Abstract
Cell stress proteins (CSPs) are a large and heterogenous family of proteins, sharing two main characteristics: their levels and/or location are modified under stress and most of them can exert a chaperon function inside the cells. Nonetheless, they are also involved in the modulation of several mechanisms, both at the intracellular and the extracellular compartments. There are more than 100 proteins belonging to the CSPs family, among them the thioredoxin (TRX) system, which is the focus of the present paper. TRX system is composed of several proteins such as TRX and peroxiredoxin (PRDX), two thiol-containing enzymes that are key players in redox homeostasis due to their ability to scavenge potential harmful reactive oxygen species. In addition to their main role as antioxidants, recent data highlights their function in several processes such as cell signalling, immune inflammatory responses, or apoptosis, all of them key mechanisms involved in atherothrombosis. Moreover, since TRX and PRDX are present in the pathological vascular wall and can be secreted under prooxidative conditions to the circulation, several studies have addressed their role as diagnostic, prognostic, and therapeutic biomarkers of cardiovascular diseases (CVDs).
1. Introduction
The vast majority of the proteins require further assistance for acquiring proper maturation and stability; this process described in the late 80s is facilitated through the activity of a family of proteins called “molecular chaperones” or cell stress proteins (CSPs) [1]. This large and diverse group of proteins composed by more than 20 families of proteins and more than 100 proteins includes the heat shock proteins (HSPs) and the thioredoxin (TRX) system. The role of HSPs in cardiovascular diseases (CVDs) has been thoroughly reviewed [2]. In the present paper, we will focus on the role of the TRX system, specifically TRX and peroxiredoxins (PRDXs), in atherothrombosis. For this purpose, we have followed the PRISMA chart displayed in Supplementary Figure 1 (see Suppplementary Figure 1 in supplementary material available online at doi:10.1155/2012/232464).
2. The Thioredoxin System
The TRX system mainly comprises TRX, TRX reductase, TRX interacting protein ((TXNIP), vitamin D3-upregulated protein-1 ((VDUP)-1) or TRX-binding protein (TRXBP)), and the PRDXs [3]. The TRX system is involved in protein assembly and plays a key role in cellular redox maintenance.
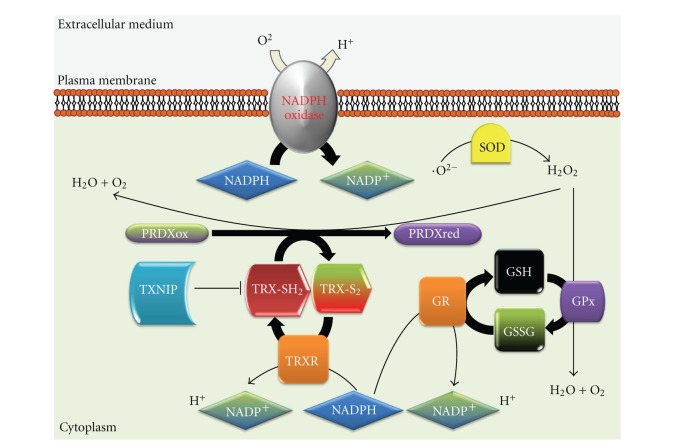
Under physiological conditions, intra- and extracellular reactive oxygen species (ROS) modulate metabolic, signaling, and transcriptional processes within the cell. However, pathological dysregulation of the redox balance could contribute to CVDs [4, 5]. Cellular redox homeostasis is tightly regulated by the coordinated action of NADPH oxidases, the TRX system and glutathione (GSH) [6], among others. The TRX system and GSH are thiol reduction systems with a key role in the defense against excessive ROS production, as well as in the modulation of signaling processes such as inflammation, cellular proliferation, and apoptosis [7–9]. These molecules maintain the intracellular milieu in a reduced state. GSH is used by the GSH peroxidase to reduce peroxides, producing oxidized GSH (GSSG) while GSH reductase reduces this oxidized form to GSH. The antioxidant properties of TRX result from PRDX action, which recycle H2O2 through reduction of several hydroperoxides into water and alcohol (Figure 1).
Figure 1.
Schematic diagram showing the maintenance of the cellular redox homeostasis by the NADPH oxidase, GSH, and the TRX system. PRDXox-oxidized PRDX; PRDXred-reduced PRDX; TRX-S2-oxidized TRX; TRX-SH2-reduced TRX; GR-GSH reductase; GPx-GSH peroxidase.
Depending on their cellular location, TRX/PRDX may exert different functions than their known chaperone and antioxidant activities. This process can be described with the so-called “Moonlighting proteins” theory [10]. This idea supports the notion that one gene = one protein = one function is simple and old-fashioned because large number of proteins have two or more functions. This theory might not apply to every protein but it seems to be right for TRX/PRDX. Under certain circumstances, mainly pro-oxidative conditions, TRX and PRDX could be released to the extracellular milieu [11, 12] although their trafficking mechanisms are not yet fully described.
2.1. Intracellular TRX
TRXs are a class of small redox molecules (~12 kDa) present in prokaryotes and eukaryotes and are essential for cellular viability [13]. So far, 3 human TRX variants have been characterized codified by different genes. TRX1 is localized under resting conditions in the cytosol but can be translocated upon stress conditions [14] (e.g., it can be found in an oxidized form in the nucleus of exponential growing cells [15] (Figure 2)), TRX2 is mitochondrial [16, 17], and SpTRX [18] is abundantly expressed in spermatozoa. TRX has a redox active disulfide/dithiol site within 2 conserved Cys residues [19], and it functions as an antioxidant molecule by protecting cells against H2O2 [20], regulating heme-oxygenase 1 (HO-1) expression [21], or inducing manganese superoxide dismutase (MnSOD) in the mitochondria [22]. Moreover, it has a protective role against nitric-oxide- (NO-) induced stress, regulating NO synthases activity [23] and through other NO regulating processes [24]. In addition to its role as antioxidant protein, TRX interacts with numerous signaling molecules (including apoptosis signal-regulating kinase 1 (ASK1) and TXNIP) and transcription factors such as nuclear factor-kappa B (NF-κB) and activating protein 1 (AP-1). TRX function can be regulated by redox modification (e.g., NO increases S-nitrosylation of TRX stimulating TRX activity [25]) or by TXNIP binding [26] (reducing TRX activity), among other mechanisms.
Figure 2.
