Summary
The Ras/Raf/MEK/ERK (or MAPK) signalling pathway relays extracellular stimuli to the nucleus, thereby regulating diverse cellular responses such as proliferation, growth, differentiation and apoptosis. Perturbation of these processes by aberrant MAPK signalling often leads to malignant transformation as indicated by the frequent occurrence in human cancers of genetic alterations affecting this pathway. In recent years, genetically modified mouse models have proven instrumental in unravelling how deregulated MAPK signalling leads to disease. Indeed, conditional activation of oncogenic K-Ras or B-Raf in mice resulted in neoplasms that closely resemble the human disease. Such tractable mouse models will enable the pursuit of basic biological mechanisms and translational applications regarding the MAPK pathway.
Introduction: the MAPK pathway
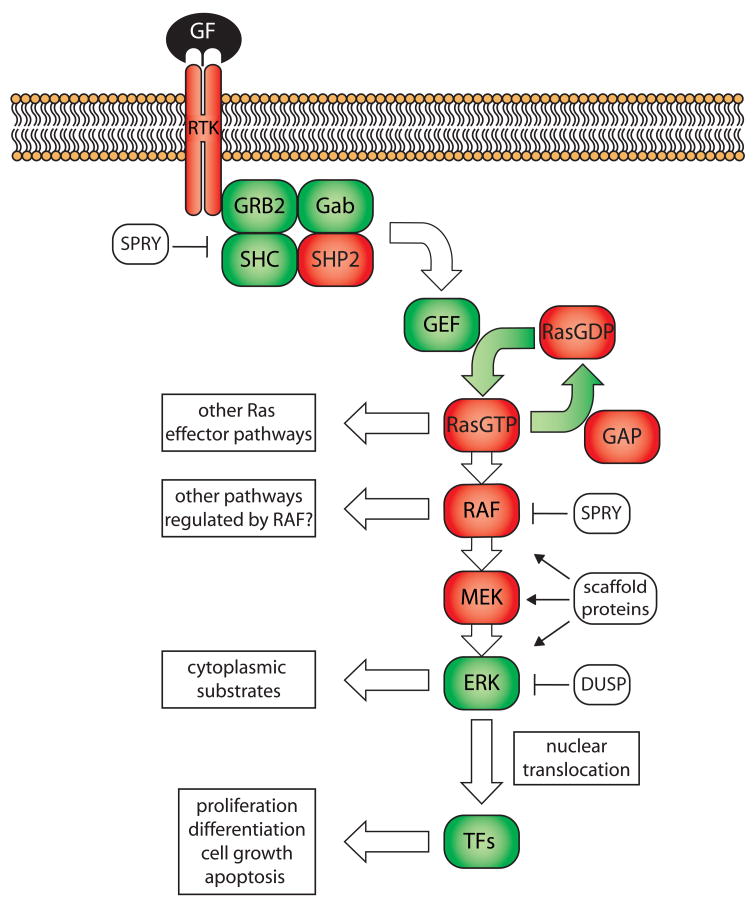
The MAPK pathway is essential for organismal development and has been elucidated through genetic and biochemical approaches over the past several decades. The pathway is proximally activated by growth factor binding to receptor tyrosine kinases (RTK), resulting in RTK phosphorylation and activation. Consequently, adaptor molecules localize to RTKs followed by recruitment and activation of guanine nucleotide-exchange factors (GEFs). GEFs catalyse the transition from GDP-bound, inactive Ras to GTP-bound, active Ras. Ras-GTP interacts with more than a dozen effector molecules to regulate a variety of biological processes [1]. GTPase-activating proteins (GAPs) allosterically stimulate the intrinsic GTPase activity of Ras, leading to GTP hydrolysis and Ras inactivation. To activate the MAPK signalling cascade, Ras recruits Raf to the cell membrane, where Raf is activated and subsequently forms complexes with MEK, ERK and scaffolding proteins. Raf then phosphorylates MEK, which in turn phosphorylates ERK. ERK both activates cytosolic substrates and translocates to the nucleus to stimulate diverse gene expression programs through transcription factors such as JUN and ELK1 (Figure 1). The recent recognition of inhibitory feedback loops through phosphatases and binding proteins with no obvious catalytic activity has added a new dimension to our understanding of the MAPK pathway. Therefore, the MAPK “pathway” is rather a complex network that is still being elucidated.
Figure 1.
The Ras/Raf/MEK/ERK pathway.
