Abstract
We introduced concurrent neoadjuvant chemoradiotherapy (CCRT) with S-1, an oral fluoropyrimidine, as treatment for oral squamous cell carcinoma (OSCC) from October 2005. The clinical usefulness and medical safety of CCRT with S-1 (S-1 group) for OSCC were analyzed and compared with CCRT using super-selective intra-arterial infusion (AI group). The subjects in the S-1 group underwent external irradiation, at a total dose of 30 Gy, with S-1 chemotherapy. The AI group received cisplatin (CDDP) or carboplatin (CBDCA) combined with daily radiotherapy at a total dose of 40 Gy. The histological effects and disease-specific survival rates were almost equivalent in the S-1 and AI groups. Adverse events were less frequent in the S-1 group, while hematological toxicity, including anemia, thrombopenia and pharyngeal edema, was observed in the AI group. The results of this study indicate that CCRT combined with S-1 is a more effective and safer treatment for OSCC than AI.
Keywords: oral squamous cell carcinoma, S-1, concurrent neoadjuvant chemoradiotherapy, super-selective intra-arterial chemotherapy, histological effect
Introduction
Concurrent chemoradiotherapy (CCRT) for oral squamous cell carcinoma (OSCC) was recently established as an effective treatment (1–5). In particular, therapy employing super-selective intra-arterial infusion for head and neck cancers has been shown to contribute to organ preservation and an increase in the survival rate (6–12). However, a number of problems remain, for example, the technical difficulty of intra-arterial infusion, limitations due to the anatomical vascular distribution, local tissue necrosis, edema, swelling and pain (6–8). S-1 is an oral anticancer drug in which the prodrug of 5-fluorouracil (5-FU), tegafur, is combined with gimeracil, a potent antagonist of the 5-FU-degrading enzyme dihydropyrimidine dehydrogenase, to increase the antitumor effect of 5-FU, and oteracil potassium to reduce digestive organ toxicity (1). Since the drug was approved for the treatment of malignant tumors in the head and neck region in 2001, the efficacy of the drug alone and in concurrent combination with radiotherapy has been reported (13–18).
Previously, we performed chemoradiotherapy employing super-selective intra-arterial infusion for patients with primary OSCC as a preoperative treatment. However, we experienced the complications of super-selective intra-arterial infusion, including pharyngeal edema and necrosis, therefore we introduced CCRT with S-1 from October 2005. In this study, to evaluate the significance of CCRT with S-1 as a preoperative treatment, we compared the efficacy and safety of the CCRTs employing S-1 and super-selective intra-arterial infusion.
Patients and methods
Patients
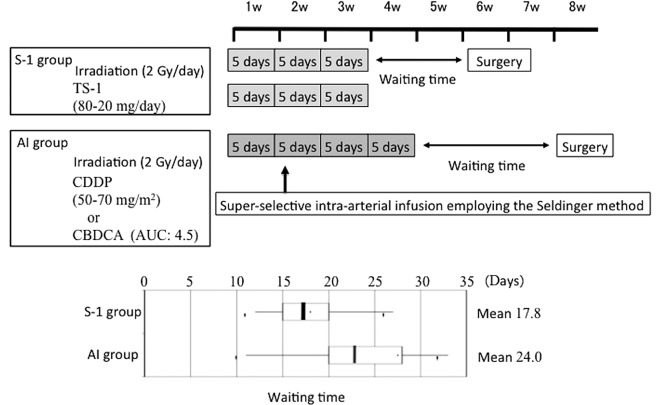
Of the patients with primary OSCC who underwent radical therapy at our department, 21 patients who underwent neoadjuvant chemoradiotherapy employing super-selective intra-arterial infusion of cisplatin (CDDP) or carboplatin (CBDCA) between July 1997 and April 2006 [intra-arterial infusion (AI) group] and 19 patients who underwent concurrent neoadjuvant chemoradiotherapy with S-1 between October 2005 and December 2009 (S-1 group) participated in this study. There were slightly more males in the AI group, but no significant difference was noted in the mean age. The most frequent location of the OSCC in the S-1 group was the tongue, observed in 8 patients (42%), followed by the gingiva in the lower jaw in 5 patients (26%). The gingiva in the lower jaw was the most frequent OSCC location in the AI group, observed in 9 patients (43%), followed by the tongue in 6 patients (28%). No patient in the AI group had an upper gingival lesion. Regarding the T and stage classifications, our department applies preoperative CCRT in late T2 or worse cases. Accordingly, all cases were T2 or worse in the S-1 group and only one case was T1 in the AI group, showing no significant difference in the distribution of T or stage classification between the two groups. The clinical characteristics of the study population are shown in Table I. Fig. 1 shows the preoperative treatment schedules at the Department of Oral and Maxillofacial Surgery, Kagoshima University. In CCRT with S-1, external X-ray irradiation employing linac at 2 Gy was performed 5 times a week for a total of 15 times, S-1 was orally administered with irradiation and radical surgery was performed ~2 weeks after the preoperative treatment. In the AI group, external X-ray irradiation employing linac at 2 Gy was performed 5 times a week for a total of 20 times and super-selective intra-arterial infusion was performed in the second week of radiotherapy, using the Seldinger method. CDDP was typically used as the chemotherapeutic drug, but CBDCA was administered to patients who were suspected of having renal dysfunction. Radical surgery was performed ~3 weeks after the completion of the preoperative treatment. The actual waiting time required for surgery is shown in the box plot of Fig. 1.
