Abstract
The aim of this study was to determine the level of expression of Mina53 in human cholangiocarcinoma (CCA) and to explore the role of Mina53 in carcinogenesis and tumor progression and its clinical significance in CCA. The level of expression of Mina53, p53 and Ki67 was investigated by immunohistochemistry in 69 surgically resected CCA tissues and 21 adjacent non-cancerous tissues. The correlation between Mina53 expression and clinicopathological characteristics and the expression of p53 and Ki67 was examined. Positive expression of Mina53 was observed in 61 of 69 CCA cases (88.4%) and 1 case (4.8%) of adjacent non-cancerous tissue. The level of expression of Mina53 in CCA was markedly higher than in the adjacent non-cancerous tissues. An increased level of expression of Mina53 in CCA was significantly associated with histological differentiation (P<0.01), TNM stage (P<0.05) and lymph node metastasis (P<0.01). There was no significant correlation between the level of Mina53 expression and gender, age or distant metastasis (P>0.05). However, the expression of Mina53 was associated with the expression of p53 in CCA (P<0.05). In addition, increased levels of expression of Mina53 in CCA were positively associated with Ki67 levels (r=0.801, P<0.01, as calculated by association analysis). Therefore, the upregulation of Mina53 expression may be significant in the carcinogenesis and development of human CCA and could have significant clinical value.
Keywords: Mina53, cholangiocarcinoma, p53, Ki67
Introduction
Cholangiocarcinoma (CCA) is a malignancy that originates in bile duct epithelial cells. The disease may be classified into three categories which are defined by the site of the carcinoma: intrahepatic, perihilar and distal extrahepatic. The location of the tumors, as well as the growth pattern of the disease and a lack of diagnostic criteria, mean that CCA is often diagnosed at an advanced stage. The only effective cure for CCA is the resection of early stage tumors, following which there is a 5-year recurrence rate of 60–90%. However, due to late diagnosis this treatment is only appropriate for <40% of patients. This, coupled with an inadequate response to chemotherapy, results in a poor prognosis for patients with CCA: 75% of patients with CCA succumb to the disease within 1 year of diagnosis. There is some difference in the prognoses of the different types of CCA, as the median survival rates for patients with intrahepatic and those with perihilar CCA are 18–30 and 12–24 months, respectively. With a 5-year survival rate of <5%, there is a clear need to study CCA in order to improve the rate of early diagnosis and also to develop new treatment options (1–9).
Similarly to most neoplasms, CCA does not result from a single genetic change, but rather a series of mutations in oncogenes and tumor-suppressor genes. These changes can cause resistance to external stimuli and therefore lead to the unregulated proliferation of cells and the transformation from epithelium to carcinoma (10). One family of proto-oncogenes whose dysregulation is known to be associated with various types of cancer is the Myc family, consisting of three main genes: C-Myc, N-Myc and L-Myc. These genes are involved in apoptosis and the differentiation and proliferation of cells and encode basic helix-loop-helix leucine zipper transcription factors. However, the mechanisms by which the transcription factors affect these phenomena and the role they play in tumorigenesis are not fully understood and certain targets of the Myc genes have yet to be identified (11–18).
One of the most widely studied proto-oncogenes is C-Myc (17,18). The expression of C-Myc is correlated with cell proliferation and is downregulated in cells which are quiescent and fully differentiated. C-Myc has been shown to directly affect the expression of Mina53 (Myc-induced nuclear antigen with a molecular weight of 53 kDa) (19,20), which is also associated with proliferation. The inhibition of Mina53 expression results in the suppression of cell proliferation in certain cell lines. Mina53 has also been found to have an increased level of expression in human colon and esophageal squamous cell carcinomas and it is therefore thought to be involved in carcinogenesis (18–24).
Thus, we detected the levels of expression of Mina53, p53 and Ki67 in CCA tissues and studied the correlation between the level of expression of Mina53 and clinicopathological characteristics, anti-oncogene expression and tumor proliferation in CCA. We also explored the role of Mina53 in carcinogenesis and tumor progression and its value in clinical application.