Binding of growth factors (GF) to receptor tyrosine kinases (RTK) leads to recruitment of adaptor proteins and guanine exchange factors (GEF). GEFs promote the active form of Ras (RasGTP). Ras signals to different pathways, one being the Raf/MEK/ERK pathway. Ras activates Raf, which may have several downstream effectors. Raf phosphorylates MEK followed by ERK phosphorylated by MEK. ERK has cytoplasmic substrates and also translocates to the nucleus to activate a variety of transcriptional programmes through several transcription factors (TFs). The signalling cascade is negatively regulated by Sprouty proteins (SPRY) and dual-specificity phosphatases (DUSP). Proteins in red have been found genetically altered in human cancers resulting in hyperactivation of the MAPK pathway.
The importance of the MAPK pathway in neoplasms is evident through the discovery of mutant alleles that hyperactivate the pathway in a variety of human cancers. First, oncogenic mutations in RTKs abnormally activate Ras and its downstream substrates [2]. Second, activating Ras mutations have been detected in approximately 30% of human cancers [3]. These mutations occur in codons 12, 13, and 61 and markedly diminish GTPase activity, thereby rendering Ras locked in the GTP-bound, active state. In mammals, the Ras family consists of three genes: K-Ras, N-Ras and H-Ras. While K-Ras is the predominantly mutated family member (in ~85% of cancers harboring Ras mutations), N-Ras and H-Ras mutations are relatively uncommon (~15% and <1%, respectively) [4]. Interestingly, each Ras family member is mutated in a specific subset of human malignancies: K-Ras is commonly mutated in epithelial cancers of the pancreas, lung and colon; N-Ras mutations occur frequently in melanoma, liver and myeloid malignancies; and H-Ras mutations have been observed in bladder cancer (Table 1). Third, activating mutations in B-Raf, a member of the Raf family, have been discovered with high frequency in melanoma (in a non-overlapping pattern with mutations in N-Ras) and, to a lesser extent, in thyroid, ovarian and colon cancer [5–7]. Importantly, activating missense mutations do not occur in the other members of the Raf family, A-Raf and Raf-1, which may be due to their more complex mode of activation [8]. Single amino acid substitutions are sufficient to promote the active conformation of B-Raf, thereby constitutively activating the MAPK pathway. Finally, loss of negative regulators, such as members of the Sprouty family and GAPs such as NF1, can indirectly hyperactivate the MAPK signalling cascade. It is estimated that most tumors exhibit deregulation of the MAPK pathway, making it an attractive target for therapeutic intervention.
Table 1.
Occurrence of Ras and B-Raf in human cancers. Data derived from COSMIC database; http://www.sanger.ac.uk/genetics/CGP/cosmic.
| Tissue | K-Ras | N-Ras | H-Ras | BRAF |
|---|---|---|---|---|
| Biliary tract | 32% | 1% | 0% | 14% |
| Bone | 1% | 0% | 2% | 0% |
| Breast | 5% | 1% | 1% | 2% |
| CNS | 1% | 2% | 0% | 3% |
| Cervix | 8% | 1% | 9% | 1% |
| Endometrium | 14% | 0% | 1% | 1% |
| GI tract (site indeterminate) | 19% | 0% | 0% | 0% |
| Haematopoietic and | 5% | 12% | 0% | 2% |
| Lymphoid tissue | ||||
| Kidney | 1% | 0% | 0% | 0% |
| Large intestine | 32% | 3% | 0% | 13% |
| Liver | 7% | 4% | 0% | 3% |
| Lung | 17% | 1% | 0% | 2% |
| Melanoma | 1% | 17% | 1% | 64% |
| Oesophagus | 4% | 0% | 0% | 2% |
| Ovary | 15% | 4% | 0% | 13% |
| Pancreas | 60% | 2% | 0% | 2% |
| Prostate | 8% | 1% | 6% | 6% |
| Salivary gland | 4% | 0% | 16% | 0% |
| Skin * | 2% | 19% | 5% | 41% |
| Small intestine | 20% | 25% | 0% | 5% |
| Soft tissue | 12% | 6% | 7% | 3% |
| Stomach | 6% | 2% | 4% | 1% |
| Testis | 5% | 4% | 0% | 0% |
| Thyroid | 3% | 7% | 4% | 37% |
| Urinary tract | 4% | 3% | 12% | 0% |
skin cancers including melanoma.
In recent years, several mouse models have been generated to establish a causal relationship between MAPK mutations found in human cancer and tumor development. These mouse models have furthered our understanding of cellular transformation by aberrant MAPK signalling and offer a powerful tool for preclinical testing of novel therapeutics. In this review, we will discuss the most recent advances of mouse cancer models that involve deregulated MAPK pathway activation.
