Abstract
Background. Several approaches have been proposed to pharmacologically ameliorate hepatic ischemia/reperfusion injury (IRI). This study was designed to evaluate the effects of a preconditioning oral nutritional supplement (pONS) containing glutamine, antioxidants, and green tea extract on hepatic warm IRI in pigs. Methods. pONS (70 g per serving, Fresenius Kabi, Germany) was dissolved in 250 mL tap water and given to pigs 24, 12, and 2 hrs before warm ischemia of the liver. A fourth dose was given 3 hrs after reperfusion. Controls were given the same amount of cellulose with the same volume of water. Two hours after the third dose of pONS, both the portal vein and the hepatic artery were clamped for 40 min. 0.5, 3, 6, and 8 hrs after reperfusion, heart rate (HR), mean arterial pressure (MAP), central venous pressure (CVP), portal venous flow (PVF), hepatic arterial flow (HAF), bile flow, and transaminases were measured. Liver tissue was taken 8 hrs after reperfusion for histology and immunohistochemistry. Results. HR, MAP, CVP, HAF, and PVF were comparable between the two groups. pONS significantly increased bile flow 8 hrs after reperfusion. ALT and AST were significantly lower after pONS. Histology showed significantly more severe necrosis and neutrophil infiltration in controls. pONS significantly decreased the index of immunohistochemical expression for TNF-α, MPO, and cleaved caspase-3 (P < 0.001). Conclusion. Administration of pONS before and after tissue damage protects the liver from warm IRI via mechanisms including decreasing oxidative stress, lipid peroxidation, apoptosis, and necrosis.
1. Introduction
During liver surgery, the inflow occlusion maneuver to prevent blood loss as well as the liver manipulation itself have been shown to induce a cascade of molecular events, referred to as ischemia-reperfusion injury (IRI). IRI leads to the activation of Kupffer cells (KCs), the release of reactive oxygen species (ROS) and proinflammatory cytokines, microcirculatory disturbances, and eventually liver dysfunction and failure [1–10]. Different strategies have been proposed to prevent or ameliorate IRI. Among others, pharmacological preconditioning has been shown to be effective via mechanisms including, but not limited to, the direct neutralization of ROS, upregulation of anti-inflammatory, and downregulation of proinflammatory signaling pathways [11–27].
During IRI, intestinal endotoxins (LPS) leak through the altered gut membrane into the portal circulation and enhance the phagocytosis in hepatic KCs [28–35]. This interrelation between intestinal LPS and hepatic KCs makes the gastrointestinal tract an attractive target for the pharmacological preconditioning strategies against hepatic IRI. We hypothesized that an oral pharmacological preconditioning supplement, tailored not only to exert direct ROS-scavenging activity but also to stabilize the gut epithelium during IRI, would tackle the warm hepatic IRI in a porcine model. To the best of our knowledge, this work is the first report of an oral pharmacological preconditioning against hepatic IRI in a larger animal model.
2. Materials and Methods
2.1. Animal Care
German landrace pigs (32.3 ± 0.9 kg) were given access to standard laboratory chow (ssniff R/M-H, ssniff Spezialdiäten, Soest, Germany) and tap water ad libitum before experiments. All experimental procedures were reviewed and approved by the responsible authority (Regierungspräsidium Karlsruhe, Baden-Württemberg, Germany) according to the animal welfare legislation (§ 8 Abs. 1 Tierschutzgesetz (TierSchG) dated 18 May, 2006 (BGBI. I S. 1206)) and were performed according to institutional guidelines at the Ruprecht-Karls University of Heidelberg.
2.2. Experimental Procedure
Pigs underwent general anesthesia. After premedication with Azaperone (Stresnil, Janssen-Cilag Pharma, Wien, Austria, 1-2 mg/kg, i.m.) and midazolamhydrochloride (Dormicum 15 mg/3 mL, Roche, Grenzach-Wyhlen, Germany, 0.5–0.7 mg/kg, i.m.), anesthesia was induced with Esketaminhydrochloride (KETANEST S 25 mg/mL, Parke-Davis, Berlin, Germany, 10 mg/kg i.v.) and midazolam hydrochloride (1–1.4 mg/kg i.v.). After endotracheal intubation, animals were ventilated with a mixture of 1.5–2.0 L/min oxygen, 0.5–1.0 L/min air, and 0.75%–1.5% isoflurane (Isofluran-Baxter, Baxter, Unterschleißheim, Germany, semiopen ventilation). For analgesia, Piritramide (Dipidolor, Janssen-Cilag, Neuss, Germany, 3.75 mg/h intravenously) was administered. Body temperature was maintained using warming blankets (WarmTouch, Maleinckrodt Medical GmbH, Hennet/Sieg, Germany) and monitored by continuous rectal temperature probes. Systemic hemodynamic parameters, including mean arterial pressure (MAP) and central venous pressure (CVP), were measured continuously (Stetham Transducer, Hellige Monitoring, Freiburg, Germany) by indwelling polypropylene catheters (Braun, Melsungen, Germany) in the common carotid artery and internal jugular vein, respectively. Heart rate (HR) was monitored by body surface electrocardiogram recordings. Experimental groups were given a preconditioning oral nutritional supplement (pONS, 70 g per serving, Fresenius Kabi, Germany) containing glutamine, green tea extract (the resource, method of extraction, and composition of green tea extract has been published elsewhere [36]), vitamin C, vitamin E, beta carotene, selenium, zinc, and carbohydrates (1 sachet = 70 g) (Table 1) dissolved in 250 mL tap water 24 hrs (p.o.) and 12 hrs (p.o.) before the operation. The animals were then fasted overnight. On the day of operation and after performing a midline laparotomy, a third dose of pONS was applied via a jejunostomy tube. The portal vein and common hepatic artery were then mobilized and encircled by elastic bands. Two hrs after the administration of the third dose of pONS, the portal vein and the common hepatic artery were closed with Yasargil clamps (Aesculap, Tübingen, Germany) for 40 min to induce warm ischemia. Common bile duct was cannulated to collect bile continuously. After 40 min, the liver was reperfused by removing the clamps. A fourth dose of pONS was given 3 hrs after reperfusion. Controls were given the same amount of cellulose with the same volume of water. Serial blood samples were drawn and spun at 0.5, 3, 6, and 8 hrs after reperfusion and serum samples were kept at −20°C for the analysis of transaminases (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) serum concentrations with standard enzymatic methods [37]. The changes in bile production during each time interval were documented and the amount of the newly produced bile was plotted at the end of each time interval to assess the bile flow rate over time. Liver tissue was taken 8 hrs after reperfusion for histology (hematoxylin and eosin (H&E) staining) and immunohistochemistry (TNF-α, myeloperoxidase, cleaved caspase-3). Hemodynamic parameters (HR, MAP, CVP, PVF, HAF) were continuously monitored throughout the experiments; ultrasonic probes (Transsonic System Inc, New York, NY, USA) were used for the measurement of portal venous flow (PVF) and hepatic arterial flow (HAF). The experimental design is outlined in Figure 1. After the completion of experimental procedures 8 hrs after reperfusion, animals were sacrificed in deep anesthesia through the intravenous application of a high dose of potassium chloride.