Materials and methods
Patients and tissue samples
Between 2006 and 2010, 69 CCA specimens and 21 adjacent non-cancerous tissues were obtained during routine surgical procedures at the Jingzhou First People’s Hospital (Jingzhou, China) and the Wuhan People’s Hospital (Wuhan, China). Informed consent was obtained from all subjects. The clinicopathological characteristics of the 69 CCA cases were as follows: mean age, 59.2 years (range, 32–86); 41 males and 28 females; 20 stage I cases, 22 stage II cases, 16 stage III cases and 11 stage IV cases according to the pathological TNM staging criteria.
Immunohistochemistry
Immunohistochemistry was performed using the labeled streptavidin-biotin immunoperoxidase technique to determine the level of expression of Mina53. Sections (4 μm) of formalin-fixed and paraffin-embedded samples were mounted on silane-coated glass slides, deparaffinized in xylene and rehydrated through a graded series of ethanols. The sections were microwaved in 10 mM citrate-phosphate buffer (pH 6.0) for antigen retrieval for 15 min, and incubated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity, followed by incubation with bovine serum albumin (BSA) for 10 min to block non-specific binding. The sections were then incubated for 2 h with mouse anti-human Mina53 monoclonal antibody (Zymed, San Francisco, CA, USA), diluted 100-fold in Tris-buffered saline (TBS, pH 7.6) with 1% BSA in a humidified chamber at room temperature. The slides were incubated in sequence with secondary biotinylated antibody for 10 min and peroxidase-labeled streptavidin for 10 min using an LSAB kit (Zhongshan Corp., Beijing, China). Finally, 3,3′-diaminobenzidine (DAB, Zhongshan Corp.) was applied as the chromogen and the sections were counterstained with Mayer’s hematoxylin and examined under a light microscope. A negative control was performed with serial sections, omitting the incubation with the primary antibody. The proliferative activity of the cholangiocarcinoma was determined by assessing the Ki67 labelling index (Ki67-LI). Briefly, sections were immunostained with the anti-Ki67 monoclonal antibody (Biolegend, San Diego, CA, USA) in the same manner as the immunohistochemical staining of Mina53 described above. Sections were also immunostained with the anti-p53 monoclonal antibody (Biolegend), but stained using the DAB kit (Zhongshan Corp.).
Evaluation of tissue staining
Tissue slides were evaluated independently by two pathologists who were blinded for the patient characteristics and outcome. Whole-tissue slides, each comprising a representative cross section of the tumor, were evaluated. To account for regional differences in staining, a semi-quantitative immunoreactivity scoring system (IRS) was applied. To obtain the IRS for each individual case, the staining intensity (0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining) as well as the percentage of cells stained (0, no cells; 1, <10% of cells; 2, 11–50% of cells; 3, 51–80% of cells; 4, >80% of cells) were evaluated and the respective scores were multiplied, resulting in an IRS range from 0 to 12. For statistical analysis, cases were grouped as either Mina53-negative (IRS 0–6) or Mina53-positive (IRS 7–12). Cases with discordant IRS values were discussed at a multiheaded microscope until consensus was achieved. The Ki67-LI was defined as the percentage of stained cells in a minimum of 1,000 counted tumor cells. Five randomly selected microscopic fields were examined at high magnification (x200) under a light microscope for this purpose, in the same manner as for Mina53. The expression of p53 was scored as positive when staining was visible in >10% of the nuclei within a specimen.
Statistical analyses
The software package SPSS version 13.0 (SPSS Inc, Chicago, IL, USA) was used for data compilation and statistical analysis. The χ2 and Fisher’s exact probability tests were used to examine the association between the level of Mina53 expression and various other parameters, including clinicopathological characteristics. The Student’s t-test was performed to examine the correlation between the Ki67-LI and the level of protein expression. The χ2 test was used to examine the correlation between the expression of Mina53 and p53. P<0.05 was considered to indicate a statistically significant result.