Oncogenic Ras mouse models
Mouse models of cancer are most valuable when they recapitulate the cardinal pathophysiological and molecular aspects of the corresponding human malignancy [9]. For example, accurate mouse models of neoplasias that utilize the conditional endogenous expression of oncogenic K-Ras have been described [10–12]. These models incorporated a loxP-flanked transcriptional stop cassette (lox-stop-lox or LSL) to conditionally permit spatiotemporal regulation of oncogenic K-Ras expression by Cre-mediated excision of the LSL. This approach allows oncogenic K-Ras expression at physiological levels; however, a potential weakness is the expression of only one copy of wildtype K-Ras in cells lacking Cre. Even though the models by Guerra et al. [10] and Jacks and colleagues [11–13] differed in their neoplastic phenotypes, in each model expression of oncogenic K-Ras was sufficient to induce hyperplasia. While Jacks, Tuveson and colleagues described pulmonary [12], pancreatic [13] and colonic [11] hyperplasia and neoplasms, Guerra et al. reported lung adenomas, histiocytic sarcomas and sarcomas [10]. Further phenotypes have been characterized in both models and will be discussed in more detail below. The phenotypic differences may be attributed to the genetic backgrounds of the mouse strains used, the amino acid substitution modelled (G12V vs. G12D in ref. 10 vs. refs 11–13, respectively), or the use of a bicistronic K-RasG12V-IRES-βgeo mRNA that could influence transcription and/or translation [10]. Using an inducible transgenic approach, it has been shown in vivo that variations in mutant Ras levels manifested different biological outcomes [14]. Indeed, high levels of mutant H-RasG12V induced a growth arrest in mammary epithelial cells in a p16INK4A/p19ARF- and p53-dependent manner, whereas expression levels similar to endogenously expressed K-RasG12D resulted in mammary epithelial hyperplasia but were insufficient to induce frank malignancy [14]. Although Ras mutations are uncommon in human breast cancer, these findings further substantiate the notion that initial expression of mutant Ras genes at physiological levels is required to accurately study Ras-induced human cancers in mice. Because mutations in K-Ras are common in many human tumor types, the development of mice expressing physiological levels of oncogenic K-Ras thus provided the opportunity to study a variety of human cancers in mice.
To this end, K-Ras mutant mice have been crossed to several tissue-specific Cre transgenic mouse strains. These mouse models displayed hyperplastic and neoplastic phenotypes in epithelial, mesenchymal and haematopoietic cells. When crossed to pancreas-specific Cre strains, LSL-K-RasG12D mice developed a preinvasive neoplasm of pancreatic ductal adenocarcinoma (PDA), termed pancreatic intraepithelial neoplasia (PanIN), which spontaneously progressed to metastatic PDA at a low frequency [13]. Furthermore, oncogenic K-Ras cooperated with p16INK4a/p19ARF deficiency [15], p53 mutation [16], perturbed TGFβ signalling [17–19], TGFα activation [20] and chronic pancreatitis [21] to accelerate the formation of PanIN and metastatic cancer. Interestingly, LSL-KrasG12D mice deficient in TGFβ signalling also developed intraductal papillary mucinous neoplasms (IPMN) [17] and mucinous cystic neoplasms (MCN) [19], which are additional preinvasive precursor lesions of PDA, suggesting that more than one route to pancreatic ductal adenocarcinoma may exist. It is currently unclear which pancreas cell type initiates PanIN in response to oncogenic K-Ras. Some reports point to the exocrine compartment [21–23]; however, it remains incompletely understood whether progenitor cells or terminally differentiated ductal or acinar cells give rise to PanIN and PDA. These pancreatic cancer models closely recapitulate the initiation and progression of the human disease and will allow further characterization of pancreatic cancer development.
In addition to pancreatic cancer, lung cancer can also be modelled with oncogenic K-Ras mice. Intranasal adenoviral Cre (Ad-Cre) instillation in LSL-K-RasG12D mice led to atypical adenomatous hyperplasia, epithelial hyperplasia and adenomas that spontaneously progressed to pulmonary adenocarcinoma [12]. Bronchioalveolar stem cells (BASCs) have been identified in the bronchioalveolar duct junction of mouse lungs [24]. Importantly, BASCs expanded in response to oncogenic K-Ras expression in early lung tumors [24], suggesting that transformed lung stem cells may possess cancer-initiating properties. Furthermore, cooperation of K-RasG12D with both loss and mutation of p53 as well as with PTEN deficiency was demonstrated [25,26]. Interestingly, MAPK activation was not seen in precursor lesions but could be detected in later stage lesions in K-RasG12D mice lacking functional p53 [12,26]. These studies suggest that oncogenic K-Ras-induced MAPK pathway activation remains low, possibly through negative feedback loops. However, in precursor lesions loss of p53 function permits the acquisition of additional mutations that attenuate those feedback loops, leading to elevated MAPK pathway signalling and overt malignancy. This model will prove useful for determining the role of oncogenic K-Ras in lung cancer as well as effector pathways relevant for therapeutic treatment.