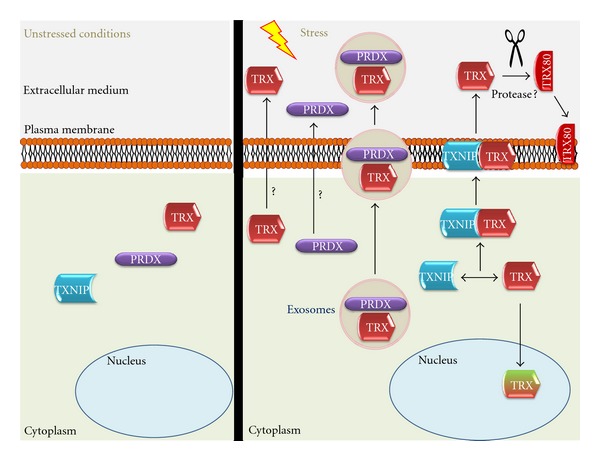
Schematic diagram showing PRDX1/TRX main cellular location under nonstress conditions (left), under oxidative stress (right) and trafficking mechanisms (right).
Regarding intracellular TRX in vivo functions, as TRX-1 KO mice are lethal, some information has been obtained from studies performed in TRX transgenic (Tg) mice [27]. TRX overexpression in mice protects placenta from oxidative stress and fetal growth by augmenting glucose availability [28], and it also functions as a shelter against apoptosis induced by extensive oxidation in diabetic embryopathy [29] or streptozotocin-induced diabetic osteopenia [30]. Furthermore, TRX is able to protect the lung injury provoked by diesel exhaust particles (DEPs) derived from diesel engine-powered automobiles and industrial machines. This protective role, played through AKT modulation, is reflected in the augmented TRX levels in control cells induced by DEP [31]. Also, mediated by AKT signaling, TRX protects neurons against apoptosis during brain focal ischemia [32].
Thus, the major role of TRX in the redox balance is supported by the numerous data regarding the protection exerted by TRX against excessive oxidative damage in different diseases and the embryonic lethality of KO mice for TRX.
2.2. Intracellular PRDX
PRDXs are a recent discovery among the peroxidases lacking the hemo group. PRDX protein levels are very abundant, around 0.1–1% of total soluble protein in mammals, and they are ubiquitously distributed in all organisms [12, 33]. PRDXs are thiol-specific enzymes lacking selenium, and they use their active sites to reduce peroxides and scavenge ROS [12]. Mammal cells express 6 PRDX isoforms (PRDX 1–6): 1, 2, and 6 are cytosolic, and 3 and 5 are mitochondrial, while 4 is described as a secretory protein in most tissues [34]. Every isoform contains a conserved Cys residue, which is the primary site for H2O2 oxidation, and they can be further classified according to their Cys residues (PRDX1-4 belongs to the 2-Cys subfamily, PRDX5 or atypical 2-Cys and PRDX6 or 1-Cys PRDX) [35].
PRDXs scavenge H2O2 more efficiently than other systems such as catalase due to its higher affinity for H2O2 [36]. PRDX can modulate NADPH oxidase activity through H2O2 inactivation [37]. In addition, PRDX can also reduce peroxynitrites levels through peroxynitrites reductases [38]. Among the mechanisms modifying PRDX functions, several posttranslational modifications have been described, such as nitrosylation [39], glutathionylation [40], phosphorylation, and the hyperoxidation of its active site [41], which stimulates its chaperone activity [42]. For example, under low H2O2 concentrations produced in conditions of cellular homeostasis PRDX forms low-molecular-weight oligomers, exerting peroxidase activity. However, under significant changes in H2O2 concentration, PRDX experiences structural changes and forms high-molecular-weight oligomers and acquires chaperone activity [43, 44]. Similarly to TRX, PRDX interacts with several proteins (e.g., cyclophilin A, macrophage inhibitory factor, etc.) and can modulate the function of these binding proteins, in a dependent or independent manner of the PRX redox status [35].
Mice lacking PRDX1 are viable with a phenotype characterized by hemolytic anemia caused by an increased ROS production by red blood cells (RBCs). Furthermore, PRDX1 has tumor suppressor properties as PRDX1 KO mice show an increased rate in malignant tumors as they age, which can be explained by the excessive accumulation of damaged tissue due to extreme ROS production [45]. Similarly to PRDX1 KO mice, targeted disruption of PRDX2 causes cysteine oxidation of several proteins on RBCs membranes, which finally results in augmented levels of denatured protein, cell toxification, and hemolysis [46]. PRDX2 also has been identified as a tumor suppressor gene [47]. Accordingly, PRDX3 (also known as MER5) KO mice are characterized by increased ROS production in macrophages and develop more severe lung injury upon Lipopolisaccharide (LPS) induction, possibly due to an excessive DNA and protein oxidative damage and inflammatory cell infiltration [48]. In fact, it has been calculated that almost 90% of H2O2 targets PRDX3 within the mitochondrial matrix, playing a major role in redox signaling in the mitochondria [49], protecting the cells against apoptosis induced by excessive damage to mitochondrial macromolecules [50]. PRDX3 absence is also involved in mitochondrial dysregulation associated with obesity, through increased protein carbonylation and ROS production [51]. It is noteworthy that PRDX3 KO adipocytes accumulate more fat than wild type due to hypertrophy and defects in the levels of enzymes implicated in glucose/lipid metabolism [51]. PRDX4 is mostly a secretory protein, while it is attached to the endoplasmic reticulum (ER) membrane of spermatogenic cells in mature testes. In these cells PRDX4 protects from cell death through its antioxidant properties, nonetheless PRDX4 KO spermatozoa shows normal fertilization [52]. To our knowledge there is no PRDX5 KO strain, although it has been described that PRDX5 overexpression prevents ROS production and p53-dependent apoptosis [53]. Nevertheless, KO mice for PRDX6, the only 1-Cys member of the peroxiredoxin family, were more vulnerable to ischemic reperfusion injury as shown by increased infarct size and higher amount oxidative stress [54]. In agreement, PRDX6 overexpression functions as a shelter in mouse lungs against toxicity of hyperoxia [55].
On the whole, the plethora of data regarding the different isoforms of PRDX demonstrate that every subunit, independently of its location, is a member of one of the major cellular systems in charge of scavenging prooxidant species and thus in the maintenance of cellular redox status.