Table I.
Clinical characteristics of patients.
| Characteristics | S-1 group (%) | AI group (%) |
|---|---|---|
| No. of patients | 19 | 21 |
| Gender (male/female) | 10/9 | 13/8 |
| Median age in years (range) | 68.5 (37–83) | 66.9 (39–84) |
| Primary tumor site | ||
| Tongue | 8 (42) | 6 (28) |
| Lower gingiva | 5 (26) | 9 (43) |
| Upper gingiva | 2 (11) | 0 (0) |
| Buccal mucosa | 1 (5) | 2 (10) |
| Soft palate | 1 (5) | 1 (5) |
| Oral floor | 2 (11) | 3 (14) |
| Tumor classification | ||
| T1 | 0 (0) | 1 (5) |
| T2 | 14 (73) | 11 (52) |
| T3 | 2 (11) | 3 (14) |
| T4 | 3 (16) | 6 (29) |
| Stage | ||
| I | 0 (0) | 1 (5) |
| II | 3 (16) | 7 (33) |
| III | 9 (47) | 6 (29) |
| IV | 7 (37) | 7 (33) |
Forty patients treated at the Department of Oral and Maxillofacial Surgery (19 in the S-1 group and 21 in the AI group) were enrolled in this study. S-1, concurrent neoadjuvant chemoradiotherapy with S-1 and a total external irradiation dose of 30 Gy; AI, concurrent neoadjuvant chemoradiotherapy using super-selective intra-arterial infusion with cisplatin or carboplatin and a total external irradiation dose of 40 Gy.
Figure 1.
Preoperative treatment schedules at the Department of Oral and Maxillofacial Surgery, Kagoshima University. The treatment schedules of the S-1 and AI groups are shown (upper panel) and the actual waiting time for surgery is shown in the box plot (lower panel). S-1, concurrent neoadjuvant chemoradiotherapy with S-1 and a total external irradiation dose of 30 Gy; AI, concurrent neoadjuvant chemoradiotherapy using super-selective intra-arterial infusion with cisplatin or carboplatin and a total external irradiation dose of 40 Gy; CDDP, cisplatin; CBDCA, carboplatin; AUC, area under the receiver operating characteristics curve.
The study was approved by the ethics committee of Kagoshima University. Written informed consent was obtained from all patients.
Areas of investigation
Histological effect following neoadjuvant chemoradiotherapy
By employing the classification established by Shimosato et al (Table II) (19), the histological effect following preoperative treatment was evaluated in each group. Grades of IIb or higher were judged to be histologically effective.
Table II.
Histological gradings of the effects of chemoradiotherapy.
| Grade | Histological findings |
|---|---|
| I | Characteristic changes are noted in the tumor cells, but the tumor structures have not been destroyed. There is no detection of tumor nests due to the lysis of individual tumor cells. |
| II | In addition to the characteristic cell changes, the tumor structures have been destroyed as a result of the disappearance of tumor cells. However, a variable number of viable cells remain. |
| IIa | The destruction of the tumor structures is mild; viable tumor cells are frequently observed. |
| IIb | The destruction of the tumor structures is severe; viable tumor cells are few in number. |
| III | Markedly altered and presumably non-viable tumor cells are present singly or in small clusters and viable tumor cells are rarely observed. |
| IV | No tumor cells remain in any sections (local cure). |
Criteria by Shimosato et al (19). We applied this classification to assess the histological effects of neoadjuvant chemoradiotherapy.
