Abstract
Plants consumed by non-human primates represent potential drug sources for human disease management. In this study, we isolated kaempferol-3-O-rhamnoside as an active compound from the leaves of Schima wallichii Korth., a plant commonly consumed by non-human primates. Its anti-cancer activities, including its ability to induce apoptotic mechanisms, were investigated in MCF-7 breast cancer cells. Results showed that in MCF-7 cells, kaempferol-3-O-rhamnoside inhibits cell proliferation in a dose-dependent manner and promotes apoptosis via the activation of the caspase signaling cascade, which includes caspase-9, caspase-3 and PARP. Our results provide a basis for further exploration of kaempferol-3-O-rhamnoside as an active compound for potential anti-cancer therapeutics.
Keywords: primates, Schima wallichii, cancer, apoptosis, caspase
Introduction
With over 12.5 million new cases and 7.5 million mortalities annually, cancer is becoming one of the most malignant diseases worldwide. Among the various cancer types, breast cancer contributes to more than 1.2 new cases and 0.5 million mortalities annually, making it the most malignant form of cancer among women (1). Due to this high incidence, the identification of novel compounds that inhibit cancer development has become a crucial objective for scientists. Of the hundreds of chemicals that have been and are being evaluated for their anti-cancer activities, natural products derived from medicinal plants rank among the most promising.
In an effort to identify novel agents that may inhibit cancer development, we have focused our investigations on discovering bioactive compounds from plants commonly consumed by primates. Due to the high degree of physiological similarity between primates and humans, primate disease often exhibits similarities to human disease, and certain human diseases are known to have originated from primates. Notably, since primate survival largely depends on daily food intake, the food consumed by primates is considered a promising source of products applicable for human disease management.
In our previous study, we found that the leaves of Schima wallichii Korth., a primate-consumed plant, demonstrated anti-tumor and anti-mutagenic properties (2,3). These preliminary studies suggest that Schima wallichii Korth. may be further developed as a source of anti-cancer agents. Thus, in this study, we investigated and characterized the pro-apoptotic activities of Schima wallichii Korth. leaf extracts.
Materials and methods
Plant materials
Schima wallichii Korth. leaves were collected from Lembang, West Java, Indonesia. The plant species was identified by the Department of Biology, Faculty of Mathematics and Natural Sciences, University of Padjadjaran, Indonesia.
Extraction and isolation
The dried leaves of Schima wallichii Korth. (5 kg) were extracted with 70% ethanol (3×24 h) at room temperature. The solvent was subsequently evaporated under reduced pressure at 50°C to yield a concentrated extract. The ethanol extract (506.05 g) was fractionated between n-hexane and water to obtain an n-hexane extract (43.90 g) and a water layer. The water layer was then extracted with ethyl acetate to obtain an ethyl acetate fraction (47.35 g) and a water fraction (104.20 g). The cytotoxicity of the fractions was assessed on MCF-7 breast cancer cells using the methyl thiazolyl tetrazolium (MTT) assay. The ethyl acetate fraction, which was the most active fraction, was chromatographed on Wakogel C-200 (Wako Pure Chemical, Japan) with a mixture of n-hexane, ethyl acetate and methanol with increased polarity. The major compound was then isolated and purified using silica G-60 with sulfuric acid-ethanol (1:9) and identified by spectroscopy methods including ultra violet and infrared spectrometry (UV-IR), nuclear magnetic resonance (NMR) and liquid chromatography mass spectrometry (LC-MS) (4).
Cell culture and treatment
The MCF-7 human breast cancer cell line (ATCC, Manassas, VA, USA) and HC-04 human hepatocytes (Siam Life Science Ltd., Thailand) used in this study were cultured in RPMI-1640 medium (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). For cell treatments, various concentrations of the sample were added to the cell culture medium. After 24 h, the cells were released from treatment, the medium was replaced, and cells were subsequently collected at the indicated times.
Drug sensitivity assay
A cell proliferation analysis was performed in the presence of various concentrations of broccoli sprout extracts using a colorimetric MTT assay, as described in a previous study (5). Briefly, the cells were plated in 96-well plates (2×104 in 50 μl/well). Following the initial cell seeding, the indicated concentrations of extracts were applied and incubated for 24 h. WST-8 assay cell-counting solution (10 μl) (Dojindo Lab., Tokyo, Japan) was added to each well and incubated at 37°C for 3 h. After the addition of 1N HCl (100 μl/well), the cell proliferation rates were determined by measuring the absorbance at a wavelength of 450 nm with a reference wavelength of 650 nm using a microtiter plate reader (Becton-Dickinson, Franklin Lakes, NJ, USA). The results were derived from triplicate experiments.