2.3. Extracellular TRX
TRX expression can be augmented very fast under stress and is secreted by normal and tumor cells although its secretion does not seem to follow a classical Golgi apparatus pathway [11]. TRX location is regulated by TXNIP binding [26] and facilitates TRX transport from cytoplasm to the membrane under oxidative stress [14]. Another mechanism that can be involved in TRX active secretion is the exosomal pathway. Exosomes are small microparticles released by cells upon activation or apoptosis. These vesicles have been implicated in thrombosis, diabetes, inflammation, atherosclerosis, and vascular cell proliferation [56]. Proteomic studies have described the presence of TRX in exosomes in B cells [57], bladder cancer cells [58], colorectal cancer cells [59], and urine [60] (Figure 2).
There is also a truncated form of TRX that corresponds to the last 80–84 amino acids from the N-terminal end, named TRX80, and it is present in plasma where it was firstly identified as a stimulating factor of eosinophils cytotoxicity [61]. It is possibly a result of protease activity but this process is still unknown (Figure 2). Recombinant TRX80 is a potent mitogenic cytokine for peripheral blood mononuclear cells (PBMCs), an effect not shared by TRX [62]. TRX80 differs from TRX because it forms a dimer lacking reductase activity and its activity is independent of the Cys residues from the TRX active site. The main cellular target for TRX80 are PBMCs in which it induces a Th1 response via IL12 production [63].
Extracellular TRX is present in the circulation and its levels are increased under oxidative stress or inflammation [64] (Figure 2). TRX has been pointed out as a biomarker in numerous oxidative and inflammatory diseases such as rheumatoid arthritis (RA) in which plasmaTRXlevels of normal subjects were significantly lower than those of RA patients and correlated with RA disease activity and C-reactive protein [65]. TRX levels were increased in patients with systemic inflammatory stress syndrome (SIRS)/sepsis compared to control subjects [66].
2.4. Extracellular PRDX
PRDX1 can be found inside the Golgi apparatus on endothelial cells (ECs) [67], and under phorbol 12-myristate 13-acetate (PMA) stimulation PRDX1 is translocated to the cellular membrane [68], as also showed for PRDX6 in polymorphonuclear cells (PMNs) [69] (Figure 2). PRDX might also be secreted by lung cancer cells through a nonclassical pathway [70, 71]. Nonetheless, the extracellular function of PRDX is still unknown. Unlike the well-described function of intracellular PRDX1, membrane PRDX6 helps in the maintenance of an optimal NADPH oxidase activity [69]. A number of chaperones, including TRX and HSPs, are released by stressed or dying cells, acting as an endogenous warning system through binding of these signals to receptors on the outer membrane [72–75]. Most of these signals are recognized by Toll-like receptor 4 (TLR4) [74, 75]. Accordingly, PRDX1 binds TLR4 and stimulates proinflammatory cytokine production in macrophages and dendritic cells, which suggests that PRDX could be acting as damage-associated molecular-pattern molecule (DAMP). Its trafficking seems to be dependent on PRDX binding to protein kinase C (PKC) through microvesicles [76]. In fact, exosomes can be participating in active transport of PRDX since proteomic studies have described PRDX in exosomes in B cells [57], bladder cancer cells [58], breast cancer cells [77], breast milk [78], colorectal cancer cells [59], and saliva [79] (Figure 2).
Thus, it is tempting to speculate that extracellular levels of PRDX/TRX result from a cellular response to high oxidant conditions in the outer milieu.
3. TRX/PRDX in Atherothrombosis
Atherothrombosis is an immune-inflammatory disease, originated by the subendothelial accumulation of LDLs, that can be oxidized by ROS. Oxidative stress is involved not only in the first stages of atherogenesis by modifying LDLs or NO, but also in later stages of atherothrombosis by modulating the expression of proteases that weakens the fibrous cap [80, 81]. ROS overproduction also produces direct damage to macromolecules such as lipids, nucleic acids, and proteins [82]. Furthermore, ROS can act as signaling molecules by inducing the activation of several cells from the vasculature. For example, through LDL oxydation and/or direct cell targeting, ROS can induce endothelial dysfunction and further leucocyte activation, deposition, and extravasation. In addition, ROS are clearly involved in the activation of vascular smooth muscle cells (VSMCs) from the lesion and sustain foam cell formation. Thus, pathological ROS overproduction is a main feature in atherogenesis and plaque rupture, which finally results in almost 70% of the clinical events [83].
Among the different systems involved in the redox maintenance in the vasculature, one of the most active is the TRX system. It is present in ECs [14, 84], VSMCs [85, 86], monocytes/macrophages [87, 88], RBCs [89, 90], and PMNs [90, 91]. Since TRX and PRDX are present in the atherosclerotic plaque and they can modulate different mechanisms involved in CVDs, several studies have addressed their role as diagnostic, prognostic, and therapeutic biomarkers.
3.1. TRX
TRX is abundantly expressed in the vasculature, and its levels are increased under oxidative stress, possibly as a response mechanism to augmented ROS production [19]. Besides, TRX expression in the endothelium and in macrophages is augmented in atherosclerotic patients [92] and in arteries damaged by the balloon model [93]. More recently, TRX has been pointed as a possible marker for unstable atherosclerotic plaques due to its association with oxidative stress and intraplaque hemorrhage in culprit lesions [94]. TRX reductase is as well overexpressed in atherosclerotic plaques, maybe synthesized by macrophages engulfing oxLDLs [95].
The antioxidant effects of TRX are shown when overexpressed in mouse hearts, protecting them from myocardial infarction and displaying significantly improved postischemic ventricular recovery [96]. The positive effects of TRX in myocardial infarction are also due to its neoangiogenic properties as shown in different murine models [97, 98].
On the other hand, TRX can function as a signaling molecule by decreasing pressure-overload cardiac hypertrophy [99], maybe through upregulation of miR-98 [100]. However, there is a controversy about this matter because almost at the same time it was published another article in which the authors described that activation of TRX participates in the development of pressure-overload cardiac hypertrophy. In this respect, animals overexpressing TXNIP developed less hypertrophy [101]. Furthermore, transverse aortic constriction increased TRX activity accompanied by a 40% reduction in TXNIP levels [101]. Whether variations in TXNIP and TRX levels/activity reflect an increase in oxidative stress or they act as signaling molecules is still a matter to elucidate [102].
Recent studies have shown that extracellular TRX can inhibit interleukin-1 expression stimulated by LPS in monocyte-derived macrophages [103]. Besides, TRX1 administration has beneficial effects on myosin-induced autoimmune myocarditis through inhibition of inflammatory mediators and macrophage infiltration [104] and TRX1 also protects from reperfusion-induced arrhythmias [105]. Furthermore, TRX administration has been also shown to be beneficial in cerebral ischemia/reperfusion injury reducing the infarcted area through its antioxidant properties [106]. As an additional support for the beneficial effects of TRX in therapeutics, it is to note that TRX-1 gene delivery protects hypertensive rats from myocardial infarction through HO-1/B-cell lymphoma 2 (BCL-2) [107].