Results
Mina53 expression in CCA tissues and adjacent non-cancerous tissues
Mouse anti-human Mina53 monoclonal antibody was used to detect the Mina53 protein immunohistochemically. Positive staining of Mina53 (yellow or brown) was mainly located in the nuclei but was also observed in the cytoplasm (Fig. 1). Adjacent non-cancerous tissues showed positive staining for Mina53 in only 1 of 21 cases (4.8%). Overexpression of the Mina53 protein was found in 61 (88.4%) of the 69 CCA tissues. The expression of Mina53 in the tumor tissues was compared with that in adjacent non-cancerous tissues. The level of Mina53 expression in the carcinoma tissues was significantly higher (P<0.01) than in the adjacent non-cancerous tissues (Table I).
Figure 1.
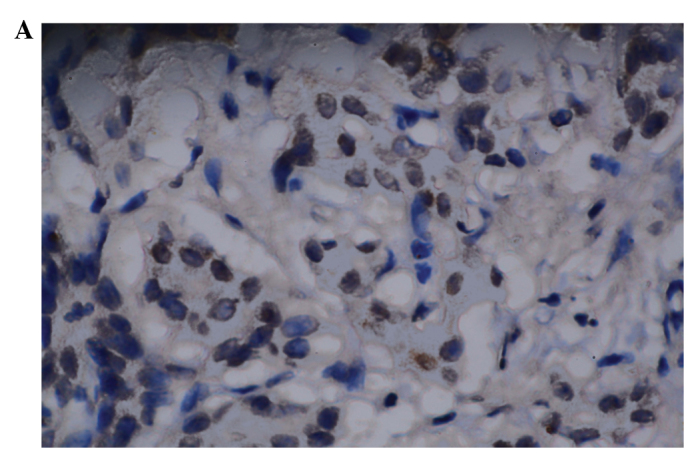
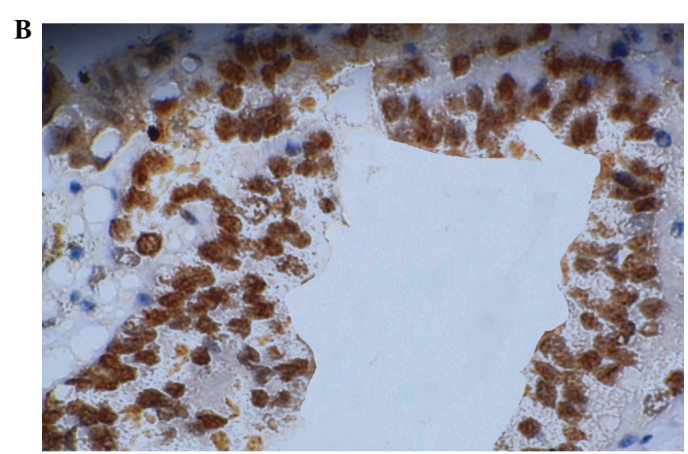
Expression of Mina53. (A) Negative expression in adjacent non-cancerous tissues (magnification, ×200, DAB); (B) Positive expression in cholangiocarcinoma (magnification, ×200, DAB). DAB, 3,3′-diaminobenzidine.
Table I.
Mina53 expression in human cholangiocarcinoma tissues and adjacent non-cancer tissues.
| Groups | Total | Negative | Positive | Cases with positive staining |
|---|---|---|---|---|
| Adjacent non-cancer tissues | 21 | 20 | 1 | 1 (4.8%) |
| Carcinoma tissues | 69 | 8 | 61 | 61 (88.4%) |
χ2=59.783, P=0.000. Negative, IRS 0–6; positive, IRS 7–12; IRS, immunoreactivity score.
Correlation between the expression of Mina53 and the clinicopathological characteristics of CCA
Significant associations were not found between the increased Mina53 expression and clinicopathological characteristics including gender and age (P>0.05). There were statistically significant associations between the increased Mina53 expression and histological differentiation (χ2=4.934, P<0.05), TNM stage (χ2=4.731, P<0.05) and lymph node metastasis (χ2=4.525, P<0.05). However, significant associations were not found between the level of Mina53 expression and distant metastasis (P>0.05, Table II).
Table II.