Cell extraction and western blot analysis
Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL, USA). Proteins (40 μg) were electrophoresed on 5–20% Tris-Tricine ReadyGel (Bio-Rad, Tokyo, Japan) and electro-transferred to a Hybond enhanced chemiluminescence membrane (Amersham, Buckinghamshire, UK). Apoptosis-related proteins were analyzed by immunoblot analysis using caspase-3, caspase-9 and PARP antibodies at a 1:1000 dilution (Cell Signaling Technology, Beverly, MA, USA). β-actin (Sigma) served as the loading control.
Results
Ethyl acetate fraction of Schima wallichii Korth. leaves inhibits MCF-7 cell proliferation
Treatment with the Schima wallichii Korth. extract was found to inhibit the proliferation of MCF-7 human breast cancer cells (IC50, 102 μg/ml) (Fig. 1). The extract was fractionated based on polarity, using n-hexane, ethyl acetate and water. The fractions were then individually applied to MCF-7 cells and were found to inhibit cell proliferation with an IC50 value of 126 μg/ml for the n-hexane fraction, 20 μg/ml for the ethyl acetate fraction and 70 μg/ml for the water fraction. Due to its low IC50 value, we then explored the ethyl acetate fraction for its anti-cancer potential.
Figure 1.
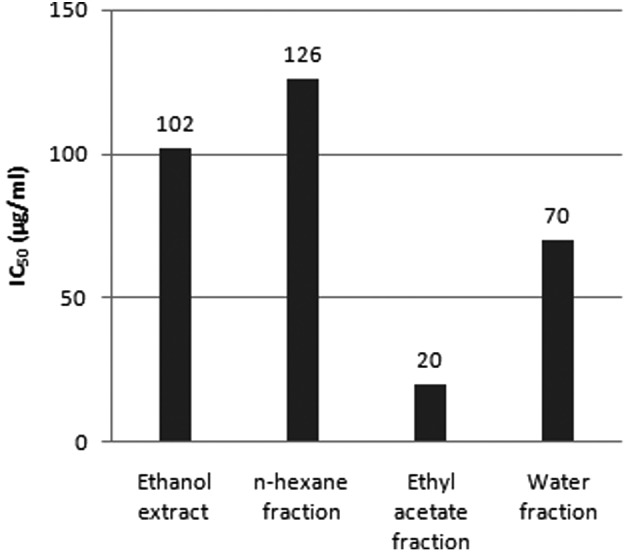
IC50 concentration of Schima wallichii Korth. extract and fractions for suppression of MCF-7 proliferation. Ethyl acetate fraction of Schima wallichii Korth. shows the strongest potential in inhibiting MCF-7 proliferation.
Kaempferol-3-O-rhamnoside is the major compound of the ethyl acetate fraction
The principle active compound of the ethyl acetate fraction of Schima wallichii Korth. extract was isolated and purified. The compound exhibited a melting point of 152.7–153°C and a molecular ion peak at m/z 432 in the LC-MS spectrum. Based on hydrogen and carbon content, the molecular ion peak and 1H and 13C NMR profiles indicated that the compound had a molecular formula of C21H20O10. The UV spectrum produced maximal absorbance peaks at λmax 265 and 342 nm, which were characteristic of a flavonoid with a flavone skeleton. The addition of NaOH produced a bathochromic shift in the absorption bands, indicating the presence of hydroxyl groups in the skeleton, one of which was attached to C-5, as indicated by a further bathochromic shift following the addition of H3BO3. Absorption bands at 1,675 and 3,197 nm of the IR spectrum indicated where the molecule harbors conjugated carbonyl and hydroxyl groups, respectively.