Regarding cardiovascular diseases, TRX levels are elevated in plasma from atherothrombotic patients [108, 109], andhigh homocysteine plus low TRX is related to CAD severity [110]. Furthermore, TRX was elevated in patients following angioplasty, and there was an association with increased TRX levels and decreased rate of restenosis at follow-up angiography (6 months) [111]. We have recently reported an increase in serum TRX from abdominal aortic aneurysm (AAA) patients compared with control subjects. Besides, TRX correlates with AAA size and expansion rate which suggests that TRX could be a good biomarker of AAA evolution [91].
3.2. PRDX
PRDX expression can be modified by prooxidative stimulus such as LPS or the phorbol ester12-O-tetradecanoylphorbol- 13-acetate (TPA) [88, 112]. Attention to PRDX as a major regulator of H2O2 homeostasis is growing [34]. In cells stimulated withplatelet-derived growth factor (PDGF) or tumor necrosis factor alpha, PRDX overexpression or silencing provoked, respectively, reduction or increase of H2O2 levels [113]. Moreover, through H2O2 scavenging, PRDX can inhibit the NF-κB pathway and consequently the inflammatory response [114]. Different PRDX isoforms seem to modulate different cellular responses. For example, transfection of VSMCs from rat pulmonary artery with an expression plasmid for PRDX1 increases proliferation rate significantly [115]. PRDX1 also diminishes leucocytes activation and adhesion to vascular endothelium. Double KO mice for PRDX1 and ApoE chow fed showed larger atherosclerotic lesions and macrophages enriched than ApoE KO mice [116].KO mice for PRDX2/ApoE showed exacerbated atherosclerotic lesion formation dependent both on vascular and hematopoietic cells. Besides, immune cells accumulation in the atherosclerotic lesions is increased due to PRDX2 absence and also redox-dependent signaling PRDX2 [117]. Moreover, PRDX2 modulates PDGF signaling, inhibiting thereby cell proliferation and migration [113, 118]. Using different in vivo models, it was shown that CD36 KO mice produce lower levels of ROS, along with an increase in heme-oxygenase1 (HO-1) and PRDX2. Furthermore, NF-E2-related factor-2 (Nrf2), a transcription factor in charge of the transcriptional regulation of HO-1 and PRDX2, knockdown led to increased ROS production and a prothrombotic phenotype under FeCl3 treatment, a similar phenotype to that of PRDX2 KO mice [119]. Regarding PRDX2 and vascular diseases, it has been recently shown that PRDX2 is overexpressed in ruptured AAA tissue compared with nonruptured [120]. This could be associated with the increased oxidative stress observed in AAA tissue, which produces 2.5 times higher superoxide than adjacent non-AAA tissue and 10 times higher than nonpathological aorta [121].
Besides, PRDX3 overexpression prevents ventricular remodeling and cardiac failure after myocardial infarction in mice [122]. As mentioned above, PRDX6 protects mice against ischemic reperfusion injury [54]. Although, little is known about circulating levels of PRDX, we have recently described high PRDX1 levels in serum from AAA patients [90]. Besides, PRDX1 levels correlated positively with size and expansion rate of AAA, suggesting its potential role as diagnostic and prognostic biomarker for AAA.
4. Conclusion
On the whole, we have summarized several findings that demonstrate the major role of the TRX system in the maintenance of the redox status in CVDs. Furthermore, the extracellular levels of PRDX/TRX seem to be related with a prooxidative scenario and there is growing data suggesting their potential role as biomarkers for oxidative-stress-related diseases. Finally, their value as useful therapeutic tools is being tested and future studies are necessary to validate thier prospective beneficial effects in CVDs.
Acknowledgments
This work was supported by the Spanish Ministerio de Ciencia y Tecnología (SAF 2010-21852), Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, Redes RECAVA (RD06/0014/0035), Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (PI10/00072), and Sociedad Española de Arterosclerosis and Fundación Lilly.
References
- 1.Hemmingsen SM, Woolford C, Van der Vies SM, et al. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 2.Madrigal-Matute J, Martin-Ventura JL, Blanco-Colio LM, Egido J, Michel JB, Meilhac O. Heat-shock proteins in cardiovascular disease. Advances in Clinical Chemistry. 2011;54:1–43. doi: 10.1016/b978-0-12-387025-4.00001-7. [DOI] [PubMed] [Google Scholar]
- 3.Powis G, Briehl M, Oblong J. Redox signalling and the control of cell growth and death. Pharmacology and Therapeutics. 1995;68(1):149–173. doi: 10.1016/0163-7258(95)02004-7. [DOI] [PubMed] [Google Scholar]
- 4.Albertini R, Moratti R, De Luca G. Oxidation of low-density lipoprotein in atherosclerosis from basic biochemistry to clinical studies. Current Molecular Medicine. 2002;2(6):579–592. doi: 10.2174/1566524023362177. [DOI] [PubMed] [Google Scholar]
- 5.Lavrovsky Y, Chatterjee B, Clark RA, Roy AK. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Experimental Gerontology. 2000;35(5):521–532. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 6.World CJ, Yamawaki H, Berk BC. Thioredoxin in the cardiovascular system. Journal of Molecular Medicine. 2006;84(12):997–1003. doi: 10.1007/s00109-006-0109-6. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxidants and Redox Signaling. 2000;2(4):811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- 8.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annual Review of Biophysics & Biomolecular Structure. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 9.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300(5619):650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 10.Jeffery CJ. Moonlighting proteins. Trends in Biochemical Sciences. 1999;24(1):8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- 11.Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. Journal of Biological Chemistry. 1992;267(34):24161–24164. [PubMed] [Google Scholar]
- 12.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radical Biology and Medicine. 2005;38(12):1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Slaby I, Holmgren A. Thioredoxin reductase-dependent insulin disulfide reduction by phage T7 DNA polymerase reflects dissociation of the enzyme into subunits. Journal of Biological Chemistry. 1989;264(28):16502–16506. [PubMed] [Google Scholar]