Table 1.
Composition of the pONS.
| Component | Dry weight per sachet (g) |
|---|---|
| Glutamine | 15 |
| Antioxidants | |
| Green tea extract | 1 |
| Vitamin C | 0.75 |
| Vitamin E | 0.25 |
| ß-carotene | 0.005 |
| Selenium | 0.00015 |
| Zinc | 0.01 |
| Carbohydrates | 50 |
1 sachet of pONS was dissolved in 250 mL tap water before use. Conditions as mentioned in Materials and Methods. pONS: preconditioning oral nutritional supplement.
Figure 1.
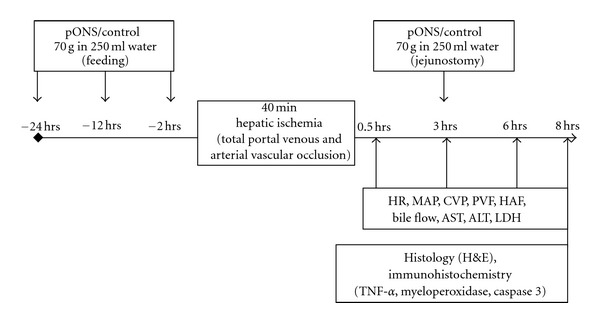
Experimental design. pONS (70 g in 250 mL tap water) was given to overnight-fasted German Landrace pigs 24, 12, and 2 hrs before warm ischemia of the liver. A fourth dose was given 3 hrs after reperfusion. Controls were given the same amount of cellulose. Two hrs after administration of the third dose, both the portal vein and the hepatic artery were clamped for 40 min. After reperfusion, hemodynamic parameters, bile production, and transaminases were measured serially. Liver tissue was taken 8 hrs after reperfusion for histology (H&E) and immunohistochemistry (TNF-a, myeloperoxidasby the Mann-Whitney rank sume, cleaved caspase-3) as described in Materials and Methods. pONS: preconditioning oral nutritional supplement; hrs: hours; min: minutes; PR: pulse rate; MAP: mean arterial pressure; CVP: central venous pressure; PVF: portal venous flow; HAF: hepatic artery flow; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
2.3. Histology
Liver samples were fixed by perfusion with 5% paraformaldehyde in Krebs-Henseleit bicarbonate buffer (118 mmol/L NaCl, 25 mmol/L NaHCO3, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 4.7 mmol/L KCl, and 1.3 mmol/L CaCl2) at pH 7.6, embedded in paraffin, and processed for light microscopy (H&E) 8 hrs after warm ischemia. In order to assess the histomorphological changes, 40 areas of 0.15 mm² were evaluated per slide using a point-counting method as described previously [38]: grade 0, minimal or no evidence of injury; grade 1, mild injury, including cytoplasmic vacuolation and focal nuclear pyknosis; grade 2, moderate to severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; grade 3, severe necrosis with disintegration of hepatic cords, hemorrhage, and neutrophil infiltration. To describe leukocyte infiltration into the hepatic tissue, a scale from 1 to 4 was used: grade 1, <10 leukocytes/field (focal infiltration); grade 2, 10–20 (mild infiltration); grade 3, 21–50; grade 4, >50 leukocytes/field.
2.4. Immunohistochemistry
Paraffin sections from liver tissue obtained 8 hrs after reperfusion were deparaffinized in xylene and rehydrated with graded ethanol. Antigen retrieval was performed via microwave pretreatment in EDTA buffer (pH 9.0) three times for 5 min. The specimens were then cooled and treated with 30% hydrogen peroxidase (H2O2) in phosphate-buffered saline (PBS)—final H2O2 concentration: 1%—to block endogenous peroxidases. Nonspecific antibody binding was blocked by normal rabbit serum. Sections were incubated with rabbit polyclonal anti-mouse tumor necrosis factor-alpha (TNF-α) antibody (Biosource Europe, Nivelles, Belgium) at the dilution of 1 : 500, and rabbit polyclonal anti-cleaved caspase-3 antibody (DCS, Hamburg, Germany) at a 1 : 200 dilution. After incubation, secondary biotinylated polyclonal rabbit anti-mouse immunoglobulin (Dako, Hamburg, Germany) was applied at a dilution of 1 : 200 for 1 hr followed by streptavidin-biotin complex. For myeloperoxidase (MPO) immunohistochemistry analysis, the sections were pretreated with proteinase K (1 : 40 dilution) and then blocked with bovine serum albumin. They were then incubated with the primary antibody, a polyclonal rabbit anti-MPO antibody (Dako, Carpinteria, CA, USA) at a dilution of 1 : 200, for 60 min at room temperature. A biotinylated swine anti-rabbit antibody (diluted 1 : 300) was used as the secondary antibody.