Residual tumor pattern
The residual tumor pattern in cases showing a GrII histological effect was investigated following the classification reported by Böheim et al (20). Malignant alveolar lesions distributed directly under the mucoepithelium with a decreasing size towards the superficial mucosal layer are designated as superficial-type and conditions with cancer cells distributed in the deep mucosa and mixed with degenerative necrotic lesions are known as deep-type.
Disease-specific survival rate
The disease-specific survival rate, as determined using the Kaplan-Meier method, was compared between the groups.
Adverse events
Adverse events of Gr2 or worse were compared between the groups following the National Cancer Institute’s Common Toxicity Criteria (NCI-CTC) v.3.0.
Statistical analysis
The Student’s t-test was used to compare the response rates following neoadjuvant chemoradiotherapy of the S-1 and AI groups. A generalized Wilcoxon test and the log-rank test were used to compare the disease-specific survival rate of the groups. Statistical evaluations were performed with JMP® statistical discovery software. P<0.05 was considered to indicate a statistically significant result.
Results
Histological effect following neoadjuvant chemoradiotherapy
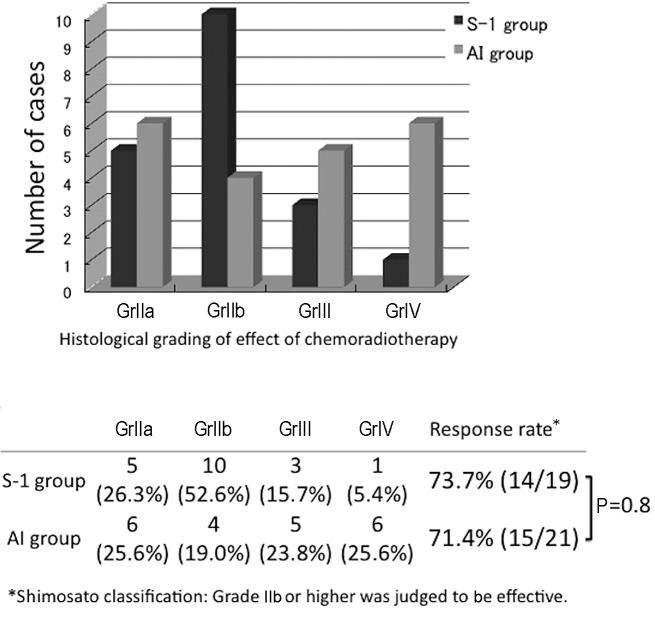
The effects of the preoperative treatments in the two groups, graded using the classification established by Shimosato et al (19) were determined (Fig. 2). The effect was GrIIa or higher in all patients. In the S-1 group, GrIIb was most frequently observed (10 cases, 52.6%) followed by GrIIa in 5 cases, GrIII in 3 and GrIV in 1 case. In the AI group, the effect was rated GrIII or GrIV in 11 cases, accounting for 52.4% of the AI group patients and indicating a high histological therapeutic effect, but this effect was rated GrIIa in 6 cases (25.6%), exhibiting a bimodal histological effect. Regarding grades of IIb or higher as effective, the response rates were 73.7 and 71.4% in the S-1 and AI groups, respectively, revealing no significant difference between the two groups.
Figure 2.
Comparison of the histological gradings of the effects of chemoradiotherapy of the S-1 and AI groups. In the S-1 group, GrIIb was most frequently observed (10 cases, 52.6%). In the AI group, the effect was rated GrIII or GrIV in 11 cases, accounting for 52.4%, showing a high histological therapeutic effect, but a bimodal histological effect. Regarding grades of IIb or above as effective, the response rates were 73.7 and 71.4% in the S-1 and AI groups, respectively, showing no significant difference between the two groups. S-1, concurrent neoadjuvant chemoradiotherapy with S-1 and a total external irradiation dose of 30 Gy; AI, concurrent neoadjuvant chemoradiotherapy using super-selective intra-arterial infusion with cisplatin or carboplatin and a total external irradiation dose of 40 Gy; Shimosato classification carried out according to the criteria of Shimosato et al (19).