- 14.World C, Spindel ON, Berk BC. Thioredoxin-interacting protein mediates TRX1 translocation to the plasma membrane in response to tumor necrosis factor-α: a key mechanism for vascular endothelial growth factor receptor-2 transactivation by reactive oxygen species. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(8):1890–1897. doi: 10.1161/ATVBAHA.111.226340. [DOI] [PubMed] [Google Scholar]
- 15.Spielberger JC, Moody AD, Watson WH. Oxidation and nuclear localization of thioredoxin-1 in sparse cell cultures. Journal of Cellular Biochemistry. 2008;104(5):1879–1889. doi: 10.1002/jcb.21762. [DOI] [PubMed] [Google Scholar]
- 16.Spyrou G, Enmark E, Miranda-Vizuete A, Gustafsson JÅ. Cloning and expression of a novel mammalian thioredoxin. Journal of Biological Chemistry. 1997;272(5):2936–2941. doi: 10.1074/jbc.272.5.2936. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Hosoi F, Yamaguchi-Iwai Y, et al. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO Journal. 2002;21(7):1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda-Vizuete A, Ljung J, Damdimopoulos AE, et al. Characterization of sptrx, a novel member of the thioredoxin family specifically expressed in human spermatozoa. Journal of Biological Chemistry. 2001;276(34):31567–31574. doi: 10.1074/jbc.M101760200. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annual Review of Immunology. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura H, Matsuda M, Furuke K, et al. Adult T cell leukemia-derived factor/human thioredoxin protects endothelial F-2 cell injury caused by activated neutrophils or hydrogen peroxide. Immunology Letters. 1994;42(1-2):75–80. doi: 10.1016/0165-2478(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 21.Trigona WL, Mullarky IK, Cao Y, Sordillo LM. Thioredoxin reductase regulates the induction of haem oxygenase-1 expression in aortic endothelial cells. Biochemical Journal. 2006;394(1):207–216. doi: 10.1042/BJ20050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das KC, Lewis-Molock Y, White CW. Elevation of manganese superoxide dismutase gene expression by thioredoxin. American Journal of Respiratory Cell and Molecular Biology. 1997;17(6):713–726. doi: 10.1165/ajrcmb.17.6.2809. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Li YD, Patel JM, Block ER. Thioredoxin overexpressin prevents NO-induced reduction of NO synthase activity in lung endothelial cells. American Journal of Physiology, Lung Cellular and Molecular Physiology. 1998;275(2):L288–L293. doi: 10.1152/ajplung.1998.275.2.L288. [DOI] [PubMed] [Google Scholar]
- 24.Shao LE, Tanaka T, Gribi R, Yu J. Thioredoxin-related regulation of NO/NOS activities. Annals of the New York Academy of Sciences. 2002;962:140–150. doi: 10.1111/j.1749-6632.2002.tb04064.x. [DOI] [PubMed] [Google Scholar]
- 25.Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S. Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: a novel vasculoprotective function of statins. Circulation. 2004;110(7):856–861. doi: 10.1161/01.CIR.0000138743.09012.93. [DOI] [PubMed] [Google Scholar]
- 26.Junn E, Han SH, Im JY, et al. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. Journal of Immunology. 2000;164(12):6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- 27.Matsui M, Oshima M, Oshima H, et al. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Developmental Biology. 1996;178(1):179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 28.Umekawa T, Sugiyama T, Kihira T, et al. Overexpression of thioredoxin-1 reduces oxidative stress in the placenta of transgenic mice and promotes fetal growth via glucose metabolism. Endocrinology. 2008;149(8):3980–3988. doi: 10.1210/en.2007-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamimoto Y, Sugiyama T, Kihira T, et al. Transgenic mice overproducing human thioredoxin-1, an antioxidative and anti-apoptotic protein, prevents diabetic embryopathy. Diabetologia. 2010;53(9):2046–2055. doi: 10.1007/s00125-010-1784-y. [DOI] [PubMed] [Google Scholar]
- 30.Hamada Y, Fujii H, Kitazawa R, Yodoi J, Kitazawa S, Fukagawa M. Thioredoxin-1 overexpression in transgenic mice attenuates streptozotocin-induced diabetic osteopenia: a novel role of oxidative stress and therapeutic implications. Bone. 2009;44(5):936–941. doi: 10.1016/j.bone.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Kaimul Ahsan M, Nakamura H, Tanito M, Yamada K, Utsumi H, Yodoi J. Thioredoxin-1 suppresses lung injury and apoptosis induced by diesel exhaust particles (DEP) by scavenging reactive oxygen species and by inhibiting DEP-induced downregulation of Akt. Free Radical Biology and Medicine. 2005;39(12):1549–1559. doi: 10.1016/j.freeradbiomed.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Zhou F, Gomi M, Fujimoto M, et al. Attenuation of neuronal degeneration in thioredoxin-1 overexpressing mice after mild focal ischemia. Brain Research. 2009;1272:62–70. doi: 10.1016/j.brainres.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Wood ZA, Schröder E, Harris JR, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends in Biochemical Sciences. 2003;28(1):32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 34.Chae HZ, Kim HJ, Kang SW, Rhee SG. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Research and Clinical Practice. 1999;45(2-3):101–112. doi: 10.1016/s0168-8227(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 35.Rhee SG, Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxidants and Redox Signaling. 2011;15(3):781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 36.Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. Journal of Biological Chemistry. 2007;282(16):11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- 37.Leavey PJ, Gonzalez-Aller C, Thurman G, et al. A 29-kDa protein associated with p67phox expresses both peroxiredoxin and phospholipase A2 activity and enhances superoxide anion production by a cell-free system of NADPH oxidase activity. Journal of Biological Chemistry. 2002;277(47):45181–45187. doi: 10.1074/jbc.M202869200. [DOI] [PubMed] [Google Scholar]
- 38.Uwayama J, Hirayama A, Yanagawa T, et al. Tissue Prx I in the protection against Fe-NTA and the reduction of nitroxyl radicals. Biochemical and Biophysical Research Communications. 2006;339(1):226–231. doi: 10.1016/j.bbrc.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 39.Fang J, Nakamura T, Cho DH, Gu Z, Lipton SA. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(47):18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chae HZ, Oubrahim H, Park JW, Rhee SG, Chock PB. Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators. Antioxidants & Redox Signaling. 2012;16(6):506–523. doi: 10.1089/ars.2011.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang KS, Kang SW, Woo HA, et al. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. Journal of Biological Chemistry. 2002;277(41):38029–38036. doi: 10.1074/jbc.M206626200. [DOI] [PubMed] [Google Scholar]