Positive cells for immunohistochemistry were counted in 10 microscopic fields per slide and slides were evaluated with a semiquantitative technique, relating the score of 0 to 4 points to the fraction of stained cells: scale 0, 0% cells; 1, <5% cells; 2, 5%–20% cells; 3, >20%–40% cells; 4, >40% positive cells as described elsewhere [12].
2.5. Statistics
Mean values ± SEM were compared using one-way ANOVA with the Students-Newman-Keuls post hoc test for the analysis of differences in hemodynamic values, vascular flow measurements, bile production, and transaminases. Differences in histological grading of injury as well as in immunohistochemical staining were tested by the Mann-Whitney rank sum test. P < 0.05 was selected prior to the investigation as the criterion for significance of differences between groups.
3. Results
3.1. General and Hemodynamic Data
Hematocrit, body weight, and temperature were not different between control and pONS groups (n = 6 in each group) (Table 2). Continuous postperfusion monitoring of the hemodynamic parameters (HR, MAP, CVP, PVF, HAF) also showed no significant differences between the two groups (Table 2).
Table 2.
Basic parameters.
| Control (n = 6) | pONS (n = 6) | P | |
|---|---|---|---|
| Body weight (kg) | 33.1 ± 1.7 | 31.4 ± 0.8 | 0.4 |
| Temperature (°C) | 36.9 ± 0.2 | 36.8 ± 0.2 | 0.7 |
| Respiratory rate (/min) | 12 ± 1 | 12 ± 1 | 0.9 |
| Hct (%) | 30 ± 0.9 | 33 ± 1.7 | 0.1 |
| HR (/min) | 177 ± 10 | 185 ± 9 | 0.6 |
| MAP (mmHg) | 90 ± 4.1 | 91 ± 3.7 | 0.9 |
| CVP (mmHg) | 15 ± 1.7 | 14.8 ± 0.5 | 0.9 |
| PVF (L/min) | 1.4 ± 0.6 | 1.9 ± 0.2 | 0.3 |
| HAF (dL/min) | 114 ± 22 | 117 ± 12 | 0.9 |
Table shows basic parameters (body weight, temperature, and respiratory rate) as well as postperfusion data for hematocrit (Hct), heart rate (HR), mean arterial pressure (MAP), central venous pressure (CVP), portal venous flow (PVF), and hepatic arterial flow (HAF). Values are mean ± SEM.
3.2. Liver Injury and Bile Production
While serum ALT increased in controls after warm ischemia/reperfusion to the liver, pONS prevented this effect; the difference between the two groups started to be significant 6 hours after reperfusion (49 ± 3 U/L in controls versus 35 ± 3 U/L in pONS; P = 0.01). This difference continued to exist until the end of experiments, 8 hrs after perfusion (50 ± 3 U/L in controls versus 33 ± 4 U/L in pONS; P = 0.02). pONS had the same effect on serum AST levels after reperfusion. The difference between the groups was significant 8 hrs after reperfusion (140 ± 52 U/L in controls versus 46 ± 7 U/L in pONS; P = 0.01) (Figure 2). There was significantly more severe necrosis with disintegration of hepatic cords, hemorrhage, and neutrophil infiltration (the median grade for necrosis and leukocyte infiltration were 3 and 4, resp.) in control tissue taken 8 hrs after reperfusion. pONS decreased the severity of the above-mentioned histomorphological changes in the liver (the median grade of necrosis and leukocyte infiltration of 1; P < 0.001) (Figure 3). Bile flow rate (mL/hr) was significantly higher in the pONS group 8 hrs after reperfusion (Figure 2).
Figure 2.
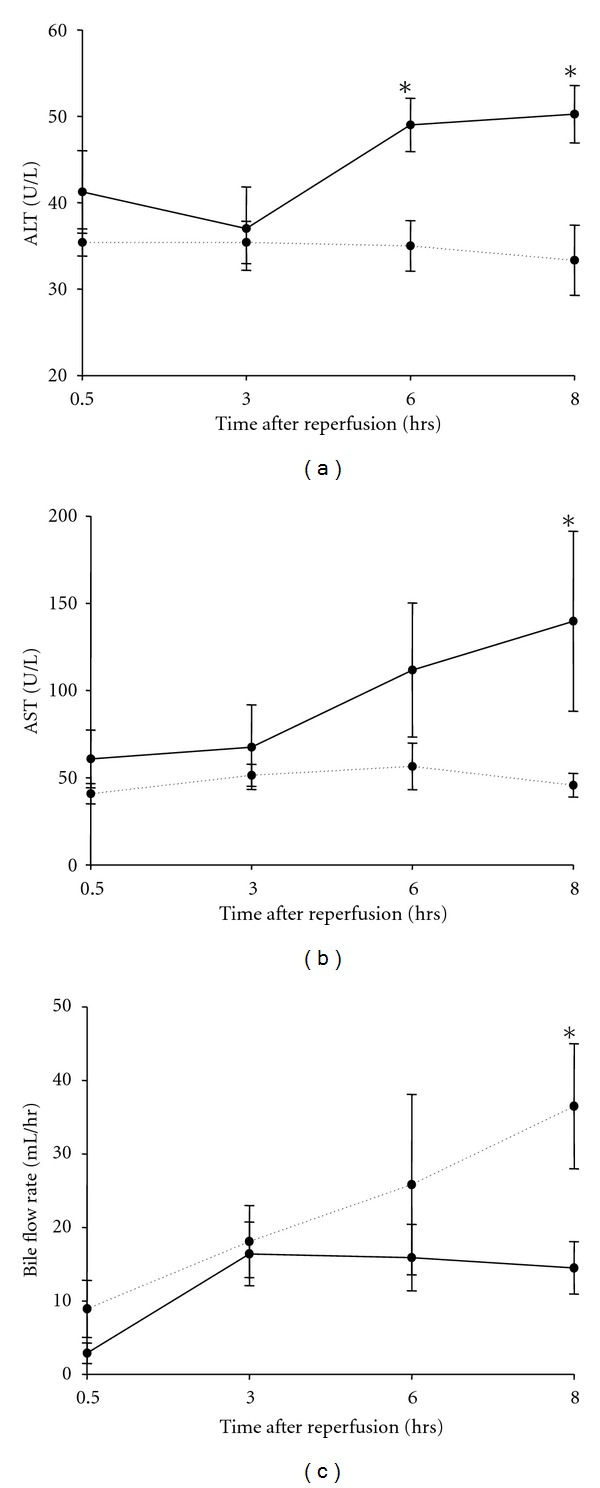
Effects of pONS on serum transaminases and bile production after reperfusion. ((a), (b)) Serial measurement of transaminases was performed after reperfusion as described in Materials and Methods. (c) Bile flow rate (mL/hr) has been depicted over time after reperfusion. Values are mean ± SEM (P < 0.05 by one-way ANOVA with Students-Newman-Keuls post hoc test, n = 6 per group); *P < 0.05 for comparison to controls; AST: aspartate aminotransferase; ALT: alanine aminotransferase; pONS: preconditioning oral nutritional supplement.
