Abstract
Background
CD4+CD25+ regulatory T cells can inhibit excessive T-cell responses in vivo. We have previously demonstrated that prophylactic administration of CD4+CD25+ regulatory T cells suppresses the development of acute allergen-induced airway inflammation in vivo.
Objective
We sought to determine the effect of therapeutic transfer of CD4+CD25+ regulatory T cells on established pulmonary inflammation and the subsequent development of airway remodeling.
Methods
CD4+CD25+ cells were transferred after the onset of allergic inflammation, and airway challenges were continued to induce chronic inflammation and airway remodeling.
Results
Administration of CD4+CD25+ regulatory T cells reduced established lung eosinophilia, TH2 infiltration, and expression of IL-5, IL-13, and TGF-β. Moreover, subsequent mucus hypersecretion and peribronchial collagen deposition were reduced after prolonged challenge. In contrast, transfer of CD4+CD25+ regulatory T cells had no effect on established airway hyperreactivity either 7 days or 4 weeks after transfer.
Conclusions
In this study we demonstrate for the first time that therapeutic transfer of CD4+CD25+ regulatory T cells can resolve features of chronic allergen-induced inflammation and prevent development of airway remodeling.
Keywords: Regulatory T cells, TH2 cells, eosinophils, TGF-β, airway remodeling, allergic inflammation
The CD4+CD25+ regulatory T-cell subset has been found to play a critical role in a number of diseases, including autoimmunity, gastritis, and cancer.1 Recent evidence proposes that the function of CD4+CD25+ regulatory T cells might be impaired in allergic patients,2 and data from mouse models suggest that regulatory T cells might play important roles in the development of allergen-induced airway inflammation.3-5
In several disease models transfer of CD4+CD25+ regulatory T cells has been found not only to prevent disease but also to reverse established disease.6,7 From a therapeutic perspective, this is of utmost importance, but the potential of CD4+CD25+ regulatory T cells to reverse existing allergen-induced airway inflammation has not yet been addressed. Because asthmatic patients present with established disease, it is important that transfer of CD4+CD25+ regulatory T cells should be able to affect lung inflammation therapeutically to be considered a target for asthma treatment. In addition to the inflammatory changes that occur after allergen challenge, chronic asthma is characterized by airway epithelial damage, goblet cell hyperplasia resulting in mucus hypersecretion, increased airway smooth muscle mass, increased airway vascularity, and deposition of extracellular matrix proteins, such as collagen, fibronectin, and tenascin.8 Current asthma therapies, at best, have only a partial effect on airway remodeling.9 Previous studies have shown that prolonged allergen challenge in sensitized mice can reproduce many of the features of asthmatic airway remodeling.10,11
Using an established protocol of chronic allergen-induced inflammation and remodeling,10,11 we administered allergen-specific CD4+CD25+ regulatory T cells therapeutically after the establishment of acute inflammation and examined their effect on inflammation and the development of airway remodeling. We show for the first time that transfer of CD4+CD25+ regulatory T cells can reduce existing inflammation and prevent subsequent development of airway remodeling. We also assessed the ability of CD4+CD25+ regulatory T cells to reverse existing airway remodeling but found that transfer of these regulatory cells was unable to resolve the structural changes present in the lung. Although these results strengthen the concept that maximizing the activity of regulatory T cells will be of benefit in suppressing allergic inflammation, they also question their therapeutic potential as an asthma therapy during established chronic disease because they might not be able to reverse airway remodeling.
METHODS
Mice
Female BALB/c mice were purchased from Harlan (Indianapolis, Ind). DO11.10 mice were kept in a breeding colony at the Imperial College animal facility. UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986 were strictly observed.
Isolation of CD4+CD25+ regulatory T cells
Ovalbumin (OVA)–specific CD4+CD25+ cells were isolated from the spleens of DO11.10 mice by using a CD4+CD25+ regulatory T-cell isolation kit according to the manufacturer’s protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). Purity was assessed by means of flow cytometric analysis (as described below) and was typically greater than 90%. We and others have previously shown that CD4+CD25+ cells isolated in this manner express FoxP3 (data not shown)5 and have suppressive activity in vitro and in vivo.3
Induction of allergen-induced airway inflammation and airway remodeling
Chronic airway inflammation and airway remodeling were induced by using an established protocol.10,11 OVA-sensitized mice received either 5 × 105 CD4+CD25+ cells administered intravenously or an equivalent volume of PBS on day 26 for one set of experiments or on day 46 for another set of experiments. Mice were killed for analysis by means of exsanguination after achievement of terminal anesthesia on day 33 or 53. Schematics of these protocols are shown in Fig E1 (available in this article’s Online Repository at www.jacionline.org).