- 42.Barranco-Medina S, Lázaro JJ, Dietz KJ. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Letters. 2009;583(12):1809–1816. doi: 10.1016/j.febslet.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 43.Lim JC, Choi HI, Park YS, et al. Irreversible oxidation of the active-site cysteine of peroxiredoxin to cysteine sulfonic acid for enhanced molecular chaperone activity. Journal of Biological Chemistry. 2008;283(43):28873–28880. doi: 10.1074/jbc.M804087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhee SG, Jeong W, Chang TS, Woo HA. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney international. Supplement. 2007;(106):S3–S8. doi: 10.1038/sj.ki.5002380. [DOI] [PubMed] [Google Scholar]
- 45.Neumann CA, Krause DS, Carman CV, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424(6948):561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 46.Lee TH, Kim SU, Yu SL, et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101(12):5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 47.Agrawal-Singh S, Isken F, Agelopoulos K, et al. Genome-wide analysis of histone H3 acetylation patterns in AML identifies PRDX2 as an epigenetically silenced tumor suppressor gene. Blood. 2012;119(10):2346–2357. doi: 10.1182/blood-2011-06-358705. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Shoji W, Takano H, et al. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochemical and Biophysical Research Communications. 2007;355(3):715–721. doi: 10.1016/j.bbrc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochemical Journal. 2010;425(2):313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 50.Chang TS, Cho CS, Park S, Yu S, Sang WK, Sue GR. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. Journal of Biological Chemistry. 2004;279(40):41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 51.Huh JY, Kim Y, Jeong J, et al. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxidants & Redox Signaling. 2012;16(3):229–243. doi: 10.1089/ars.2010.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iuchi Y, Okada F, Tsunoda S, et al. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochemical Journal. 2009;419(1):149–158. doi: 10.1042/BJ20081526. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Y, Kok KH, Chun ACS, et al. Mouse peroxiredoxin V is a thioredoxin peroxidase that inhibits p53-induced apoptosis. Biochemical and Biophysical Research Communications. 2000;268(3):921–927. doi: 10.1006/bbrc.2000.2231. [DOI] [PubMed] [Google Scholar]
- 54.Nagy N, Malik G, Fisher AB, Das DK. Targeted disruption of peroxiredoxin 6 gene renders the heart vulnerable to ischemia-reperfusion injury. American Journal of Physiology, Heart and Circulatory Physiology. 2006;291(6):H2636–H2640. doi: 10.1152/ajpheart.00399.2006. [DOI] [PubMed] [Google Scholar]
- 55.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radical Biology and Medicine. 2005;38(11):1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Azevedo LCP, Pedro MA, Laurindo FRM. Circulating microparticles as therapeutic targets in cardiovascular diseases. Recent Patents on Cardiovascular Drug Discovery. 2007;2(1):41–51. doi: 10.2174/157489007779606121. [DOI] [PubMed] [Google Scholar]
- 57.Buschow SI, Van Balkom BWM, Aalberts M, Heck AJR, Wauben M, Stoorvogel W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunology and Cell Biology. 2010;88(8):851–856. doi: 10.1038/icb.2010.64. [DOI] [PubMed] [Google Scholar]
- 58.Welton JL, Khanna S, Giles PJ, et al. Proteomics analysis of bladder cancer exosomes. Molecular and Cellular Proteomics. 2010;9(6):1324–1338. doi: 10.1074/mcp.M000063-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi DS, Lee JM, Gun WP, et al. Proteomic analysis of microvesicles derived from human colorectal cancer cells. Journal of Proteome Research. 2007;6(12):4646–4655. doi: 10.1021/pr070192y. [DOI] [PubMed] [Google Scholar]
- 60.Gonzales PA, Pisitkun T, Hoffert JD, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. Journal of the American Society of Nephrology. 2009;20(2):363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dessein AJ, Lenzi HL, Bina JC. Modulation of eosinophil cytotoxicity by blood mononuclear cells from healthy subjects and patients with chronic schistosomiasis mansoni. Cellular Immunology. 1984;85(1):100–113. doi: 10.1016/0008-8749(84)90282-x. [DOI] [PubMed] [Google Scholar]
- 62.Pekkari K, Gurunath R, Arner ESJ, Holmgren A. Truncated thioredoxin is a mitogenic cytokine for resting human peripheral blood mononuclear cells and is present in human plasma. Journal of Biological Chemistry. 2000;275(48):37474–37480. doi: 10.1074/jbc.M001012200. [DOI] [PubMed] [Google Scholar]
- 63.Pekkari K, Avila-Carino J, Bengtsson A, Gurunath R, Scheynius A, Holmgren A. Truncated thioredoxin. (Trx80) induces production of interleukin-12 and enhances CD14 expression in human monocytes. Blood. 2001;97(10):3184–3190. doi: 10.1182/blood.v97.10.3184. [DOI] [PubMed] [Google Scholar]
- 64.Kondo N, Ishii Y, Kwon YW, et al. Redox-sensing release of human thioredoxin from t lymphocytes with negative feedback loops. Journal of Immunology. 2004;172(1):442–448. doi: 10.4049/jimmunol.172.1.442. [DOI] [PubMed] [Google Scholar]
- 65.Jikimoto T, Nishikubo Y, Koshiba M, et al. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Molecular Immunology. 2002;38(10):765–772. doi: 10.1016/s0161-5890(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 66.Leaver SK, MacCallum NS, Pingle V, et al. Increased plasma thioredoxin levels in patients with sepsis: positive association with macrophage migration inhibitory factor. Intensive Care Medicine. 2010;36(2):336–341. doi: 10.1007/s00134-009-1640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mowbray AL, Kang DH, Sue GR, Sang WK, Jo H. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. Journal of Biological Chemistry. 2008;283(3):1622–1627. doi: 10.1074/jbc.M707985200. [DOI] [PubMed] [Google Scholar]
- 68.Lehel C, Oláh Z, Petrovics G, Jakab G, Anderson WB. Influence of various domains of protein kinase C ∈ on its PMA-induced translocation from the golgi to the plasma membrane. Biochemical and Biophysical Research Communications. 1996;223(1):98–103. doi: 10.1006/bbrc.1996.0852. [DOI] [PubMed] [Google Scholar]