Figure 3.
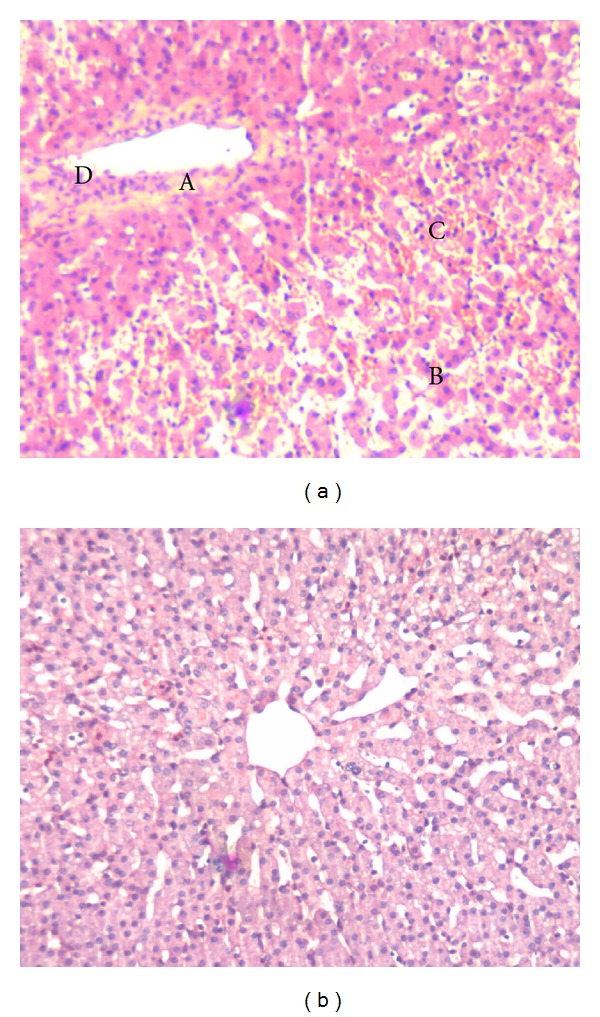
Liver injury eight hours after reperfusion. Liver tissue was taken 8 hrs after reperfusion and processed for light microscopy by H&E staining. (a), control; (b), pONS; controls displayed severe focal necrosis (A) with disintegration of hepatic cords (B), hemorrhage (C), and neutrophil infiltration (D) 8 hrs after reperfusion; this effect was significantly blunted by pONS. Pictures depict typical pattern of pathology; pONS: preconditioning oral nutritional supplement.
3.3. Immunohistochemistry
The immunohistochemical analysis of sections obtained 8 hrs after reperfusion indicated positive staining for TNF-α, MPO, and cleaved Caspase-3. pONS reduced the number of positively stained hepatocytes against all of three above enzymes (Figure 4). The quantitative assessment of immunohistochemical findings is presented in Table 3.
Figure 4.
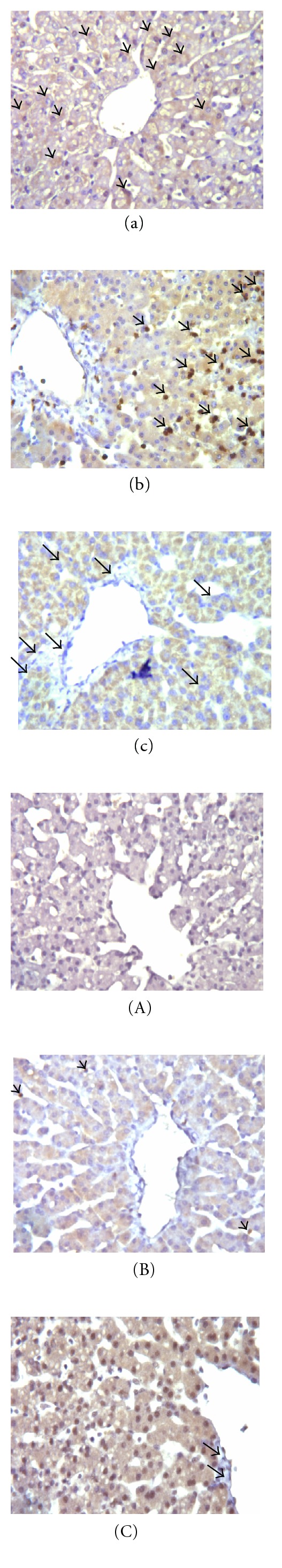
Immunohistochemistry for TNF-α ((a), (A)), MPO ((b), (B)), and cleaved Caspase-3 ((c), (C)). Conditions as described in Materials and Methods. Eight hrs after reperfusion, tissue was collected and processed for immunohistochemical analysis with light microscopy. The intensity of TNF-α expression (brown staining, black arrows), MPO expression (brown staining, black arrows), and cleaved Caspase-3 (blue halo, black arrows) was significantly higher in controls ((a), (b), (c), resp.) compared to their pONS counterparts ((A), (B), (C), resp.). Pictures depict typical pattern of staining (original magnification: ×200). TNF-α: tumor necrosis factor-alpha; MPO: myeloperoxidase; pONS: preconditioning oral nutritional supplement.