Residual tumor pattern following preoperative treatment
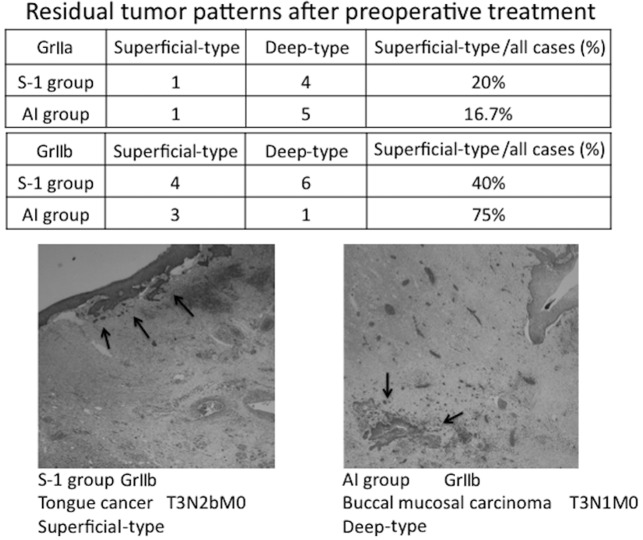
The residual tumor patterns following the preoperative treatment are shown in Fig. 3.
Figure 3.
Residual tumor patterns following preoperative treatment. The residual tumor patterns in cases with GrII histological effects are shown. The ratios of superficial- and deep-type patterns in GrIIa and GrIIb cases in the S-1 and AI groups are shown in the upper and lower panels, respectively. The images show H&E pathological preparations (magnification, ×20) of superficial- and deep-type GrIIb cases. The arrows indicate tumor cells. S-1, concurrent neoadjuvant chemoradiotherapy with S-1 and a total external irradiation dose of 30 Gy; AI, concurrent neoadjuvant chemoradiotherapy using super-selective intra-arterial infusion with cisplatin or carboplatin and a total external irradiation dose of 40 Gy; histological grading according to the criteria of Shimosato et al (19); residual tumor pattern classification according to the criteria of Böheim et al (20).
The residual tumor patterns of the GrII cases, in which viable cancer cells remained, were classified as superficial- or deep-type based on the classification reported by Böheim et al (20). The GrIIa cases were mostly deep-type in the two groups. The evaluation of the GrIIb cases was limited due to the small number of cases, but 4 and 6 cases were superficial- and deep-type in the S-1 group, respectively, showing that the superficial type accounted for 40% of the GrIIb cases in the S-1 group. In the AI group, 3 cases were superficial-type, accounting for 75% of the GrIIb cases. The lower panel of Fig. 3 shows the pathological findings in a superficial-type GrIIb case in the S-1 group and a deep-type GrIIb case in the AI group. The superficial-type tumor was reduced towards the mucoepithelium, whereas the deep-type tumor cells remained in the degenerative tissue.
Disease-specific survival rate
The disease-specific survival rates were determined using the Kaplan-Meier method (Fig. 4). The disease-specific survival rates were 88.8 and 86.4% in the S-1 and AI groups, respectively, showing a similar therapeutic outcome. Regarding the causes of mortality, 1 patient succumbed to the primary lesion and another to distant metastasis in the S-1 group and 3 patients succumbed to the primary lesion in the AI group.
Figure 4.
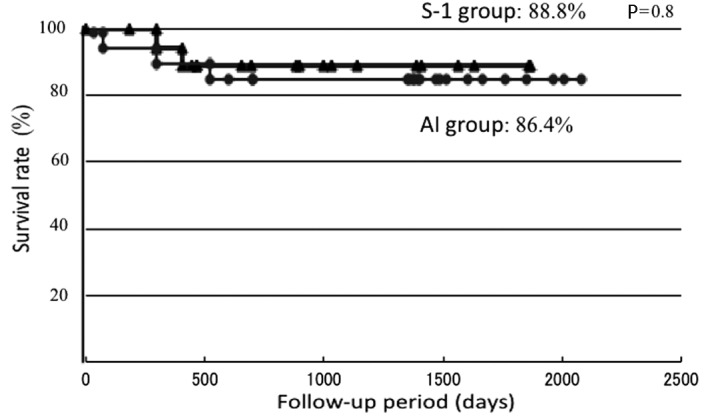
Five-year disease-specific survival rates. The disease-specific survival rates were 88.8 and 86.4% in the S-1 and AI groups, respectively, showing a similar survival curve with no significant difference in the survival rate. S-1, concurrent neoadjuvant chemoradiotherapy with S-1 and a total external irradiation dose of 30 Gy; AI, concurrent neoadjuvant chemoradiotherapy using super-selective intra-arterial infusion with cisplatin or carboplatin and a total external irradiation dose of 40 Gy.