Measurement of airway hyperresponsiveness
Airway hyperresponsiveness (AHR) was measured by means of direct measurement of lung resistance and dynamic compliance in a modified version of previously described methods.3
Cell recovery
Bronchoalveolar lavage (BAL) was performed, and cells were counted and quantified, as previously described.3 Leukocyte numbers were assessed in lung tissue digest, as previously described.3
Lung tissue histopathology
Lungs were fixed in 10% normal buffered formalin. Paraffin-embedded sections (4 μm) were stained with hematoxylin and eosin, periodic acid–Schiff, or Sirius red.
Assessment of mucus production
Goblet cells were counted on periodic acid–Schiff–stained sections by using an arbitrary scoring system, as previously described.12 Goblet cells in the airway epithelium were quantified according to a scoring system as follows: 0, less than 0.5%; 1, 5% to 25%; 2, 25% to 50%; 3, 50% to 75%; and 4, greater than 75%. The sum of all airway scores was divided by the number of airways examined (typically 10-30 per section). This value was expressed as mucus index in arbitrary units.
Assessment of peribronchiolar collagen deposition
Peribronchiolar collagen deposition was quantified on Sirius red–stained sections viewed under polarized light with the Scion Image analysis software package (Scion Corp, Frederick, Md) in a method adapted from the literature.13 Digital photographs of 4 intact bronchioles per lung section were taken at 340 magnification. Bronchioles were selected randomly by a blinded reviewer. These images were converted into monochrome, and 10 measurements of 20 μm in length perpendicular to the basement membrane were taken for each bronchiole. The mean density of collagen staining was calculated as follows: (pixels/μm2).
Staining of leukocytes for flow cytometric analysis
Antibodies for mouse CD4, mouse T1/ST2, and mouse KJ1-26 were purchased from PharMingen (San Diego, Calif), Morwell Diagnostics (Zurich, Switzerland), and Caltag (Carlsbad, Calif). Lung digest cells were stained and analyzed, as previously described.3
Measurement of IgE levels
Levels of total IgE were measured in serum by means of ELISA with paired antibodies, according to the manufacturer’s instructions (PharMingen). Levels of anti-OVA IgE were measured in sera by means of ELISA, as described previously.14
Cytokine and collagen analysis
Cytokines were analyzed in BAL fluid samples and lung tissue homogenate supernatants. Lung tissue was homogenized at 50 mg/mL in HBSS (Invitrogen, Carlsbad, Calif) containing protease inhibitors (Roche Diagnostics, Mannheim, Germany) and centrifuged, and the supernatant was collected. Paired antibodies for murine IFN-γ and TGF-β (PharMingen), IL-5 (Endogen), and CCL22/monocyte-derived chemokine (MDC), CCL17/thymus and activation-regulated chemokine (TARC), and CCL1/T-cell activator 3 (TCA-3) (R&D Systems, Minneapolis, Minn) were used in standardized sandwich ELISAs, according to the manufacturer’s protocol. ELISA kits to measure IL-13 (R&D Systems) and IL-10 (eBioscience, San Diego, Calif) levels were used according to the manufacturer’s protocol. Total lung collagen was quantified in lung homogenate supernatant by using a Sircoll kit (Biocolor, Carrickfergus, United Kingdom).
Data analysis
Data are expressed as means ± SEMs, with 8 to 25 mice per group from 2 separate experiments (unless stated). Statistical significance between OVA-sensitized mice that received PBS instead of regulatory T cells was tested by using a Mann-Whitney U test. A P value of less than .05 was considered significant. Graph generation and statistical analysis was performed by using Prism v4.00 software (GraphPad, La Jolla, Calif).
RESULTS
Transfer of CD4+CD25+ regulatory T cells reverses existing allergen-induced inflammation
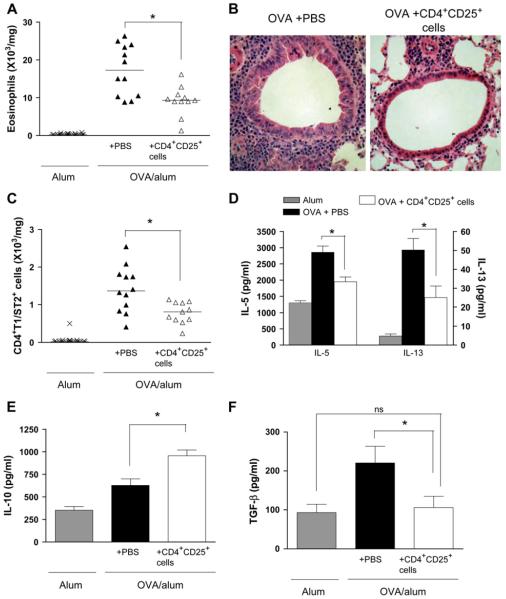
We set out to determine whether therapeutic transfer of allergen-specific CD4+CD25+ regulatory T cells could reverse existing inflammation and AHR. OVA-sensitized and challenged mice received either CD4+CD25+ regulatory T cells or an equivalent volume of PBS on day 26. In this model this represents the peak of acute inflammation before the onset of airway remodeling (see Fig E2 available in this article’s Online Repository at www.jacionline.org).10 Mice were then killed after further OVA challenge 7 days later on day 33 (therapeutic inflammation protocol; see Fig E1, A). We found that eosinophil numbers in both the airway lumen (BAL) and lung parenchyma were significantly decreased after therapeutic administration of CD4+CD25+ regulatory T cells (Fig 1, A and B, and see Fig E3, A, available in this article’s Online Repository at www.jacionline.org).