The 1H NMR spectrum of the compound showed two aromatic hydrogen signals with ‘meta coupling’ at δ 6.35 (1H, d, J= 2.2 Hz) and 6.18 (1H, d, J=2.2 Hz), which was predicted by the hydrogens at C-6 and C-8 of the A ring of the flavone skeleton. Accordingly, this compound was suggested to have a hydroxyl group at C-5 and C-7. Furthermore, its 1H NMR spectrum revealed two signals with ‘ortho coupling’ at δ 6.92 (2H, d, J=6.7 Hz) and 7.74 (2H, d, J=6.7 Hz), the signals of which were approximated from the hydrogens at C-2′, C-3′, C-5′ and C-6′ of the B ring. The absence of a specific signal for an olefinic hydrogen at C-3 and the presence of an anomeric hydrogen signal at δ 5.37 (1H, d, J=7.2 Hz) suggested that the compound was a flavonol glycoside. The appearance of an anomeric carbon signal at δ 94.9 in the 13C NMR spectrum indicated the presence of a sugar moiety. Due to a correlation between the anomeric hydrogen signal (δ 5.37) and the anomeric carbon signal (δ 94.9) that was revealed by analysis of the HMBC spectral data, the position of the sugar moiety was assigned to the C-3 hydroxyl group. The methyl signal observed at δ 0.93 (3H, s) in the 1H NMR spectrum and at δ 17.7 in the 13C NMR spectrum indicated that the sugar moiety was rhamnose. Based on the accumulated data above, the compound was identified as kaempferol-3-O-rhamnoside (Fig. 2B).
Figure 2.
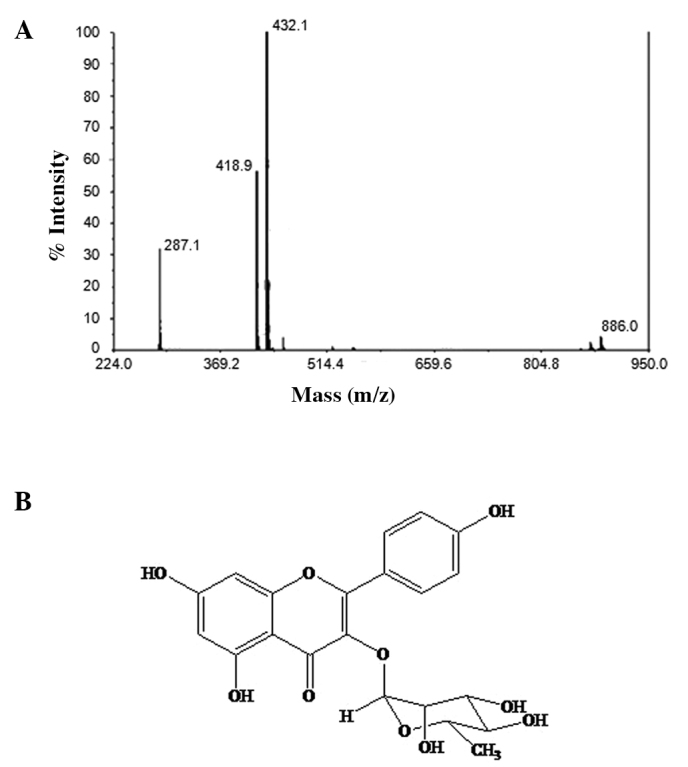
Identification of the major compound within the ethyl acetate fraction of Schima wallichii. (A) LC-MS profile of the isolated compound. (B) Structure of kaempferol-3-O-rhamnoside.
Kaempferol-3-O-rhamnoside inhibits MCF-7 cell proliferation in a dose-dependent manner
The effect of kaempferol-3-O-rhamnoside on the viability of MCF-7 and HC-04 cells was evaluated. The treatment of cancer (MCF-7) and non-cancer (HC-04) cell lines with kaempferol-3-O-rhamnoside resulted in a dose-dependent inhibition of cell growth, as demonstrated by the MTT assay (Fig. 3). Twenty-four hours of treatment with kaempferol-3-O-rhamnoside inhibited the proliferation of MCF-7 cells with an IC50 value of 227 μM. By contrast, the inhibition of proliferation in HC-04 cells with kaempferol-3-O-rhamnoside demonstrated an IC50 of 448 μM, ~2-fold higher than that with MCF-7 cells, suggesting that kaempferol-3-O-rhamnoside may exhibit fewer cytotoxic effects on non-cancer cells. Subsequent immunoblot-based investigation applying the IC50 dose of 227 μM kaempferol-3-O-rhamnoside was performed on MCF-7 cells.
Figure 3.
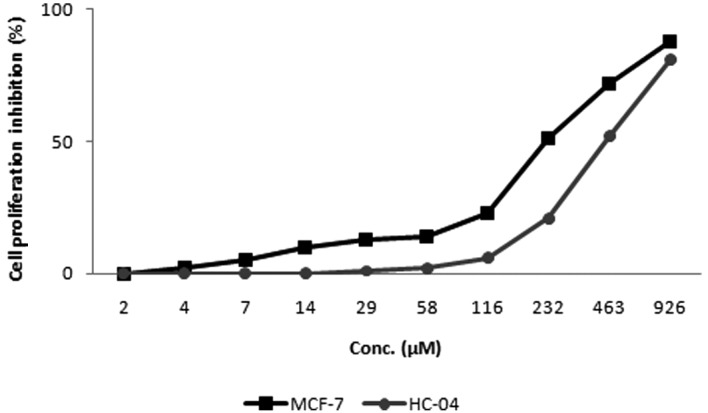
Effect of the 24-h treatment of kaempferol-3-O-rhamnoside on cancer (MCF-7) and non-cancer (HC-04) cells.