Adverse events
S-1 was withdrawn due to neutropenia (1 case) and diarrhea (1 case) in the S-1 group. All patients recovered following the discontinuation of administration and no patients had their surgery postponed or canceled.
Table III shows NCI-CTC v.3.0 Gr2 or above adverse events. The most frequent adverse event in the two groups was stomatitis, with incidences of 73.7 and 85.7% in the S-1 and AI groups, respectively. Gr3 stomatitis was noted in 1 patient in the S-1 group. Regarding hematological toxicity, leukopenia occurred in 2 patients (10.5%) and neutropenia in 1 patient (5.2%) in the S-1 group, whereas hypochromia occurred in 2 patients (14.2%), leukopenia in 2 (14.2%) and neutropenia in 2 (14.2%) in the AI group, and Gr3 neutropenia was noted in 1 patient. In addition, complications of pharyngeal edema and necrosis were noted in the AI group.
Table III.
NCI-CTC v.3.0 grade 2 or above adverse events.
| Adverse events | Gr2 | Gr3 | Gr4 | % |
|---|---|---|---|---|
| S-1 group | ||||
| Leukopenia | 2 | 0 | 0 | 10.5 |
| Neutropenia | 1a | 0 | 0 | 5.2 |
| Fatigue | 3 | 0 | 0 | 15.7 |
| Vomiting | 2 | 0 | 0 | 10.5 |
| Diarrhea | 0 | 1a | 0 | 5.2 |
| Stomatitis | 13 | 1 | 0 | 73.7 |
| Elevation of bilirubin level | 1 | 0 | 0 | 5.2 |
| AI group | ||||
| Hypochromia | 2 | 0 | 0 | 14.2 |
| Leukopenia | 2 | 0 | 0 | 14.2 |
| Neutropenia | 1 | 1 | 0 | 14.2 |
| Thrombocytopenia | 1 | 0 | 0 | 7.1 |
| Fatigue | 3 | 0 | 0 | 21.4 |
| Nausea | 2 | 0 | 0 | 14.2 |
| Vomiting | 0 | 1 | 0 | 7.1 |
| Stomatitis | 12 | 0 | 0 | 57.1 |
| Soft tissue impairment | 1b | 1c | 0 | 9.5 |
Withdrawal of S-1;
pharyngeal edema;
pharyngeal necrosis.
S-1, concurrent neoadjuvant chemoradiotherapy with S-1 and a total external irradiation dose of 30 Gy; AI, concurrent neoadjuvant chemoradiotherapy using super-selective intra-arterial infusion with cisplatin or carboplatin and a total external irradiation dose of 40 Gy; NCI-CTC, National Cancer Institute’s Common Toxicity Criteria.
Discussion
Successful treatment for oral cancer should retain esthetic appearance and conserve function, unlike the treatment of malignant tumors in other regions, in addition to facilitating a high survival rate. The response rate of primary lesions and the organ conservation rate have increased with the development of potent chemotherapeutic drugs and regimens for concomitant drug administration and advancements in administration methods, for example chemotherapy by super-selective intra-arterial infusion (8–12). According to the oral cancer treatment guidelines in Japan, platinum-based drugs, including CDDP, are widely used in chemotherapy as the standard, but S-1 is also used. In a meta-analysis of 32 clinical studies on CCRT for advanced head and neck cancers reported in Germany in 2006 (18), 5-FU alone and in combination with other drugs, including CDDP, exhibited a marked effect. However, the incidence of oral cancer in the elderly has increased in Japan. Selecting the effective dose and administration method of chemotherapeutic drugs for elderly patients who may have a number of underlying problems is difficult due to the severe adverse events and complications caused by super-selective intra-arterial infusion, including hemorrhage and edema (21). S-1 is an oral anticancer drug in which the antitumor effect of 5-FU is strengthened and digestive organ toxicity is reduced. Since the administration of S-1 is relatively simple, the drug has been administered for advanced and non-advanced oral cancer in elderly patients, and cases which responded to S-1 alone and CCRT (13) and its efficacy as a preoperative treatment (16) have been reported. However, there have been few studies that have performed a comparison with other chemoradiotherapies, for instance chemoradiotherapy using super-selective intra-arterial infusion. To evaluate CCRT with S-1 as preoperative treatment for OSCC, we compared the efficacy and safety of CCRT employing super-selective intra-arterial infusion previously administered at our department and CCRT with S-1.