Correlation between the expression of Mina53 and the clinicopathological characteristics of cholangiocarcinoma cases.
| Clinical characteristics | Total | Mina53 expression | P-value | |
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| All cases | 69 | 8 | 61 | |
| Gender | ||||
| Male | 41 | 5 | 36 | 0.850 |
| Female | 28 | 3 | 25 | |
| Age | ||||
| ≤60 years | 39 | 4 | 35 | 0.692 |
| >60 years | 30 | 4 | 26 | |
| Histological differentiation | ||||
| Well | 34 | 7 | 27 | 0.026 |
| Moderate | 22 | 1 | 21 | |
| Poor | 13 | 0 | 13 | |
| TNM stage | ||||
| I | 20 | 4 | 16 | 0.030 |
| II | 22 | 4 | 18 | |
| III | 16 | 0 | 16 | |
| IV | 11 | 0 | 11 | |
| pN (lymph node metastasis) | ||||
| pN0 | 46 | 8 | 38 | 0.033 |
| pN1 | 23 | 0 | 23 | |
| Distant metastasis | ||||
| M0 | 59 | 8 | 51 | 0.216 |
| M1 | 10 | 0 | 10 | |
| p53 expression | ||||
| Positive | 58 | 2 | 56 | 0.000 |
| Negative | 11 | 6 | 5 | |
Negative Mina53 expression, IRS 0–6; positive Mina53 expression, IRS 7–12; IRS, immunoreactivity score.
Correlation between the expression of Mina53 and p53 in CCA
Positive staining of p53 was mainly localized to the nuclei (Fig. 2A). Accumulation of p53 was detected in 58 of the 69 CCA tissues (84.1%). The expression of Mina53 in CCA tissues was significantly associated with the expression of p53 (χ2=23.553, P<0.01). Of the 69 CCA tissue samples, 56 (81.2%) had simultaneous upregulation of Mina53 and p53.
Figure 2.
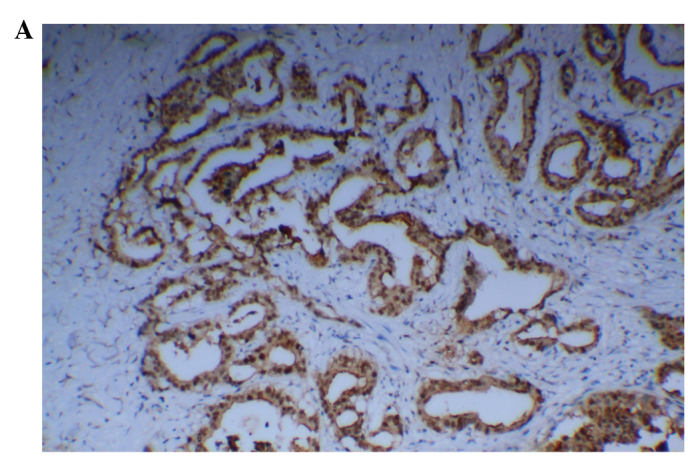
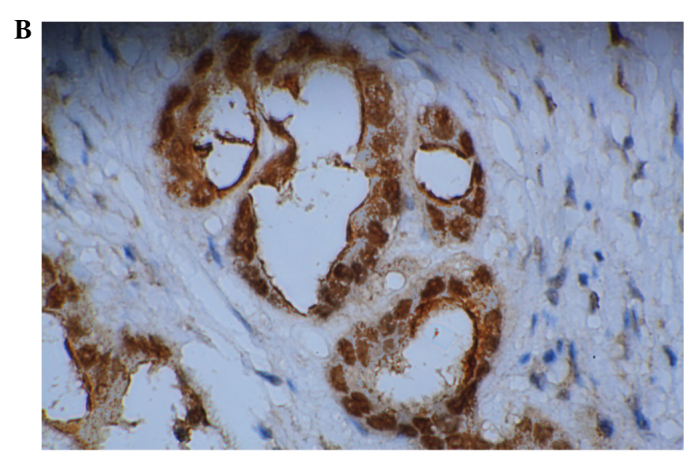
Expression of p53 and Ki67 in cholangiocarcinoma. (A) Positive expression of p53 (magnification, ×100, DAB); (B) Positive expression of Ki67 (magnification, ×200, DAB). DAB, 3,3′-diaminobenzidine.