- 69.Ambruso DR, Ellison MA, Thurman GW, Leto TL. Peroxiredoxin 6 translocates to the plasma membrane during neutrophil activation and is required for optimal NADPH oxidase activity. Biochimica et Biophysica Acta. 2012;1823(2):306–315. doi: 10.1016/j.bbamcr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Chang JW, Lee SH, Lu Y, Yoo YJ. Transforming growth factor-β1 induces the non-classical secretion of peroxiredoxin-I in A549 cells. Biochemical and Biophysical Research Communications. 2006;345(1):118–123. doi: 10.1016/j.bbrc.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 71.Jong WC, Seung HL, Ju YJ, et al. Peroxiredoxin-I is an autoimmunogenic tumor antigen in non-small cell lung cancer. FEBS Letters. 2005;579(13):2873–2877. doi: 10.1016/j.febslet.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida S, Katoh T, Tetsuka T, Uno K, Matsui N, Okamoto T. Involvement of thioredoxin in rheumatoid arthritis: its costimulatory roles in the TNF-α-induced production of IL-6 and IL-8 from cultured synovial fibroblasts. Journal of Immunology. 1999;163(1):351–358. [PubMed] [Google Scholar]
- 73.Hunter-Lavin C, Davies EL, Bacelar MMFVG, Marshall MJ, Andrew SM, Williams JHH. Hsp70 release from peripheral blood mononuclear cells. Biochemical and Biophysical Research Communications. 2004;324(2):511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 74.Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70. Role of toll-like receptor (TLR) 2 and TLR4. Journal of Biological Chemistry. 2002;277(17):15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 75.Lotze MT, Zeh HJ, Rubartelli A, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunological Reviews. 2007;220(1):60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 76.Westermann P, Knoblich M, Maier O, Lindschau C, Haller H. Protein kinase C bound to the Golgi apparatus supports the formation of constitutive transport vesicles. Biochemical Journal. 1996;320(2):651–658. doi: 10.1042/bj3200651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Staubach S, Razawi H, Hanisch FG. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics. 2009;9(10):2820–2835. doi: 10.1002/pmic.200800793. [DOI] [PubMed] [Google Scholar]
- 78.Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk. Journal of Immunology. 2007;179(3):1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez-Begne M, Lu B, Han X, et al. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) Journal of Proteome Research. 2009;8(3):1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. American Journal of Cardiology. 2003;91(3):A7–A11. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 81.Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-κB activity in lipopolysaccharide-activated human primary monocytes. Journal of Immunology. 2005;175(8):5423–5429. doi: 10.4049/jimmunol.175.8.5423. [DOI] [PubMed] [Google Scholar]
- 82.Blumberg J. Use of biomarkers of oxidative stress in research studies. Journal of Nutrition. 2004;134(11):3188S–3189S. doi: 10.1093/jn/134.11.3188S. [DOI] [PubMed] [Google Scholar]
- 83.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the Vulnerable Plaque. Journal of the American College of Cardiology. 2006;47(8, supplement):C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 84.Jo H, Song H, Mowbray A. Role of NADPH oxidases in disturbed flow- and BMP4-induced inflammation and atherosclerosis. Antioxidants and Redox Signaling. 2006;8(9-10):1609–1619. doi: 10.1089/ars.2006.8.1609. [DOI] [PubMed] [Google Scholar]
- 85.Qu A, Jiang C, Xu M, et al. PGC-1α attenuates neointimal formation via inhibition of vascular smooth muscle cell migration in the injured rat carotid artery. American Journal of Physiology, Cell Physiology. 2009;297(3):C645–C653. doi: 10.1152/ajpcell.00469.2008. [DOI] [PubMed] [Google Scholar]
- 86.Popowich DA, Vavra AK, Walsh CP, et al. Regulation of reactive oxygen species by p53: implications for nitric oxide-mediated apoptosis. American Journal of Physiology, Heart and Circulatory Physiology. 2010;298(6):H2192–H2200. doi: 10.1152/ajpheart.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sahaf B, Rosén A. Secretion of 10-kDa and 12-kDa thioredoxin species from blood monocytes and transformed leukocytes. Antioxidants and Redox Signaling. 2000;2(4):717–726. doi: 10.1089/ars.2000.2.4-717. [DOI] [PubMed] [Google Scholar]
- 88.Hess A, Wijayanti N, Neuschäfer-Rube AP, Katz N, Kietzmann T, Immenschuh S. Phorbol ester-dependent activation of peroxiredoxin i gene expression via a protein kinase C, Ras, p38 mitogen-activated protein kinase signaling pathway. Journal of Biological Chemistry. 2003;278(46):45419–45434. doi: 10.1074/jbc.M307871200. [DOI] [PubMed] [Google Scholar]
- 89.Cha MK, Kim IH. Thioredoxin-linked peroxidase from human red blood cell: evidence for the existence of thioredoxin and thioredoxin reductase in human red blood cell. Biochemical and Biophysical Research Communications. 1995;217(3):900–907. doi: 10.1006/bbrc.1995.2856. [DOI] [PubMed] [Google Scholar]
- 90.Martinez-Pinna R, Ramos-Mozo P, Madrigal-Matute J, et al. Identification of peroxiredoxin-1 as a novel biomarker of abdominal aortic aneurysm. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(4):935–943. doi: 10.1161/ATVBAHA.110.214429. [DOI] [PubMed] [Google Scholar]
- 91.Martinez-Pinna R, Lindholt JS, Blanco-Colio LM, et al. Increased levels of thioredoxin in patients with abdominal aortic aneurysms (AAAs). A potential link of oxidative stress with AAA evolution. Atherosclerosis. 2010;212(1):333–338. doi: 10.1016/j.atherosclerosis.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 92.Okuda M, Inoue N, Azumi H, et al. Expression of glutaredoxin in human coronary arteries: its potential role in antioxidant protection against atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(9):1483–1487. doi: 10.1161/hq0901.095550. [DOI] [PubMed] [Google Scholar]
- 93.Takagi Y, Gon Y, Todaka T, et al. Expression of thioredoxin is enhanced in atherosclerotic plaques and during neointima formation in rat arteries. Laboratory Investigation. 1998;78(8):957–966. [PubMed] [Google Scholar]