Several conclusions were derived from our clinical analyses: i) no significant difference was found in the histological response rate of the two groups; ii) the residual tumor pattern was slightly superior in the AI group, although we should be cautious due to the limited number of surgeries; iii) no significant difference in the disease-specific survival rates of the groups was found; and iv) no serious adverse event occurred.
In the S-1 group, the histological effect was rated GrIII or GrIV in only 4 patients (21.4%), but GrIIb effects were observed in 10 cases, accounting for the greatest proportion (52.6%). Regarding grades of IIb or higher as effective, the histological response rate was 73.7%, which is similar to the findings reported by Matsui et al (16). Enhancement of the effect of radiotherapy by S-1 in clinical head and neck cancer cases has been reported (13,16) and Zeng et al (22) investigated its mechanism in a basic experiment in which S-1 inhibited the activation of irradiation-induced HIF-1. This inhibition resulted in a reduced microvascular density and increased apoptosis, which significantly increased the irradiation sensitivity in the presence of S-1 compared with that in radiotherapy alone. GrIII and IV curative effects were observed in more patients in the AI compared to the S-1 group, but GrIIa with a residual tumor was also noted in a number of cases. This finding is not contradictory to improvements in the response rate of primary lesions and the organ conservation rate, but the effect of the chemotherapeutic drug may have been insufficient in certain regions due to the technical difficulty of intra-arterial infusion depending on the location of the primary lesion and limitations caused by the anatomical vascular distribution and the number of feeder blood vessels. Therefore, the histological response rate to CCRT with S-1 is high, although fewer cases showed a marked improvement compared with the AI group. The potentiation mechanism of concomitant treatment with irradiation has been elucidated and suggests that the therapy may be used to enhance the histological therapeutic effect.
Regarding the residual tumor pattern following CDDP- based preoperative CCRT, Kirita et al (23) reported that the residual tumor cells were mostly localized in the central superficial layer of the primary lesion in complete response cases of tongue carcinoma, suggesting the possibility of limited surgery. By contrast, Böheim et al (20) analyzed the residual pattern of viable cancer cells and found that it could be classified into 2 types: superficial, in which the tumor cell distribution narrows towards the mucosal superficial layer, and deep, in which the tumor cells are present in the deep mucosa. In our GrII cases, the deep type was most frequently observed in cases that achieved a GrIIa histological effect in the AI and S-1 groups, showing no significant difference between the groups. However, in GrIIb cases, 4 (40%) were superficial-type in the S-1 group, whereas the superficial type accounted for 75% in the AI group, although the number of cases was small. The therapeutic effect on the primary lesion was suggested to be higher in the AI group when an effect was obtained, retaining the possibility of limited surgery. However, caution should be exercised with regard to its application in the two groups, as indicated by the findings.
The disease-specific survival rates during the follow-up period were 88.8 and 86.4% in the S-1 and AI groups, respectively, showing a similar survival curve with no significant difference in the survival rate (Fig. 4). Few studies have compared two regimens of chemoradiotherapy performed at the same facility during the same period in Japan. In their study, Kuratomi et al (24) compared chemoradiotherapies with S-1 and low-dose CDDP venous injection for resectable pharyngeal and laryngeal cancers and the disease-specific survival rates were found to be 77 and 76%, respectively. Although a simple comparison with the results of this study is impossible as the location of the primary lesions was different, the effect of chemoradiotherapy with S-1 for head and neck cancer may be comparable to therapy employing super- selective intra-arterial infusion.