Table 3.
Quantitative assessment of immunohistochemical findings.
| Expression | Control (n = 6) | pONS (n = 6) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | median | 25% | 75% | n | median | 25% | 75% | ||
| TNF-α | 84 | 2 | 2 | 3 | 102 | 1 | 1 | 1 | <0.001 |
| MPO | 79 | 4 | 3 | 4 | 99 | 2 | 2 | 3 | <0.001 |
| Caspase-3 | 81 | 4 | 3 | 4 | 100 | 2 | 1 | 2 | <0.001 |
Conditions as described in Materials and Methods; n = 6 in each group; median values of indices for immunohistochemical expression of TNF-α, MPO, and cleaved caspase-3 with interquartile range 8 hrs after warm ischemia/reperfusion have been compared with Mann-Whitney rank sum. TNF-α: tumor necrosis factor-alpha; MPO: myeloperoxidase; pONS: preconditioning oral nutritional supplement.
4. Discussion
The manipulation of the liver during hepatic surgery activates Kupffer cells, which leads to the release of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interlukin-1 (IL-1) as well as free radicals, thus initiating an inflammatory cascade [2–7]. This phenomenon is usually further complicated through the application of the Pringle maneuver (hepatic inflow occlusion) in an attempt to prevent blood loss during the hepatic transection [39]. The cumulative effect induces warm IRI to the liver, which results, depending on the duration of ischemia, in microcirculatory disturbances, liver cell damage, and—in severe cases—liver failure [40]. Several approaches have been proposed to pharmacologically tackle hepatic IRI; none, however, has found its way into clinical routine yet.
To prevent IRI, it is important to neutralize reactive oxygen and nitrogen species, either by administering radical scavengers or by enhancing the capacity of endogenous redox defense systems [41]. A vast variety of dietary constituents can exert radical scavenging effects in vivo. Among all, hydrophilic ascorbic acid (vitamin C) and lipophilic α-tocopherol (vitamin E) are the important components of the human antioxidant system [42]. Carotenoids, the principal dietary source of vitamin A in humans [43], polyphenolic compounds in green tea extract [36, 44], selenium [45], and zinc [46] all exert antioxidant action; a synergism among the different antioxidants as part of an antioxidant network has been shown [47–49].
Large amounts of Gram-negative bacteria and endotoxins (LPS) are normally present in the intestines. A reduction in splanchnic blood flow and ischemia damages the intestinal wall and changes the permeability of the gut membrane, leading to excessive leakage of LPS and bacterial translocation into the portal circulation. It has been shown that LPS can activate KCs directly [50]. Scavenger receptors, including the scavenger receptor cystein-rich (SRCR) superfamily members that are expressed on KCs, are involved in the bactericidal action by binding and endocytosis of endotoxin [51, 52]. The CD11/CD18 receptor of KCs, the pattern recognition receptors (PRRs) CD14, and the Toll-like receptor 4 (TLR4) in combination with the adaptor protein MD2 are reported to be essentially involved in the LPS-associated KC activation [50, 53]. This may reflect an evolutionary adaptation by KCs to their local hepatic environment and strategic anatomic position in the portal circuit, which is optimal for the removal of endotoxin and, thus, for the protection of the host [54]. Glutamine has been shown to have a positive impact on the intestinal barrier by reducing permeability and bacterial translocation and preserving mucosal integrity [55, 56]. It can, therefore, prevent Kupffer cell activation and results in a more favorable outcome [57].
However, we did not measure blood LPS levels or histology of intestine, which might have further documented the protective effects of pONS on intestine.
In many models of liver injury, TNF-α levels are elevated and correlate with injury; the inhibition of TNF-α activity can attenuate liver injury, protect hepatic morphology, and decrease mortality [54]. MPO has been shown to be largely responsible for the neutrophil-induced parenchymal cell killing [58]. Released from the neutrophil's azurophilic granules, MPO can generate hypochlorous acid, a diffusible oxidant and chlorinating agent that gives rise to other toxic species, such as chloramines [59]. Apoptotic cell death can trigger neutrophil transmigration, severely aggravating apoptotic cell injury; caspase inhibitors can have a significant overall protective effect on hepatic IRI [60]. In the present study, we have shown that the oral administration of consecutive doses of a preconditioning supplement in experimental pigs significantly reduced the transaminases compared to controls after hepatic warm IRI. Furthermore, pONS resulted in significantly milder histological changes as well as a significant increase in bile production. The milder histological changes as well as improved sinusoidal bile production after pONS represents reduced postreperfusion injury. This has been further proved by the immunohistochemical analysis of the tissues obtained 8 hrs postreperfusion; pONS reduced the expression of TNF-α, MPO, and cleaved Caspase-3. pONS most likely exerts these protective effects via different mechanisms including direct antioxidative effects of its various antioxidant constituents including vitamin C, vitamin E, β-carotene, polyphenolic compounds in green tea extract, selenium and zinc. Furthermore, pONS most likely exerts an inhibitory effect on LPS-associated KC activation through glutamine.