In contrast to the lung and pancreas, K-Ras mutations are not an initiating event in colon cancer [27]. Expression of K-RasG12V-IRES-βgeo alone was not sufficient to induce colonic hyperplasia; however, cooperation with loss of Apc was observed [28]. In other studies, activation of K-RasG12D in colonic epithelium promoted hyperproliferation [11,29,30], which could be rescued by pharmacological MEK inhibition, thus demonstrating dependence of hyperplasias on the MAPK pathway [30]. Furthermore, K-RasG12D expression accelerated colon cancer progression induced by Apc deficiency [30]. The discrepancies in the induction of hyperplasia observed in the two models may be attributed to the reasons discussed above. Nevertheless, both models demonstrated promotion of colonic tumor progression by oncogenic K-Ras.
As opposed to pancreas, lung and colon cancer, K-Ras mutations occur infrequently in the endometrium and soft tissue. Interestingly, injection of Ad-Cre in the bursal cavity of LSL-K-RasG12D mice induced endometriotic-like lesions and peritoneal endometriosis [31]. In addition, concomitant loss of PTEN led to endometrioid ovarian adenocarcinoma, suggesting that K-RasG12D-induced endometriosis may represent a precursor lesion to ovarian adenocarcinoma [31]. K-RasG12D was also reported to be oncogenic in mesenchymal cells, as intramuscular Ad-Cre injection in K-RasG12D mice lacking either functional p53 or p16INK4A/p19ARF promoted the formation of soft tissue sarcoma [32]. Thus, LSL-K-RasG12D mice appear to recapitulate the full spectrum of human malignancies carrying K-Ras mutations.
In contrast to solid tumors, K-Ras is not the predominant Ras oncogene in haematopoietic malignancies. Nevertheless, expression of K-RasG12D in haematopoietic cells caused a fatal myeloproliferative disease similar to human juvenile myelomonocytic leukaemia and chronic myelomonocytic leukaemia [33,34] that never progressed to acute myeloid leukaemia. In contrast, mice concomitantly expressing oncogenic K-Ras and a PML-RARα translocation transgene succumbed to a rapid onset, highly penetrant acute promyelocytic leukaemia-like disease [35], suggesting cooperation of K-RasG12D and secondary mutations in leukaemogenesis. These K-Ras mouse models provided experimental support to a model in which mutation of K-Ras is the neoplasia-initiating event and progression to malignant cancer requires additional genetic changes such as inactivation of tumor suppressor genes.
Compared to oncogenic K-Ras, studies on mutant N-Ras and H-Ras function have largely remained limited in their use of transgenic approaches. For instance, retroviral infection of bone marrow cells with mutant N-Ras [36] as well as H-Ras and K-Ras [37] alleles, followed by transplantation into lethally irradiated hosts, revealed the leukemogenic potential of all Ras isoforms. In another study, melanocyte-specific expression of H-RasG12V promoted melanomagenesis, which was accelerated by p16INK4a/p19ARF deficiency [38]. In addition, mice expressing an H-RasG12V transgene in livers deficient for β-catenin developed hepatocellular carcinomas [39]. Although these transgenic mouse models have contributed to the understanding of Ras-mediated tumorigenesis, they are limited by non-physiological expression levels and the fact that H-RAS mutations are not found in human leukaemia, melanoma or hepatocellular carcinoma.
The successful development of conditional K-Ras mouse models prompted the generation of mouse models carrying cancer-associated mutations in the other Ras family members. Mice conditionally expressing endogenous mutant N-Ras were generated in a fashion similar to LSL-K-RasG12D mice and crossed to colonic epithelia-specific Cre mice. In contrast to K-RasG12D mice, colonic expression of N-RasG12D neither induced hyperplasia nor cooperated with loss of Apc to promote tumor progression [30], providing a rationale for the absence of mutations in colon cancer. The phenotypic differences could reflect that the G12D mutation confers a weaker oncogenic potential on N-Ras than on K-Ras or could reflect different expression levels of the two oncogenes. Accordingly, N-Ras Q61 mutations are more frequently found in human cancers than G12 mutations (COSMIC database; http://www.sanger.ac.uk/genetics/CGP/cosmic). Given the lack of a neoplastic phenotype in the mouse colon it will be of future interest to evaluate the oncogenic potential of N-Ras in cell types that show frequent N-RAS mutations in humans such as melanocytes and haematopoietic cells.