Kaempferol-3-O-rhamnoside induces caspase cascade protein expression in MCF-7 cells
The caspase-inducing activity of kaempferol-3-O-rhamnoside was assessed in MCF-7 cells. During kaempferol-3-O-rhamnoside treatment, caspase-9 and caspase-3 were upregulated in a time-dependent manner (Fig. 4). The changes in the expression levels of poly (ADP-ribose) polymerase (PARP), a hallmark biomarker of apoptosis, suggested that kaempferol-3-O-rhamnoside induced apoptosis through the activation of caspase-dependent signaling pathways as early as 24 h after treatment.
Figure 4.
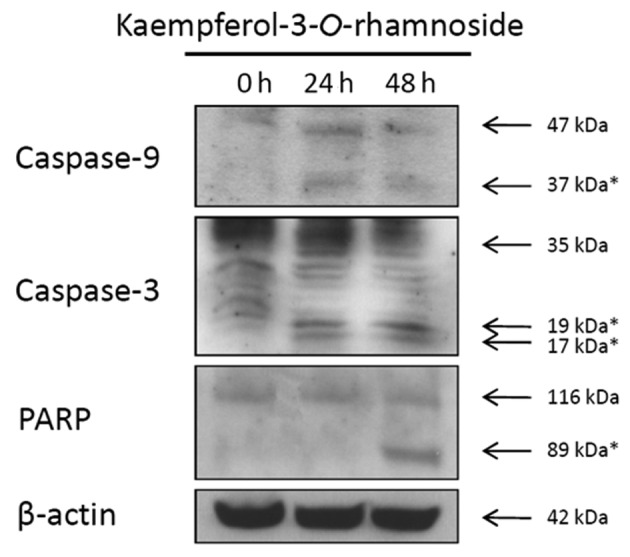
Western blot analysis of apoptotic proteins that are upregulated in MCF-7 cells treated with kaempferol-3-O-rhamnoside for 24 h and collected at the indicated times; * is the cleaved form.
Discussion
Plants consumed by primates are sources of drugs with a potential for application in the treatment and management of human disease. In a 1999 review, Milton established foods routinely consumed by primates as good sources of hexoses, cellulose, hemicellulose, pectic substances, vitamin C, minerals, essential fatty acids and protein, suggesting that non-human primate diets may be an important research area for human health (6). In this study, we evaluated this hypothesis using Schima wallichii Korth. as an example of a primate-consumed plant that exhibits anti-cancer properties by activating the caspase cascade pathway.
Schima wallichii Korth. is a tree of 5–30 m in height and is widely found in the tropical areas of Nepal, Sikkim, Assam, Myanmar, South China, the Malay Peninsula, Indonesia and the Philippines (7). It is predominantly found in primate conservation areas (8,9), where it is consumed by non-human primates. Schima wallichii Korth. was identified as a food commonly consumed by primates in our previous study on primate-consumed plants in the Pangandaran Conservation Area of West Java, Indonesia (2).
In this study, we isolated kaempferol-3-O-rhamnoside, the major active compound in Schima wallichii Korth. extracts, of which the ethyl acetate fractions exhibited the most activity against MCF-7 human breast cancer cells. kaempferol-3-O-rhamnoside is a polyphenol that was reported by Vareed et al to inhibit lipid peroxidation and cyclooxygenase enzymes (COX-1 and COX-2) (10). Results of the present study have shown that kaempferol-3-O-rhamnoside is also able to inhibit the proliferation of MCF-7 cells in a dose-dependent manner. Additionally, the caspase cascade pathway is involved in the kaempferol-3-O-rhamnoside-mediated suppression of proliferation. Our results suggest that kaempferol-3-O-rhamnoside triggers cell death intrinsically in MCF-7 cells via the mitochondrial caspase-9 pathway and activation of PARP, a hallmark biomarker of apoptosis (11). The N-terminal fragment of PARP, which is generated from the cleavage of full-length PARP (116 kDa), is an 89-kDa peptide and was detected as early as 24 h after kaempferol-3-O-rhamnoside treatment. These results suggest that kaempferol-3-O-rhamnoside induces apoptosis of MCF-7 cells, thus demonstrating inhibitory activity towards cancer development and progression.