To the best of our knowledge, this work is the first report of an oral pharmacological preconditioning against hepatic IRI in a larger animal model. The application of an oral nutritional substance in pigs is safe, reproducible, and well-deals with the current obstacles faced within the context of hepatic surgery and warm IRI. Tailoring such clinically-oriented experiments may finally help improve bench-to-bedside preconditioning protocols.
Acknowledgments
A. Nickkholgh together with Z. Li, R. Liang, and S. Mikalauskas carried out the experiments; A. Nickkholgh wrote the paper. E. Mohr performed the histological and immunohistochemical preparations. X. Yi and M.-L. Gross reviewed the histological and immunohistochemical part of the study. H. Schneider and S. Benzing supported the study regarding the experimental product and the design. M. Zorn was consulted regarding the biochemical measurements. M. Büchler and P. Schemmer supported the design of the study with their knowledge and experience. Further, P. Schemmer conceived and designed the study based on his experimental experience. The paper has been seen and approved by all authors listed above. The authors thank Katherine Hughes for editing the paper as a native speaker. The authors have declared no conflict of interest.
References
- 1.Bremer C, Bradford BU, Hunt KJ, et al. Role of Kupffer cells in the pathogenesis of hepatic reperfusion injury. American Journal of Physiology. 1994;267(4):630–636. doi: 10.1152/ajpgi.1994.267.4.G630. [DOI] [PubMed] [Google Scholar]
- 2.Schemmer P, Schoonhoven R, Swenberg JA, Bunzendahl H, Thurman RG. Gentle in situ liver manipulation during organ harvest decreases survival after rat liver transplantation: role of Kupffer cells. Transplantation. 1998;65(8):1015–1020. doi: 10.1097/00007890-199804270-00001. [DOI] [PubMed] [Google Scholar]
- 3.Schemmer P, Connor HD, Arteel GE, et al. Reperfusion injury in livers due to gentle in situ organ manipulation during harvest involves hypoxia and free radicals. Journal of Pharmacology and Experimental Therapeutics. 1999;290(1):235–240. [PubMed] [Google Scholar]
- 4.Schemmer P, Bradford BU, Rose ML, et al. Intravenous glycine improves survival in rat liver transplantation. American Journal of Physiology. 1999;276(4):924–932. doi: 10.1152/ajpgi.1999.276.4.G924. [DOI] [PubMed] [Google Scholar]
- 5.Schemmer P, Schoonhoven R, Swenberg JA, et al. Gentle organ manipulation during harvest as a key determinant of survival of fatty livers after transplantation in the rat. Transplant International. 1999;12(5):351–359. doi: 10.1007/s001470050239. [DOI] [PubMed] [Google Scholar]
- 6.Schemmer P, Bunzendahl H, Raleigh JA, Thurman RG. Graft survival is improved by hepatic denervation before organ harvesting. Transplantation. 1999;67(10):1301–1307. doi: 10.1097/00007890-199905270-00002. [DOI] [PubMed] [Google Scholar]
- 7.Schemmer P, Enomoto N, Bradford BU, et al. Activated Kupffer cells cause a hypermetabolic state after gentle in situ manipulation of liver in rats. American Journal of Physiology. 2001;280(6):1076–1082. doi: 10.1152/ajpgi.2001.280.6.G1076. [DOI] [PubMed] [Google Scholar]
- 8.Hanboon BK, Ekataksin W, Alsfasser G, et al. Microvascular dysfunction in hepatic ischemia-reperfusion injury in pigs. Microvascular Research. 2010;80(1):123–132. doi: 10.1016/j.mvr.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Schemmer P, Golling M, Kraus T, et al. Extended experience with glycine for prevention of reperfusion injury after human liver transplantation. Transplantation Proceedings. 2002;34(6):2307–2309. doi: 10.1016/s0041-1345(02)03247-5. [DOI] [PubMed] [Google Scholar]
- 10.Schemmer P, Mehrabi A, Kraus T, et al. New aspect on reperfusion injury to liver—impact of organ harvest. Nephrology Dialysis Transplantation. 2004;19(4):26–35. doi: 10.1093/ndt/gfh1038. [DOI] [PubMed] [Google Scholar]
- 11.Luntz SP, Unnebrink K, Seibert-Grafe M, et al. HEGPOL: randomized, placebo controlled, multicenter, double-blind clinical trial to investigate hepatoprotective effects of glycine in the postoperative phase of liver transplantation [ISRCTN69350312] BMC Surgery. 2005;5, article 18 doi: 10.1186/1471-2482-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schemmer P, Liang R, Kincius M, et al. Taurine improves graft survival after experimental liver transplantation. Liver Transplantation. 2005;11(8):950–959. doi: 10.1002/lt.20501. [DOI] [PubMed] [Google Scholar]
- 13.Kincius M, Liang R, Nickkholgh A, et al. Taurine protects from liver injury after warm ischemia in rats: the role of Kupffer cells. European Surgical Research. 2007;39(5):275–283. doi: 10.1159/000102982. [DOI] [PubMed] [Google Scholar]
- 14.Jahnke C, Mehrabi A, Golling M, et al. Evaluation of microperfusion disturbances in the transplanted liver after Kupffer cell destruction using GdCl3: an experimental porcine study. Transplantation Proceedings. 2006;38(5):1588–1595. doi: 10.1016/j.transproceed.2006.02.067. [DOI] [PubMed] [Google Scholar]
- 15.Nickkholgh A, Schneider H, Encke J, Büchler MW, Schmidt J, Schemmer P. PROUD: effects of preoperative long-term immunonutrition in patients listed for liver transplantation. Trials. 2007;8, article 20 doi: 10.1186/1745-6215-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickkholgh A, Barro-Bejarano M, Liang R, et al. Signs of reperfusion injury following CO2 pneumoperitoneum: an in vivo microscopy study. Surgical Endoscopy and Other Interventional Techniques. 2008;22(1):122–128. doi: 10.1007/s00464-007-9386-6. [DOI] [PubMed] [Google Scholar]