H-RAS mutations are uncommon in spontaneous human cancer. However, germline expression of mutant H-RAS is associated with Costello syndrome (CS) and CS patients display a certain predisposition to neuroblastomas, rhabdomyosarcomas, and bladder carcinomas [1]. Mice expressing germline H-RasG12V phenocopied certain features of CS; however tumors were extremely rare [40]. This phenotype suggests that H-Ras is a poor initiating oncogene and may be mutationally activated in spontaneous cancers only at later stages during tumor development when cooperating genetic lesions have already occurred. Alternatively, H-Ras could be a less potent oncogene in mice compared to humans.
Oncogenic Raf mouse models
Following the identification of BRAF mutations in human malignancies, novel mouse strains modelling those mutations have been developed. Pritchard and colleagues created a B-RafV600E mouse by employing a strategy in which mice are heterozygous mutant for B-Raf in cells expressing Cre and homozygous wildtype for B-Raf in all other cells, thus closely resembling B-Raf status in human neoplasms as well as in surrounding normal tissues [41]. B-RafV600E activation in a variety of somatic tissues by Mx1-Cre caused death in all animals within a month due to the development of nonlymphoid leukaemia of the histiocytic type [41]. Interestingly, human leukaemia has not been associated with BRAF mutations thus far. This discrepancy may represent distinct susceptibility of haematopoietic cells to oncogenic stimuli in humans and mice. A second study using a similar mouse model reported lung adenoma formation in B-RafV600E mice after intranasal Ad-Cre instillation [42]. Even though adenomas developed with complete penetrance, they rarely progressed to adenocarcinoma, and rather demonstrated a senescence-like growth arrest following hyperplasia [42]. Interestingly, no growth arrest was observed in KrasG12D-induced lung adenomas, which eventually progressed to malignancy [12], suggesting that activation of other Ras effector pathways may be required for tumor progression (or example see ref. 57). B-RafV600E could, however, cooperate with loss of p53 or p16INK4A/p19Arf, known mediators of senescence, to induce adenocarcinomas [42]. These studies provided experimental evidence for murine tumor initiation by mutation of B-Raf. It is expected that oncogenic B-Raf mice will prove valuable for investigating B-Raf-mediated transformation in a variety of other cancers such as melanoma, thyroid and colon cancer.
Further evidence for the oncogenic properties of B-Raf was provided by a forward genetic screen employing transposon-mediated mutagenesis. In a p19Arf-deficient background, B-Raf was the most frequently disrupted gene, leading to acceleration of sarcoma formation [43]. In the majority of cases, the T2/Onc transposon integrated in intron 9, in the orientation that directed expression of the C-terminal B-Raf kinase domain [43]. Similarly, transgenic expression of the kinase domain of Raf-1 sufficed to induce lung adenomas [44], and additional deletion of E-cadherin led to metastatic progression [45]. Furthermore, Raf-1 lacking the N-terminal negative regulatory domain cooperated with Akt activation and p19Arf loss to promote the formation of gliomas [46]. However, expression of truncated forms of B-Raf or Raf-1 has not been associated with sporadic human malignancies and activating mutations in CRAF have not been found in cancer thus far. Even though the preclinical potential of these models may be limited for the above reasons, they have confirmed the importance of MAPK pathway activation in tumor development.