FIG 1.
Therapeutic administration of CD4+CD25+ regulatory T cells reverses allergen-induced eosinophilic airway inflammation and the lung TH2 response. Lung eosinophils (A), lung inflammation (B; hematoxylin and eosin-stained sections), TH2 (CD4+T1/ST2+) cells (C), IL-5 and IL-13 levels (D), and IL-10 (E) and TGF-β1 levels (F) in lung tissue were measured at day 33 (therapeutic inflammation protocol).
We have demonstrated previously that prophylactic transfer of CD4+CD25+ regulatory T cells can reduce the TH2 response in the lung.3 To determine whether CD4+CD25+ regulatory T cell transfer could reduce an established TH2 response, we measured numbers of TH2 cells in the lung tissue and found them to be significantly reduced after therapeutic administration of CD4+CD25+ regulatory T cells (Fig 1, C). Concomitantly, expression of the TH2 cytokines IL-5 and IL-13 in the lung (Fig 1, D) and BAL fluid (see Fig E3, B) was decreased, although levels of the TH1 cytokine IFN-γ were found to be unaffected by transfer of CD4+CD25+ regulatory T cells (data not shown).
Suppression of airway inflammation by prophylactic transfer of CD4+CD25+ regulatory T cells during acute allergen challenge occurs through induction of IL-10.3 Similarly, in this setting IL-10 expression was increased in both BAL fluid and the lungs of OVA-sensitized mice that received CD4+CD25+ regulatory T cells (Fig 1, E, and see Fig E3, C).
TGF-β is a cytokine that has been shown to have regulatory activity in some settings but that might also contribute to the development of airway remodeling.15-17 Although TGF-β levels in the BAL fluid were unaffected, TGF-β expression in the lung tissue was reduced almost to control levels by means of therapeutic transfer of CD4+CD25+ regulatory T cells (Fig 1, F, and see Fig E3, D).
Increased IgE levels are thought to be important in the development of an allergic response. We measured serum levels of IgE and OVA-specific IgE but found them to be unaffected by transfer of CD4+CD25+ regulatory T cells (see Fig E4 available in this article’s Online Repository at www.jacionline.org).
Administration of CD4+CD25+ regulatory T cells after induction of acute inflammation reduces subsequent development of airway remodeling during chronic challenge
Airway remodeling is a feature of chronic asthma. At day 26, when the CD4+CD25+ regulatory T cells were transferred, extensive inflammation is present, but airway remodeling has not yet developed (see Fig E2). To determine whether therapeutic transfer of CD4+CD25+ regulatory T cells would affect development of airway remodeling, we continued allergen challenge for a further 4 weeks after transfer (airway remodeling protocol; see Fig E1, B).
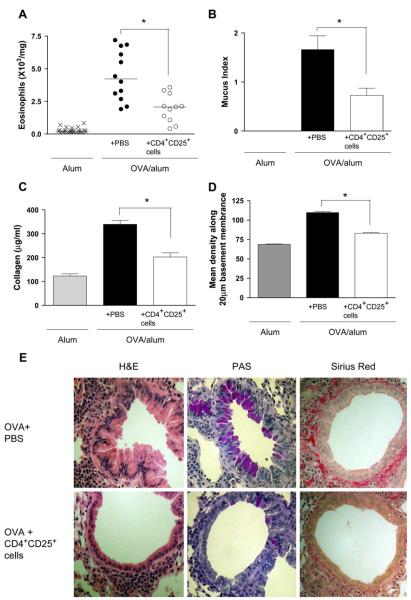
Using this protocol, we observed that transfer of CD4+CD25+ regulatory T cells at day 26 significantly decreased inflammation at day 53 (Fig 2, A and E). Excess mucus secretion was also found to be significantly reduced after administration of CD4+CD25+ regulatory T cells on day 26 (Fig 2, B and E).
FIG 2.
Therapeutic administration of CD4+CD25+ regulatory T cells prevents the development of airway remodeling after prolonged allergen challenge. Lung eosinophils (A), mucus production (B), total lung collagen (C), and peribronchial collagen deposition (D) were assessed at day 53 after chronic challenge (airway remodeling protocol). E, Representative lung sections stained with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), and Sirius red for each treatment group are shown.