- 17.Schemmer P, Nickkholgh A, Schneider H, et al. PORTAL: pilot study on the safety and tolerance of preoperative melatonin application in patients undergoing major liver resection: a double-blind randomized placebo-controlled trial. BMC Surgery. 2008;8, article 2 doi: 10.1186/1471-2482-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan X, Dei-Anane G, Liang R, et al. Donor preconditioning with taurine protects kidney grafts from injury after experimental transplantation. Journal of Surgical Research. 2008;146(1):127–134. doi: 10.1016/j.jss.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Liang R, Nickkholgh A, Hoffmann K, et al. Melatonin protects from hepatic reperfusion injury through inhibition of IKK and JNK pathways and modification of cell proliferation. Journal of Pineal Research. 2009;46(1):8–14. doi: 10.1111/j.1600-079X.2008.00596.x. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Nickkholgh A, Yi X, et al. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. Journal of Pineal Research. 2009;46(4):365–372. doi: 10.1111/j.1600-079X.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 21.Liang R, Bruns H, Kincius M, et al. Danshen protects liver grafts from ischemia/reperfusion injury in experimental liver transplantation in rats. Transplant International. 2009;22(11):1100–1109. doi: 10.1111/j.1432-2277.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- 22.Guan X, Dei-Anane G, Bruns H, et al. Danshen protects kidney grafts from ischemia/reperfusion injury after experimental transplantation. Transplant International. 2009;22(2):232–241. doi: 10.1111/j.1432-2277.2008.00770.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann K, Büchler MW, Schemmer P. Supplementation of amino acids to prevent reperfusion injury after liver surgery and transplantation—where do we stand today? Clinical Nutrition. 2011;30(2):143–147. doi: 10.1016/j.clnu.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Bruns H, Watanpour I, Gebhard MM, et al. Glycine and taurine equally prevent fatty livers from Kupffer cell-dependent injury: an in vivo microscopy study. Microcirculation. 2011;18(3):205–213. doi: 10.1111/j.1549-8719.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 25.Ceyhan GO, Timm AK, Bergmann F, et al. Prophylactic glycine administration attenuates pancreatic damage and inflammation in experimental acute pancreatitis. Pancreatology. 2011;11(1):57–67. doi: 10.1159/000325972. [DOI] [PubMed] [Google Scholar]
- 26.Mikalauskas S, Mikalauskiene L, Bruns H, et al. Dietary glycine protects from chemotherapy-induced hepatotoxicity. Amino Acids. 2011;40(4):1139–1150. doi: 10.1007/s00726-010-0737-6. [DOI] [PubMed] [Google Scholar]
- 27.Nickkholgh A, Schneider H, Sobirey M, et al. The use of high-dose melatonin in liver resection is safe: first clinical experience. Journal of Pineal Research. 2011;50(4):381–388. doi: 10.1111/j.1600-079X.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- 28.Nolan JP. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981;1(5):458–465. doi: 10.1002/hep.1840010516. [DOI] [PubMed] [Google Scholar]
- 29.Cowper KB, Currin RT, Dawson TL, Lindert KA, Lemasters JJ, Thurman RG. A new method to monitor Kupffer-cell function continuously in the perfused rat liver: dissociation of glycogenolysis from particle phagocytosis. Biochemical Journal. 1990;266(1):141–147. doi: 10.1042/bj2660141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schemmer P, Enomoto N, Bradford BU, Bunzendahl H, Raleigh JA, Thurman RG. Autonomic nervous system and gut-derived endotoxin: involvement in activation of Kupffer cells after in situ organ manipulation. World Journal of Surgery. 2001;25(4):399–406. doi: 10.1007/s002680020070. [DOI] [PubMed] [Google Scholar]
- 31.Monden K, Arii S, Itai S, et al. Enhancement of hepatic macrophages in septic rats and their inhibitory effect on hepatocyte function. Journal of Surgical Research. 1991;50(1):72–76. doi: 10.1016/0022-4804(91)90012-b. [DOI] [PubMed] [Google Scholar]
- 32.Schultze RL, Gangopadhyay A, Cay O, Lazure D, Thomas P. Tyrosine kinase activation in LPS stimulated rat Kupffer cells. Cell Biochemistry and Biophysics. 1999;30(2):287–301. doi: 10.1007/BF02738071. [DOI] [PubMed] [Google Scholar]
- 33.Tokyay R, Zeigler ST, Traber DL, et al. Postburn gastrointestinal vasoconstriction increases bacterial and endotoxin translocation. Journal of Applied Physiology. 1993;74(4):1521–1527. doi: 10.1152/jappl.1993.74.4.1521. [DOI] [PubMed] [Google Scholar]
- 34.Morris SE, Navaratnam N, Townsend CM, Herndon DN. Decreased mesenteric blood flow independently promotes bacterial translocation in chronically instrumented sheep. Surgical Forum. 1989;40:88–91. [Google Scholar]
- 35.Golling M, Jahnke C, Fonouni H, et al. Distinct effects of surgical denervation on hepatic perfusion, bowel ischemia, and oxidative stress in brain dead and living donor porcine models. Liver Transplantation. 2007;13(4):607–617. doi: 10.1002/lt.21069. [DOI] [PubMed] [Google Scholar]
- 36.Liang R, Nickkholgh A, Kern M, et al. Green tea extract ameliorates reperfusion injury to rat livers after warm ischemia in a dose-dependent manner. Molecular Nutrition and Food Research. 2011;55(6):855–863. doi: 10.1002/mnfr.201000643. [DOI] [PubMed] [Google Scholar]
- 37.Bergmeyer HU. Methods of Enzymatic Analysis. New York, NY, USA: Academic Press; 1988. [Google Scholar]