Mouse models of regulators of the MAPK pathway
The MAPK pathway is subjected to extensive regulation by posttranslational processing enzymes, phosphatases and negative feedback loops. Disruption of this regulation leads to pathway hyperactivation that mimics activating Ras mutations as has been demonstrated in mouse models lacking the Ras GAP neurofibromin (NF1) [reviewed in 47,48]. In addition to GAPs for Ras, other proteins function to attenuate the MAPK pathway such as the dual-specificity phosphatases (DUSPs) and Sprouty (Spry) proteins. Spry proteins inhibit the MAPK pathway by binding to Raf as well as to Grb2, an adaptor protein important for the recruitment of Ras GEFs [49]. Expression of Spry-1, Spry-2, and Spry-4 was increased upon K-RasG12D expression in the lung [50], indicating that negative feedback loops are upregulated in response to oncogenic K-Ras to compensate for augmented MAPK signalling. Furthermore, Spry-2-deficiency increased the size of K-RasG12D-induced lung tumors [50], suggesting that negative feedback regulation has tumor suppressive activity in the context of oncogenic K-Ras. Besides Spry proteins, MAPK phosphatases represent another way of negatively regulating the MAPK pathway through dephosphorylation of ERK1 and ERK2. Tyrosine, serine/threonine as well as dual-specificity phosphatases that act on ERK proteins have been identified and knockout mice have been generated to several of these [reviewed in 51]. However, the effect of phosphatase depletion on oncogenic Ras and Raf signalling has not yet been determined. In addition to regulation of its downstream signalling effectors, Ras itself is regulated through post-translational modification. To increase membrane affinity and promote protein interaction, Ras is modified at the carboxy-terminal CAAX motif. First, the cysteine residue is isoprenylated by farnesyltransferase (FTase), followed by endoproteolytic release by Ras converting enzyme 1 (RCE1) of the three amino acids downstream of the isoprenylcysteine. Finally, the isoprenylcysteine residue is carboxyl methylated by isoprenylcysteine carboxyl methyltransferase (ICMT). However, in the absence of FTase activity, K-Ras and N-Ras can be isoprenylated by geranylgeranyltransferase type 1 (GGTase-I), a phenomenon providing an explanation for the disappointing performance of FTase inhibitors in clinical trials. Accordingly, genetic ablation of FNTB, the gene encoding FTase, in K-RasG12V-expressing cells did not prevent lung tumorigenesis [52]. Surprisingly, the K-RasG12D-induced myeloproliferative disease was accelerated upon genetic deletion of RCE1 [53], suggesting that RCE1 may have a poorly understood tumor suppressor role in cells and that RCE1 is not required for Ras oncogenesis. In contrast, the lack of either GGTase-I or ICMT ameliorated the lung tumor and myeloproliferative disease-like phenotypes in K-RasG12D mutant mice [54,55]. As K-Ras is still farnesylated in the setting of GGTase-I ablation, other CAAX proteins that undergo geranylgeranylation must also be required for K-RasG12D-mediated transformation. These findings imply that GGTase-I or ICMT may represent more suitable targets RCE1 for therapeutic intervention in Ras associated malignancies, either alone or in combination with FTase inhibitors. However, the suitability of Ras-modifying enzymes as therapeutic targets needs further investigation.
Conclusions and future outlook
The development of preclinical mouse models has confirmed a causal role of oncogenic K-Ras expression in a variety of tumor types. Oncogenic K-Ras expression can initiate multiple malignancies, consistent with the demonstration of K-Ras mutations in different types of human preneoplasms. The cancer-initiating cell type described by Kim et al. for pulmonary adenocarcinoma [24] should now be pursued for other K-Ras-driven cancers.
The finding that N-Ras and H-Ras have weak oncogenic abilities in mice was somewhat surprising and may not reflect observations made in human malignancies. Alternatively and in contrast to K-Ras, N-Ras and H-Ras may not be initiating oncogenes and may be mutated only at later stages during tumor progression. Therefore, to distinguish between these two possibilities, the acceleration of tumor progression by N-Ras or H-Ras in combination with tumor suppressor deficiency needs to be investigated.
Each Ras family member is associated with different kinds of cancer. Moreover, mutant B-Raf-harbouring cancers do not completely overlap with cancers carrying Ras mutations (e.g. mutant B-Raf has not been detected in myeloid malignancies where N-Ras is the most commonly mutated Ras gene; see Table 1), indicating that activation of the MAPK pathway alone is unlikely to promote cancer. In fact, it has been shown in mouse models [56,57] and cell culture [58] that activation of other Ras effector pathways is important for tumorigenesis. The ability of endogenously expressed, mutant Ras to activate certain effector pathways may vary between the three family members and could be influenced by factors such as subcellular localization of Ras and cell type. Additionally, the oncogenic mutations may confer different activity onto Ras and Raf, which may also depend on cell type. Understanding the difference in oncogenic activities between mutant K-Ras, N-Ras, H-Ras and B-Raf as well as the cellular context in which they are transforming should ultimately explain the tropism of these oncogenes. Finally, the development of preclinical mouse models of oncogenic Ras/Raf signalling will prove instrumental for developing novel therapeutics in a timely manner.