Correlation between the expression of Mina53 and cell proliferation
Ki67 is a widely used biomarker of cell proliferation. Positive staining of Ki67 was mainly localized to the nuclei (Fig. 2B). The examined carcinoma specimens showed definite positive nuclear staining for Ki67 and some adjacent non-cancer tissues also showed positive staining for Ki67. The values of the Ki67-LI for the carcinoma samples and the adjacent non-cancerous tissues were 58.84±15.72% and 29.63±14.52%, respectively (mean ± SD). The level of Ki67 expression in the CCA tissues was higher than that in the adjacent non-cancerous tissues (P<0.05). An increased level of expression of Mina53 was positively associated with the Ki67 level (r=0.801, P<0.01, as calculated by association analysis).
Discussion
Tsuneoka et al previously identified Mina53 as a Myc target gene and revealed a clear correlation between Mina53 expression and cell proliferation (18,19). Certain studies have reported that Mina53 is overexpressed in a number of tumor cells and tissues, including colon carcinoma, esophageal squamous cell carcinoma and gingival squamous cell carcinoma (18–24), which led us to suspect that Mina53 may be involved in the abnormal cell growth observed in neoplastic diseases, including CCA. In this study, we used mouse anti-human Mina53 monoclonal antibody to study the level of expression of Mina53 in CCA tissues. Our results revealed that while almost all the CCA tissues examined exhibited elevated levels of expression of Mina53, only one adjacent non-cancerous tissue sample showed weak positive staining. We also observed that significant associations were not found between the level of Mina53 expression and clinicopathological characteristics including gender, age and distant metastasis. Statistically significant associations were found between increased levels of Mina53 expression and lymph node metastasis, histological differentiation and TNM stage. Therefore, Mina53 may play a role in biliary tract carcinogenesis and may be used as a marker for CCA.
The p53 gene, a tumor suppressor gene or anti-oncogene, is associated with apoptosis. Mutations in the p53 gene are the most common genetic alterations in a number of human carcinomas. The loss of p53 accelerates tumorigenesis associated with Myc activation by preventing apoptosis (25–30). In our study, the accumulation of mutated p53 was significantly associated with Mina53 expression in CCA. As Mina53 is a Myc target gene, Mina53 may suppress the activity of p53 via the Myc pathway. Therefore, Mina53 may contribute to biliary tract carcinogenesis by suppressing apoptosis.
Ki67 is a widely used biomarker of cell proliferation (31). Using association analysis, we found that the overexpression of Mina53 in CCA tissues was positively associated with the level of Ki67. Cell proliferation was promoted with the increasing expression of Mina53. Therefore, the overexpression of Mina53 may be involved in the proliferation of CCA cells and have certain functions in carcinogenesis.
In conclusion, Mina53 was overexpressed in CCA and was associated with tumor proliferation and anti-oncogene expression. Mina53 was important in the carcinogenesis and development of CCA. We suggest that Mina53 may be used as a marker for CCA and could be exploited as a target for the treatment of CCA.
References
- 1.Skipworth JR, Olde Damink SW, Imber C, et al. Review article: surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Aliment Pharmacol Ther. 2011;34:1063–1078. doi: 10.1111/j.1365-2036.2011.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto M, Ariizumi S. Surgical outcomes of intrahepatic cholangiocarcinoma. Surg Today. 2011;41:896–902. doi: 10.1007/s00595-011-4517-z. [DOI] [PubMed] [Google Scholar]
- 4.Wadsworth CA, Dixon PH, Wong JH, et al. Genetic factors in the pathogenesis of cholangiocarcinoma. Dig Dis. 2011;29:93–97. doi: 10.1159/000324688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akamatsu N, Sugawara Y, Hashimoto D. Surgical strategy for bile duct cancer: advances and current limitations. World J Clin Oncol. 2011;2:94–107. doi: 10.5306/wjco.v2.i2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friman S. Cholangiocarcinoma - current treatment options. Scand J Surg. 2011;100:30–34. doi: 10.1177/145749691110000106. [DOI] [PubMed] [Google Scholar]
