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozkan M, Er O, Karahan IO, Deniz K, Coşkun R, Küçük C, Yurci A, Altinbaş M. Rectal carcinoid tumor with bone marrow and osteoblastic bone metastasis: a case report. Turk J Gastroenterol. 2007;18:111–114. [PubMed] [Google Scholar]
- 17.Nguyen BD, Ram PC. Bronchopulmonary carcinoid tumor and related cervical vertebral metastasis with PET-positive and octreotide-negative scintigraphy. Clin Nucl Med. 2006;31:101–103. doi: 10.1097/01.rlu.0000196603.90748.a0. [DOI] [PubMed] [Google Scholar]
- 18.Goddard MJ, Atkinson C. Cardiac metastasis from a bronchial carcinoid: report of a case presenting with diffuse thickening of the left ventricular wall. J Clin Pathol. 2004;57:778–779. doi: 10.1136/jcp.2004.016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashraf MH. Bronchial carcinoid with osteoblastic metastases. Thorax. 1977;32:509–511. doi: 10.1136/thx.32.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaka T, Maruno M, Suzuki T, Sato M, Yoshimine T. Skull metastases from atypical pulmonary carcinoid tumor in a 19-year-old man. Neurol Med Chir (Tokyo) 2006;46:609–613. doi: 10.2176/nmc.46.609. [DOI] [PubMed] [Google Scholar]
- 21.Pellini R, Ruggieri M, Pichi B, Covello R, Danesi G, Spriano G. A case of cervical metastases from temporal bone carcinoid. Head Neck. 2005;27:644–647. doi: 10.1002/hed.20197. [DOI] [PubMed] [Google Scholar]
- 22.Blondet E, Dulou R, Camparo P, Pernot P. Lumbar intradural metastasis of a primary carcinoid tumor of the lung. Case illustration. J Neurosurg Spine. 2005;2:231. doi: 10.3171/spi.2005.2.2.0231. [DOI] [PubMed] [Google Scholar]
- 23.Lim KH, Huang MJ, Yang S, Hsieh RK, Lin J. Primary carcinoid tumor of prostate presenting with bone marrow metastases. Urology. 2005;65:174. doi: 10.1016/j.urology.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Tanabe M, Akatsuka K, Umeda S, Shomori K, Taniura S, Okamoto H, Kamitani H, Watanabe T. Metastasis of carcinoid to the arch of the axis in a multiple endocrine neoplasia patient: a case report. Spine J. 2008;8:841–844. doi: 10.1016/j.spinee.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Brown LR, Aughenbaugh GL, Wick MR, Baker BA, Salassa RM. Roentgenologic diagnosis of primary corticotropin-producing carcinoid tumors of the mediastinum. Radiology. 1982;142:143–148. doi: 10.1148/radiology.142.1.6273965. [DOI] [PubMed] [Google Scholar]
- 26.Gowitt GT, Mirra SS. Malignant carcinoid causing spinal cord compression. Neurosurgery. 1985;17:801–806. doi: 10.1227/00006123-198511000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Noshiro H, Satoh K, Gondoh T, Iwata T, Fujii T, Mezuki M, Masuda H, Inoue T. A case of rectal carcinoid with extensive sacral breakage. Jpn J Surg. 1990;20:443–447. doi: 10.1007/BF02470829. [DOI] [PubMed] [Google Scholar]
- 28.Sato K, Higaki Y, Sakaguchi S, Hirano M, Tanimura A, Sasaguri Y. Carcinoid tumor of the larynx. Auris Nasus Larynx. 1991;18:39–53. doi: 10.1016/s0385-8146(12)80249-5. [DOI] [PubMed] [Google Scholar]