Acknowledgments
We apologize to our colleagues whose data could not be discussed due to space constriction. We are grateful to Dr. A. Cox and members of the Tuveson laboratory for critical comments on the manuscript. DAT is supported by US National Institutes of Health grants CA101973, CA111292, CA084291, CA105490 and the Lustgarten Foundation for Pancreatic Cancer Research. DAT is a group leader of Cancer Research UK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 2.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 3.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 4.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 5.Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, Einhorn E, Herlyn M, Minna J, Nicholson A, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 6.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 7.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 8.Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 9.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 10.Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, Campuzano V, Barbacid M. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 11.Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 12.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 14••.Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. This study provides the first in vivo evidence for the cell biological responses to different expression levels of oncogenic Ras. High levels engaged the p19ARF-p53 tumor suppressor pathway to induce senescence, while low levels promoted hyperplastic growth of mammary epithelial cells. [DOI] [PubMed] [Google Scholar]
- 15.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV, Moses HL. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, Deng CX, Hruban RH, Adsay NV, Tuveson DA, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11:229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Siveke JT, Einwachter H, Sipos B, Lubeseder-Martellato C, Kloppel G, Schmid RM. Concomitant pancreatic activation of Kras(G12D) and Tgfa results in cystic papillary neoplasms reminiscent of human IPMN. Cancer Cell. 2007;12:266–279. doi: 10.1016/j.ccr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Carriere C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2007;104:4437–4442. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuveson DA, Zhu L, Gopinathan A, Willis NA, Kachatrian L, Grochow R, Pin CL, Mitin NY, Taparowsky EJ, Gimotty PA, et al. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006;66:242–247. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- 24.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Iwanaga K, Yang Y, Raso MG, Ma L, Hanna AE, Thilaganathan N, Moghaddam S, Evans CM, Li H, Cai WW, et al. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res. 2008;68:1119–1127. doi: 10.1158/0008-5472.CAN-07-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, Jacks T. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- 27.Grady WM, Markowitz SD. Genetic and epigenetic alterations in colon cancer. Annu Rev Genomics Hum Genet. 2002;3:101–128. doi: 10.1146/annurev.genom.3.022502.103043. [DOI] [PubMed] [Google Scholar]
- 28.Sansom OJ, Meniel V, Wilkins JA, Cole AM, Oien KA, Marsh V, Jamieson TJ, Guerra C, Ashton GH, Barbacid M, et al. Loss of Apc allows phenotypic manifestation of the transforming properties of an endogenous K-ras oncogene in vivo. Proc Natl Acad Sci U S A. 2006;103:14122–14127. doi: 10.1073/pnas.0604130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calcagno SR, Li S, Colon M, Kreinest PA, Thompson EA, Fields AP, Murray NR. Oncogenic K-ras promotes early carcinogenesis in the mouse proximal colon. Int J Cancer. 2008;122:2462–2470. doi: 10.1002/ijc.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. Haigis et al. describe oncogene-specific effects in the colon, where K-Ras but not N-Ras induced hyperplasia, suggesting that Ras family members are oncogenic only in cell type-specific contexts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 32.Kirsch DG, Dinulescu DM, Miller JB, Grimm J, Santiago PM, Young NP, Nielsen GP, Quade BJ, Chaber CJ, Schultz CP, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- 33.Braun BS, Tuveson DA, Kong N, Le DT, Kogan SC, Rozmus J, Le Beau MM, Jacks TE, Shannon KM. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101:597–602. doi: 10.1073/pnas.0307203101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan IT, Kutok JL, Williams IR, Cohen S, Kelly L, Shigematsu H, Johnson L, Akashi K, Tuveson DA, Jacks T, et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004;113:528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan IT, Kutok JL, Williams IR, Cohen S, Moore S, Shigematsu H, Ley TJ, Akashi K, Le Beau MM, Gilliland DG. Oncogenic K-ras cooperates with PML-RAR alpha to induce an acute promyelocytic leukemia-like disease. Blood. 2006;108:1708–1715. doi: 10.1182/blood-2006-04-015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parikh C, Subrahmanyam R, Ren R. Oncogenic NRAS rapidly and efficiently induces CMML- and AML-like diseases in mice. Blood. 