Regarding adverse events, stomatitis had the highest incidence in the S-1 group, as previously reported (16,17), with Gr2 or worse stomatitis noted in 73.7% of the patients (Table III). Gr3 stomatitis was noted in 1 patient, but it was reversible and treatment was not discontinued due to stomatitis in any patient during the observation period. In the AI group, the frequency of stomatitis was 57.1%, similar to that in the S-1 group, but no Gr3 case was present. Regarding hematological toxicity in the S-1 group, Ohnishi et al (17) reported that the incidences of Gr3 or worse leukopenia and anemia were 5.2 and 2.6%, respectively, and Shirasaki et al (15) reported that Gr2 adverse events were hypochromia (35%), leukopenia (35%) and thrombocytopenia (5%), but no Gr3 or worse event occurred. In our patients, no Gr3 or worse hematological toxicity was observed and the frequency of adverse events was slightly lower than those reported. Kishimoto et al (21) reported that treatment of dermatitis and stomatitis was necessary in CCRT with S-1 for elderly patients, but the treatment method was suitable for elderly patients as the drug could be administered while observing adverse events. However, since renal function is generally reduced in the elderly, the lower level of renal excretion of gimeracil results in an increased 5-FU level. This may then influence the therapeutic effect and lead to severe bone marrow inhibition and lung disorders, including interstitial pneumonia (25), demonstrating that S-1 administration should be carefully managed. In the AI group, the effects of bone marrow inhibition, including anemia, leukopenia, neutropenia and thrombocytopenia, were observed. Gr3 neutropenia was noted in 1 patient, but the condition was improved with the administration of a G-CSF preparation. Bone marrow inhibition was also widely observed in a study reported by Furutani et al (26), in which numerous Gr3 and 4 adverse events occurred, showing the necessity of investigating the optimum dose of chemotherapeutic drugs. Moreover, severe complications induced by super-selective intra-arterial infusion chemotherapy, including laryngeal edema and mucosal necrosis, have been reported (6,7). As pharyngeal edema and necrosis were also evident in our previous study (?), sufficient attention should be paid to the application of super-selective intra-arterial infusion chemotherapy. Regarding the S-1 administration method, Harada et al (13) altered the regimen from a 4-week administration followed by a 2-week withdrawal to a 2-week administration followed by a 1-week withdrawal, thereby improving the adverse events and avoiding the suspension or dose reduction of S-1 and the withdrawal of irradiation. In our S-1 group, the drug was administered for 15 days with irradiation and the mean waiting time for surgery was 17.8 days. A wait of longer than 3 weeks was necessary for certain patients, but the treatment was mostly completed as scheduled, suggesting that 3-week administration does not cause marked changes in adverse events.
The comparison between the S-1 and AI groups was limited as the number of cases was small and the location and stage of the primary lesion varied. An analysis involving an increased number of cases is necessary. However, the response and survival rates were not significantly different between the S-1 and AI groups, although the proportion of markedly improved cases was slightly different. The frequency of hematological toxicity and the incidence of complications were low in CCRT with S-1, suggesting that this therapy was effective and sufficiently beneficial for oral cancer as a preoperative treatment.
References
- 1.Shirasaka T. Development history and concept of an oral anticancer agent S-1(TS-1®): its clinical usefulness and future vistas. Jpn J Clin Oncol. 2009;39:2–15. doi: 10.1093/jjco/hyn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuwa N. Current and future state of chemoradiotherapy for head and neck cancer. Nihon Igaku Hoshasen Gakkai Zasshi. 2002;62:65–72. (In Japanese) [PubMed] [Google Scholar]
- 3.Choong N, Vokes E. Expanding role of the medical oncologist in the management of head and neck cancer. CA Cancer J Clin. 2008;58:32–58. doi: 10.3322/CA.2007.0004. [DOI] [PubMed] [Google Scholar]
- 4.Bernier J, Domenge C, Ozsahlin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 5.Klug C, Berzaczy D, Voracek M, et al. Preoperative chemoradiotherapy in the management of oral cancer: A review. J Craniomaxillofac Surg. 2008;36:75–88. doi: 10.1016/j.jcms.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Yoshizaki T, Tanaka F, Shiga H, et al. Superselective intra-arterial chemotherapy for head and neck squamous cell carcinoma. Head and Neck Cancer. 2003;29:445–449. [Google Scholar]