- 94.Nishihira K, Yamashita A, Imamura T, et al. Thioredoxin in coronary culprit lesions: possible relationship to oxidative stress and intraplaque hemorrhage. Atherosclerosis. 2008;201(2):360–367. doi: 10.1016/j.atherosclerosis.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 95.Furman C, Rundlöf AK, Larigauderie G, et al. Thioredoxin reductase 1 is upregulated in atherosclerotic plaques: specific induction of the promoter in human macrophages by oxidized low-density lipoproteins. Free Radical Biology and Medicine. 2004;37(1):71–85. doi: 10.1016/j.freeradbiomed.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 96.Turoczi T, Chang VWH, Engelman RM, Maulik N, Ho YS, Das DK. Thioredoxin redox signaling in the ischemic heart: an insight with transgenic mice overexpressing Trx1. Journal of Molecular and Cellular Cardiology. 2003;35(6):695–704. doi: 10.1016/s0022-2828(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 97.Samuel SM, Thirunavukkarasu M, Penumathsa SV, et al. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121(10):1244–1255. doi: 10.1161/CIRCULATIONAHA.109.872481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adluri RS, Thirunavukkarasu M, Zhan L, et al. Thioredoxin 1 enhances neovascularization and reduces ventricular remodeling during chronic myocardial infarction: a study using thioredoxin 1 transgenic mice. Journal of Molecular and Cellular Cardiology. 2011;50(1):239–247. doi: 10.1016/j.yjmcc.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamamoto M, Yang G, Hong C, et al. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. Journal of Clinical Investigation. 2003;112(9):1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang Y, Ago T, Zhai P, Abdellatif M, Sadoshima J. Thioredoxin 1 negatively regulates angiotensin II-Induced cardiac hypertrophy through upregulation of miR-98/let-7. Circulation Research. 2011;108(3):305–313. doi: 10.1161/CIRCRESAHA.110.228437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshioka J, Schulze PC, Cupesi M, et al. Thioredoxin-interacting protein controls cardiac hypertrophy through regulation of thioredoxin activity. Circulation. 2004;109(21):2581–2586. doi: 10.1161/01.CIR.0000129771.32215.44. [DOI] [PubMed] [Google Scholar]
- 102.Lowenstein CJ. Exogenous thioredoxin reduces inflammation in autoimmune myocarditis. Circulation. 2004;110(10):1178–1179. doi: 10.1161/01.CIR.0000143048.05940.0D. [DOI] [PubMed] [Google Scholar]
- 103.Billiet L, Furman C, Larigauderie G, et al. Extracellular human thioredoxin-1 inhibits lipopolysaccharide-induced interleukin-1β expression in human monocyte-derived macrophages. Journal of Biological Chemistry. 2005;280(48):40310–40318. doi: 10.1074/jbc.M503644200. [DOI] [PubMed] [Google Scholar]
- 104.Liu W, Nakamura H, Shioji K, et al. Thioredoxin-1 ameliorates myosin-induced autoimmune myocarditis by suppressing chemokine expressions and leukocyte chemotaxis in mice. Circulation. 2004;110(10):1276–1283. doi: 10.1161/01.CIR.0000141803.41217.B6. [DOI] [PubMed] [Google Scholar]
- 105.Aota M, Matsuda K, Isowa N, Wada H, Yodoi J, Ban T. Protection against reperfusion-induced arrhythmias by human thioredoxin. Journal of Cardiovascular Pharmacology. 1996;27(5):727–732. doi: 10.1097/00005344-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 106.Hattori I, Takagi Y, Nakamura H, et al. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxidants and Redox Signaling. 2004;6(1):81–87. doi: 10.1089/152308604771978372. [DOI] [PubMed] [Google Scholar]
- 107.Koneru S, Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik N. Thioredoxin-1 gene delivery induces heme oxygenase-1 mediated myocardial preservation after chronic infarction in hypertensive rats. American Journal of Hypertension. 2009;22(2):183–190. doi: 10.1038/ajh.2008.318. [DOI] [PubMed] [Google Scholar]
- 108.Miyamoto S, Sakamoto T, Soejima H, et al. Plasma thioredoxin levels and platelet aggregability in patients with acute myocardial infarction. American Heart Journal. 2003;146(3):465–471. doi: 10.1016/S0002-8703(03)00311-9. [DOI] [PubMed] [Google Scholar]
- 109.Hokamaki J, Kawano H, Soejima H, et al. Plasma thioredoxin levels in patients with unstable angina. International Journal of Cardiology. 2005;99(2):225–231. doi: 10.1016/j.ijcard.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 110.Wu Y, Yang L, Zhong L. Decreased serum levels of thioredoxin in patients with coronary artery disease plus hyperhomocysteinemia is strongly associated with the disease severity. Atherosclerosis. 2010;212(1):351–355. doi: 10.1016/j.atherosclerosis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 111.Wahlgren CM, Pekkari K. Elevated thioredoxin after angioplasty in peripheral arterial disease. European Journal of Vascular and Endovascular Surgery. 2005;29(3):281–286. doi: 10.1016/j.ejvs.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 112.Wijayanti N, Naidu S, Kietzmann T, Immenschuh S. Inhibition of phorbol ester-dependent peroxiredoxin I gene activation by lipopolysaccharide via phosphorylation of RelA/p65 at serine 276 in monocytes. Free Radical Biology and Medicine. 2008;44(4):699–710. doi: 10.1016/j.freeradbiomed.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Choi MH, Lee IK, Kim GW, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435(7040):347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 114.Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Current Opinion in Cell Biology. 2005;17(2):183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 115.Ihida-Stansbury K, McKean DM, Gebb SA, et al. Regulation and functions of the paired-related homeobox gene PRX1 in pulmonary vascular development and disease. Chest. 2005;128(6, supplement):p. S591. doi: 10.1378/chest.128.6_suppl.591S. [DOI] [PubMed] [Google Scholar]
- 116.Kisucka J, Chauhan AK, Patten IS, et al. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circulation Research. 2008;103(6):598–605. doi: 10.1161/CIRCRESAHA.108.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park J-G, Yoo J-Y, Jeong S-J, et al. Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Circulation Research. 2011;109(7):739–749. doi: 10.1161/CIRCRESAHA.111.245530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends in Molecular Medicine. 2005;11(12):571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. Journal of Clinical Investigation. 2010;120(11):3996–4006. doi: 10.1172/JCI42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Urbonavicius S, Lindholt JS, Vorum H, Urbonaviciene G, Henneberg EW, Honoré B. Proteomic identification of differentially expressed proteins in aortic wall of patients with ruptured and nonruptured abdominal aortic aneurysms. Journal of Vascular Surgery. 2009;49(2):455–463. doi: 10.1016/j.jvs.2008.08.097. [DOI] [PubMed] [Google Scholar]
- 121.Miller FJ, Jr., Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(4):560–565. doi: 10.1161/01.atv.0000013778.72404.30. [DOI] [PubMed] [Google Scholar]
- 122.Matsushima S, Ide T, Yamato M, et al. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113(14):1779–1786. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]