Potential anti-cancer compounds should demonstrate inhibitory activity and specificity for cancer cells without causing excessive damage to normal cells, as reported for several plant products that induce apoptosis in cancer cells with minimal damage to normal cells (12,13). In our study, although kaempferol-3-O-rhamnoside affects the proliferation of non-cancer HC-04 cells, the IC50 concentration required was also significantly higher than that required for cancer MCF-7 cells, suggesting that kaempferol-3-O-rhamnoside may be less cytotoxic to non-cancer cells.
In conclusion, accumulating evidence suggests that products derived from plants hold potential in the treatment and prevention of cancer. Therefore, it is important to identify apoptotic inducers from plants, either as crude extracts or as active isolated compounds. Furthermore, understanding their exact mechanisms of action may provide valuable information for their possible application in cancer therapy and prevention. We report the active properties of kaempferol-3-O-rhamnoside in the inhibition of cancer cell proliferation through the induction of apoptosis. Although further studies are required to evaluate its toxicity, its detailed mechanism(s) of anti-cancer action, and the possibility of its structural modification to enhance its anti-cancer activities, our results emphasize the potential application of kaempferol-3-O-rhamnoside in the management and treatment of cancer.
Acknowledgements
This study was supported by Grants-in-Aid from the Ministry of National Education of Indonesia to A.D. (National Competitive Research Grant and PAR-C Grant for Academic Recharging) and to A.S. (Grant for International Collaborations and Publications).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v12, Cancer Incidence and Mortality Worldwide. International Agency for Research on Cancer; 2010. [Accessed August 27, 2011.]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Koshimizu K, Murakami A, Hayashi H, Ohigashi H, Subarnas A, Gurmaya KJ, Ali AM. The Tokyo International Forum on Conservation and Sustainable Use of Tropical Bioresources. Japan Bioindustry Association; Tokyo: 1998. Biological activities of edible and medicinal plants from Indonesia and Malaysia; pp. 203–208. [Google Scholar]
- 3.Subarnas A, Hadiansyah C, Gurmaya KJ, Muhtadi A. Characterization of antimutagenic compound from primates-consumed plant Schima wallichii. Biotika. 2003;2:7–13. [Google Scholar]
- 4.Silverstein RM, Webster FX, Kiemle DJ. Spectrometric Identification of Organic Compounds. John Wiley & Sons. Inc; New Jersey: 2005. [Google Scholar]
- 5.Abdulah R, Faried A, Kobayashi K, Yamazaki C, Suradji EW, Ito K, Suzuki K, Murakami M, Kuwano H, Koyama H. Selenium enrichment of broccoli sprout extract increases chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC Cancer. 2009;9:414. doi: 10.1186/1471-2407-9-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milton K. Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us? Nutrition. 1999;15:488–498. doi: 10.1016/s0899-9007(99)00078-7. [DOI] [PubMed] [Google Scholar]
- 7.Keng H. Flora malesianae precursores - LVIII, part four. The genus Schima (Theaceae) in Malesia. Gard Bull Singapore. 1994;46:77–88. [Google Scholar]
- 8.Chalise MK. Assamese Macaques (Macaca assamensis) in Nepal. Primate Conservation. 2003;19:99–107. [Google Scholar]
- 9.Swapna N, Gupta A, Radhakrishna S. Distribution survey of Bengal slow loris Nycticebus Bengalensis in Tripura, Northeastern India. Asian Primates J. 2008;1:37–40. [Google Scholar]
- 10.Vareed SK, Schutzki RE, Nair MG. Lipid peroxidation, cyclooxygenase enzyme and tumor cell proliferation inhibitory compounds in Cornus kousa fruits. Phytomedicine. 2007;14:706–709. doi: 10.1016/j.phymed.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Konopleva M, Zhao S, Xie Z, Segall H, Younes A, Claxton DF, Estrov Z, Kornblau SM, Andreeff M. Apoptosis. Molecules and mechanisms. Adv Exp Med Biol. 1999;457:217–236. [PubMed] [Google Scholar]
- 12.Hirano T, Abe K, Gotoh M, Oka K. Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br J Cancer. 1995;72:1380–1388. doi: 10.1038/bjc.1995.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiao C, Carothers AM, Grunberger D, Solomon G, Preston GA, Barrett JC. Apoptosis and altered redox state induced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res. 1995;55:3576–3583. [PubMed] [Google Scholar]