- 38.Thurman RG, Marzi I, Seitz G, Thies J, Lemasters JJ, Zimmerman F. Hepatic reperfusion injury following orthotopic liver transplantation in the rat. Transplantation. 1988;46(4):502–506. doi: 10.1097/00007890-198810000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Rahbari NN, Wente MN, Schemmer P, et al. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. British Journal of Surgery. 2008;95(4):424–432. doi: 10.1002/bjs.6141. [DOI] [PubMed] [Google Scholar]
- 40.Jaeschke H, Farhood A. Kupffer cell activation after no-flow ischemia versus hemorrhagic shock. Free Radical Biology and Medicine. 2002;33(2):210–219. doi: 10.1016/s0891-5849(02)00867-5. [DOI] [PubMed] [Google Scholar]
- 41.Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transplantation. 2005;11(9):1031–1047. doi: 10.1002/lt.20504. [DOI] [PubMed] [Google Scholar]
- 42.Sharma MK, Buettner GR. Interaction of vitamin C and vitamin E during free radical stress in plasma: an ESR study. Free Radical Biology and Medicine. 1993;14(6):649–653. doi: 10.1016/0891-5849(93)90146-l. [DOI] [PubMed] [Google Scholar]
- 43.Nagel E, Meyer zu Vilsendorf A, Bartels M, Pichlmayr R. Antioxidative vitamins in prevention of ischemia/reperfusion injury. International Journal for Vitamin and Nutrition Research. 1997;67(5):298–306. [PubMed] [Google Scholar]
- 44.Zhong Z, Froh M, Connor HD, et al. Prevention of hepatic ischemia-reperfusion injury by green tea extract. American Journal of Physiology. 2002;283(4):957–964. doi: 10.1152/ajpgi.00216.2001. [DOI] [PubMed] [Google Scholar]
- 45.Zapletal C, Heyne S, Breitkreutz R, Gebhard MM, Golling M. The influence of selenium substitution on microcirculation and glutathione metabolism after warm liver ischemia/reperfusion in a rat model. Microvascular Research. 2008;76(2):104–109. doi: 10.1016/j.mvr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Horie Y, Wolf R, Flores SC, McCord JM, Epstein CJ, Granger DN. Transgenic mice with increased copper/zinc-superoxide dismutase activity are resistant to hepatic leukostasis and capillary no-reflow after gut ischemia/reperfusion. Circulation Research. 1998;83(7):691–696. doi: 10.1161/01.res.83.7.691. [DOI] [PubMed] [Google Scholar]
- 47.Vertuani S, Angusti A, Manfredini S. The antioxidants and pro-antioxidants network: an overview. Current Pharmaceutical Design. 2004;10(14):1677–1694. doi: 10.2174/1381612043384655. [DOI] [PubMed] [Google Scholar]
- 48.Wijnen MH, Roumen RMH, Vader HL, Goris RJA. A multiantioxidant supplementation reduces damage from ischaemia reperfusion in patients after lower torso ischaemia. A randomised trial. European Journal of Vascular and Endovascular Surgery. 2002;23(6):486–490. doi: 10.1053/ejvs.2002.1614. [DOI] [PubMed] [Google Scholar]
- 49.Schindler G, Kincius M, Liang R, et al. Fundamental efforts toward the development of a therapeutic cocktail with a manifold ameliorative effect on hepatic ischemia/reperfusion injury. Microcirculation. 2009;16(7):593–602. doi: 10.1080/10739680903110779. [DOI] [PubMed] [Google Scholar]
- 50.Su GL, Goyert SM, Fan MH, et al. Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. American Journal of Physiology. 2002;283(3):640–645. doi: 10.1152/ajpgi.00253.2001. [DOI] [PubMed] [Google Scholar]
- 51.van Amersfoort ES, van Berkel TJC, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clinical Microbiology Reviews. 2003;16(3):379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Oosten M, van de Bilt E, van Berkel TJC, Kuiper J. New scavenger receptor-like receptors for the binding of lipopolysaccharide to liver endothelial and Kupffer cells. Infection and Immunity. 1998;66(11):5107–5112. doi: 10.1128/iai.66.11.5107-5112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsung A, Hoffman RA, Izuishi K, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. Journal of Immunology. 2005;175(11):7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 54.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiological Reviews. 2009;89(4):1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- 55.dos Santos RG, Viana ML, Generoso SV, Arantes RE, Davisson Correia MI, Cardoso VN. Glutamine supplementation decreases intestinal permeability and preserves gut mucosa integrity in an experimental mouse model. Journal of Parenteral and Enteral Nutrition. 2010;34(4):408–413. doi: 10.1177/0148607110362530. [DOI] [PubMed] [Google Scholar]
- 56.Schroeder J, Alteheld B, Stehle P, Cayeux MC, Chioléro RL, Berger MM. Safety and intestinal tolerance of high-dose enteral antioxidants and glutamine peptides after upper gastrointestinal surgery. European Journal of Clinical Nutrition. 2005;59(2):307–310. doi: 10.1038/sj.ejcn.1602073. [DOI] [PubMed] [Google Scholar]
- 57.Kul M, Vurucu S, Demirkaya E, et al. Enteral glutamine and/or arginine supplementation have favorable effects on oxidative stress parameters in neonatal rat intestine. Journal of Pediatric Gastroenterology and Nutrition. 2009;49(1):85–89. doi: 10.1097/MPG.0b013e318198cd36. [DOI] [PubMed] [Google Scholar]
- 58.Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. American Journal of Physiology. 2006;290(6):1083–1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 59.El-Benna J, Dang PMC, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Archivum Immunologiae et Therapiae Experimentalis. 2005;53(3):199–206. [PubMed] [Google Scholar]
- 60.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. American Journal of Physiology. 2003;284(1):15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]