2006;108:2349–2357. doi: 10.1182/blood-2004-08-009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh C, Subrahmanyam R, Ren R. Oncogenic NRAS, KRAS, and HRAS exhibit different leukemogenic potentials in mice. Cancer Res. 2007;67:7139–7146. doi: 10.1158/0008-5472.CAN-07-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner JW, 2nd, DePinho RA. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada N, Oshima H, Katoh M, Tamai Y, Oshima M, Taketo MM. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 40•.Schuhmacher AJ, Guerra C, Sauzeau V, Canamero M, Bustelo XR, Barbacid M. A mouse model for Costello syndrome reveals an Ang II-mediated hypertensive condition. J Clin Invest. 2008;118:2169–2179. doi: 10.1172/JCI34385. This reference reports the generation of an endogenous H-RasG12V germline mouse model, which did not develop cancer but displayed phenotypes resembling human Costello syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercer K, Giblett S, Green S, Lloyd D, DaRocha Dias S, Plumb M, Marais R, Pritchard C. Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts. Cancer Res. 2005;65:11493–11500. doi: 10.1158/0008-5472.CAN-05-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–384. doi: 10.1101/gad.1516407. References 41 and 42 describe the generation of conditional oncogenic B-Raf mutant mice. Upon activation by Cre in the appropriate cell type, these mice developed haematopoietic and pulmonary malignancies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collier LS, Carlson CM, Ravimohan S, Dupuy AJ, Largaespada DA. Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature. 2005;436:272–276. doi: 10.1038/nature03681. [DOI] [PubMed] [Google Scholar]
- 44.Kerkhoff E, Fedorov LM, Siefken R, Walter AO, Papadopoulos T, Rapp UR. Lung-targeted expression of the c-Raf-1 kinase in transgenic mice exposes a novel oncogenic character of the wild-type protein. Cell Growth Differ. 2000;11:185–190. [PubMed] [Google Scholar]
- 45.Ceteci F, Ceteci S, Karreman C, Kramer BW, Asan E, Gotz R, Rapp UR. Disruption of tumor cell adhesion promotes angiogenic switch and progression to micrometastasis in RAF-driven murine lung cancer. Cancer Cell. 2007;12:145–159. doi: 10.1016/j.ccr.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Lyustikman Y, Momota H, Pao W, Holland EC. Constitutive activation of Raf-1 induces glioma formation in mice. Neoplasia. 2008;10:501–510. doi: 10.1593/neo.08206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26:4609–4616. doi: 10.1038/sj.onc.1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubin JB, Gutmann DH. Neurofibromatosis type 1 - a model for nervous system tumour formation? Nat Rev Cancer. 2005;5:557–564. doi: 10.1038/nrc1653. [DOI] [PubMed] [Google Scholar]
- 49.Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- 50.Shaw AT, Meissner A, Dowdle JA, Crowley D, Magendantz M, Ouyang C, Parisi T, Rajagopal J, Blank LJ, Bronson RT, et al. Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis. Genes Dev. 2007;21:694–707. doi: 10.1101/gad.1526207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salojin K, Oravecz T. Regulation of innate immunity by MAPK dual-specificity phosphatases: knockout models reveal new tricks of old genes. J Leukoc Biol. 2007;81:860–869. doi: 10.1189/jlb.1006639. [DOI] [PubMed] [Google Scholar]
- 52.Mijimolle N, Velasco J, Dubus P, Guerra C, Weinbaum CA, Casey PJ, Campuzano V, Barbacid M. Protein farnesyltransferase in embryogenesis, adult homeostasis, and tumor development. Cancer Cell. 2005;7:313–324. doi: 10.1016/j.ccr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Wahlstrom AM, Cutts BA, Karlsson C, Andersson KM, Liu M, Sjogren AK, Swolin B, Young SG, Bergo MO. Rce1 deficiency accelerates the development of K-RAS-induced myeloproliferative disease. Blood. 2007;109:763–768. doi: 10.1182/blood-2006-05-024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sjogren AK, Andersson KM, Liu M, Cutts BA, Karlsson C, Wahlstrom AM, Dalin M, Weinbaum C, Casey PJ, Tarkowski A, et al. GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer. J Clin Invest. 2007;117:1294–1304. doi: 10.1172/JCI30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Wahlstrom AM, Cutts BA, Liu M, Lindskog A, Karlsson C, Sjogren AK, Andersson KM, Young SG, Bergo MO. Inactivating Icmt ameliorates K-RAS-induced myeloproliferative disease. Blood. 2008;112:1357–1365. doi: 10.1182/blood-2007-06-094060. References 52–55 report the genetic ablation of Ras processing enzymes. In the case of GGTase-I and Icmt deficiency, K-RasG12D-driven tumorigenesis was impaired, while loss of Rce1 enhanced tumorigenicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 57.Kissil JL, Walmsley MJ, Hanlon L, Haigis KM, Bender Kim CF, Sweet-Cordero A, Eckman MS, Tuveson DA, Capobianco AJ, Tybulewicz VL, et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res. 2007;67:8089–8094. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- 58•.Lim KH, Baines AT, Fiordalisi JJ, Shipitsin M, Feig LA, Cox AD, Der CJ, Counter CM. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7:533–545. doi: 10.1016/j.ccr.2005.04.030. The studies referenced in 56–58 showed the importance of different Ras effector pathways for Ras-mediated tumorigenesis, distinguishing these pathways as relevant therapeutic targets. [DOI] [PubMed] [Google Scholar]