- 7.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morise Z, Sugioka A, Tokoro T, et al. Surgery and chemotherapy for intrahepatic cholangiocarcinoma. World J Hepatol. 2010;2:58–64. doi: 10.4254/wjh.v2.i2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis MC, Cassera MA, Vetto JT, et al. Surgical treatment of intrahepatic cholangiocarcinoma: outcomes and predictive factors. HPB (Oxford) 2011;13:59–63. doi: 10.1111/j.1477-2574.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fava G. Molecular mechanisms of cholangiocarcinoma. World J Gastrointest Pathophysiol. 2010;1:12–22. doi: 10.4291/wjgp.v1.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz W, Leon J, Eilers M. Contributions of Myc to tumorigenesis. Biochim Biophys Acta. 2002;16:61–71. doi: 10.1016/s0304-419x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 12.Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henriksson M, Luscher B. Proteins and the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- 14.Cowling VH, Cole MD. HATs off to capping: a new mechanism for Myc. Cell Cycle. 2007;6:307–309. doi: 10.4161/cc.6.8.4123. [DOI] [PubMed] [Google Scholar]
- 15.Prochownik EV, Li Y. The ever expanding role for c-Myc in promoting genomic instability. Cell Cycle. 2007;6:1024–1029. doi: 10.4161/cc.6.9.4161. [DOI] [PubMed] [Google Scholar]
- 16.Schick B, Wemmert S, Jung V, et al. Genetic heterogeneity of the MYC oncogene in advanced juvenile angiofibromas. Cancer Genet Cytogenet. 2006;164:25–31. doi: 10.1016/j.cancergencyto.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Jain M, Arvanitis C, Chu K, et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 18.Tsuneoka M, Koda Y, Soejima M, et al. A novel myc target gene, mina53, that is involved in cell proliferation. J Biol Chem. 2002;277:35450–35459. doi: 10.1074/jbc.M204458200. [DOI] [PubMed] [Google Scholar]
- 19.Tsuneoka M, Nishimune Y, Ohta K, et al. Expression of Mina53, a product of a Myc target gene in mouse testis. Int J Androl. 2006;29:323–330. doi: 10.1111/j.1365-2605.2005.00572.x. [DOI] [PubMed] [Google Scholar]
- 20.Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18:2967–2987. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- 21.Obaya AJ, Mateyak MK, Sedivy JM. Mysterious liaisons: the relationship between c-Myc and the cell cycle. Oncogene. 1999;18:2934–2941. doi: 10.1038/sj.onc.1202749. [DOI] [PubMed] [Google Scholar]
- 22.Kuratomi K, Yano H, Tsuneoka M, et al. Immunohistochemical expression of Mina53 and Ki67 proteins in human primary gingival squamous cell carcinoma. Kurume Med J. 2006;53:71–78. doi: 10.2739/kurumemedj.53.71. [DOI] [PubMed] [Google Scholar]
- 23.Tsuneoka M, Fujita H, Arima N, et al. Mina53 as a potential prognostic factor for esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:7347–7356. doi: 10.1158/1078-0432.CCR-03-0543. [DOI] [PubMed] [Google Scholar]
- 24.Teye K, Tsuneoka M, Arima N, et al. Increased expression of a Myc target gene Mina53 in human colon cancer. Am J Pathol. 2004;164:205–216. doi: 10.1016/S0002-9440(10)63111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vousden KH. P53: death star. Cell. 2000;103:691–694. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 26.Levine AJ. P53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 27.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 28.Jiang XH, Chun YWB, Yuen ST, et al. Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of p53 and activation of caspase-3. Int J Cancer. 2001;91:173–179. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1039>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Yin XY, Grove L, Datta NS, et al. C-myc overexpression and p53 loss cooperate to promote genomic instability. Oncogene. 1999;18:1177–1184. doi: 10.1038/sj.onc.1202410. [DOI] [PubMed] [Google Scholar]
- 30.Hong S, Pusapati RV, Powers JT, Johnson DG. Oncogenes and the DNA damage response: Myc and E2F1 engage the ATM signaling pathway to activate p53 and induce apoptosis. Cell Cycle. 2006;5:801–803. doi: 10.4161/cc.5.8.2638. [DOI] [PubMed] [Google Scholar]
- 31.Schlüter C, Duchrow M, Wohlenberg C, et al. The cell proliferation-associated antigen of antibody Ki67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell-cycle-maintaining proteins. J Cell Biol. 1993;123:513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]