- 7.Shiga K, Yokoyama J, Tateda M, et al. Superselective intraarterial chemotherapy for patients with head and neck tumor. Head and Neck Cancer. 2003;29:457–462. [Google Scholar]
- 8.Imai S, Gyoten M, Kajihara Y, et al. Superselective intraarterial chemotherapy using cisplatin(CDDP)-carboplatin(CBDCA) combined with radiotherapy for head and neck cancer. Head and Neck Cancer. 2003;29:463–467. [Google Scholar]
- 9.Endo S, Suzuki S, Tsuji K, et al. Intraarterial concomitant chemoradiation for tongue cancer: analysis of 20 patients. Nippon Jibiinkoka Gakkai Kaiho. 2005;108:689–693. doi: 10.3950/jibiinkoka.108.689. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 10.Tohnai I. Intra-arterial chemotherapy for head and neck cancer. Gan To Kagaku Ryoho. 2005;32:2024–2029. (In Japanese) [PubMed] [Google Scholar]
- 11.Tonai I, Mitsudo K, Nishiguchi H, et al. Daily concurrent chemoradiotherapy using superselective intra-arterial infusion via superficial temporal artery-preoperative therapy for stage III, IV oral cancer. Head and Neck Cancer. 2005;31:413–418. [Google Scholar]
- 12.Mitsudo K, Shigetomi T, Fukui T, et al. Daily concurrent chemoradiotherapy with docetaxel (DOC) and cisplatin (CDDP) using superselective intra-arterial infusion via superficial temporal artery for advanced oral cancer. J Clin Oncol. 2009:abse17020. [Google Scholar]
- 13.Harada K, Kawashima Y, Uchida D, et al. A case of advanced oral squamous cell carcinoma responding to concurrent radiotherapy. Gan To Kagaku Ryoho. 2007;34:745–747. (In Japanese) [PubMed] [Google Scholar]
- 14.Tsukuda M, Kida A, Fujii M, et al. Long-term results of S-1 administration as adjuvant chemotherapy for advanced head and neck cancer. Gan To Kagaku Ryoho. 2007;34:1215–1225. (In Japanese) [PubMed] [Google Scholar]
- 15.Shirasaki T, Maruya S, Namba A, et al. Treatment results of chemotherapy with S-1 for head and neck cancer. Gan To Kagaku Ryoho. 2009;36:237–240. (In Japanese) [PubMed] [Google Scholar]
- 16.Matsui R, Mukai H, Imamura H, et al. Clinical evaluation of anti-tumor effect in concurrent radiotherapy with TS-1 for oral cancer (abstract) Jpn J Oral Maxillofac Surg. 2009;55:57. [Google Scholar]
- 17.Ohnishi K, Shioyama Y, Nakamura K, et al. Concurrent chemoradiotherapy with S-1 as first-line treatment for patients with oropharyngeal cancer. J Radiat Res. 2011;52:47–53. doi: 10.1269/jrr.10081. [DOI] [PubMed] [Google Scholar]
- 18.Budach W, Hehr T, Budach V, et al. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresectable locally advanced squamous cell head and neck cancer. BMC Cancer. 2006;6:28. doi: 10.1186/1471-2407-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimosato Y, Oboshi S, Baba K. Histological evaluation of effects of radiotherapy and chemotherapy for carcinoma. Jpn J Clin Oncol. 1971;1:19–35. [Google Scholar]
- 20.Böheim K, Spoendkin H. The effect of chemotherapy in relation to pathohistological tumor grading in head and neck cancer. Acta Otorhinolaryngol. 1983;238:197–204. doi: 10.1007/BF00453929. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto K, Yoshida S, Domaem S, et al. Concurrent chemoradiotherapy with TS-1 for advanced oral cancer-Feasibility, problems, and countermeasures for elderly patients with complication. Jpn J Oral Maxillofac Surg. 2009;55:177–183. (In Japanese) [Google Scholar]
- 22.Zeng L, Ou G, Itasaka S, et al. TS-1 enhances the effect of radiotherapy by suppressing radiation-induced hypoxia-inducible factor-1 activation and inducing endothelial cell apoptosis. Cancer Sci. 2008;99:2327–2335. doi: 10.1111/j.1349-7006.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirita T, Ohgi K, Kawakami M, et al. Primary tumor resection of tongue carcinoma based on response to preoperative therapy. Int J Oral Maxillofac Surg. 2002;31:267–272. doi: 10.1054/ijom.2002.0241. [DOI] [PubMed] [Google Scholar]
- 24.Kuratomi Y, Satoh S, Monji M, et al. A comparative study of concurrent chemoradiotherapy with S-1 or CDDP for pharyngeal or laryngeal cancer. Gan To Kagaku Ryoho. 2010;37:1471–1476. (In Japanese) [PubMed] [Google Scholar]
- 25.Yamamo N, Ohshima T, Sato T, et al. A case of interstitial pneumonia after S-1 administration for gastric cancer. Gan To Kagaku Ryoho. 2008;35:1935–1937. (In Japanese) [PubMed] [Google Scholar]
- 26.Furutani K, Fuwa N, Kodaira T, et al. Combination therapy of continuous intra-arterial chemotherapy and radiotherapy for stage III, IV tongue cancer. Head and Neck Cancer. 2005;37:419–423. [Google Scholar]