Another hallmark feature of airway remodeling is increased matrix deposition, and therefore we quantified collagen deposition within the lung. Analysis of total lung collagen revealed that transfer of CD4+CD25+ regulatory T cells significantly reduced this value at day 53 (Fig 2, C). In addition, peribronchial collagen deposition was also decreased at day 53 after transfer of CD4+CD25+ regulatory T cells at day 26 (Fig 2, D and E). Thus therapeutic transfer of CD4+CD25+ regulatory T cells to mice with established allergic inflammation resulted in downregulation of lung inflammation and prevention of multiple parameters of remodeling.
Decreased airway inflammation and airway remodeling after transfer of CD4+CD25+ regulatory T cells is not associated with diminished AHR
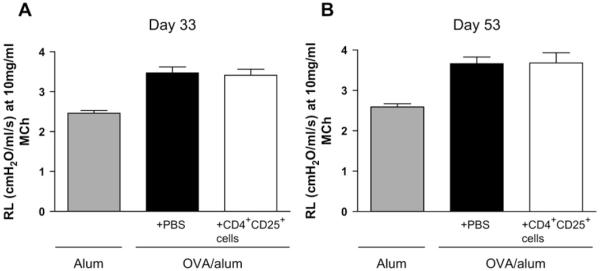
We previously showed that transfer of CD4+CD25+ T cells to sensitized mice before allergen challenge could prevent development of AHR.3 However, transfer of CD4+CD25+ T cells after establishment of airway inflammation by means of inhaled challenge was not able to reduce AHR, either 7 days after transfer, when airway inflammation was decreased, or at 4 weeks after transfer, when airway remodeling was prevented (Fig 3, A and B, and see Fig E5 available in this article’s Online Repository at www.jacionline.org).
FIG 3.
Therapeutic transfer of CD4+CD25+ regulatory T cells has no effect on established AHR. Cells were administered on day 26, and mice were killed on either day 33 (A; therapeutic inflammation protocol) or day 53 (B; airway remodeling protocol). AHR was measured and expressed as mean lung resistance (RL), as summarized for the 10 mg/mL methacholine dose.
Transfer of CD4+CD25+ regulatory T cells during the chronic phase had no effect on existing allergen-induced inflammation, AHR, or airway remodeling
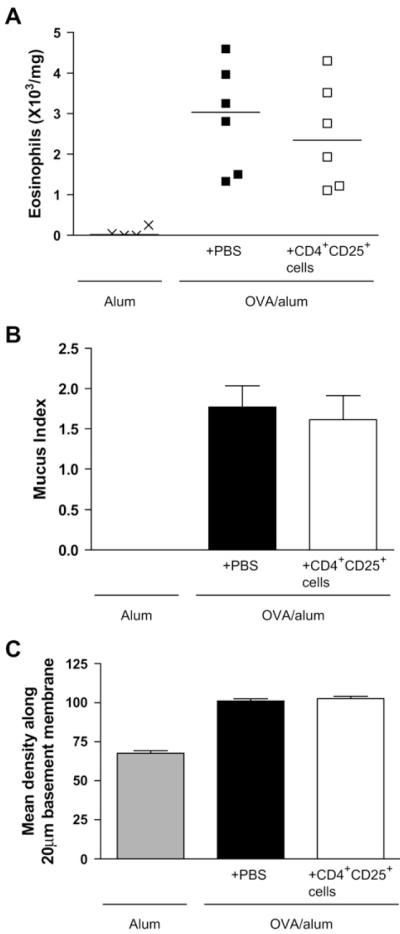
Because we had demonstrated that therapeutic administration of CD4+CD25+ regulatory T cells during acute inflammation (day 26) could reverse existing inflammation and prevent airway remodeling, we sought to determine whether transfer of these regulatory cells can also reverse established remodeling (established remodeling protocol; see Fig E1, C). To this end, we transferred CD4+CD25+ regulatory T cells on day 46, when remodeling changes are well established in the lungs with sustained eosinophilic inflammation (see Fig E2).10 However, CD4+CD25+ regulatory T cells transferred at day 46 had no effect on any parameter measured (Fig 4, A-C, and see Fig E6 available in this article’s Online Repository at www.jacionline.org).
FIG 4.
Transfer of CD4+CD25+ regulatory T cells during established remodeling (day 46) has no effect on allergen-induced airway inflammation or remodeling. Lung eosinophils (A), mucus production (B), and peribronchial collagen deposition (C) were quantified at day 53 after transfer of regulatory T cells by using the established remodeling protocol (n = 4-6 mice per group).
CD4+CD25+ regulatory T cells might not be recruited to the lung after transfer during the chronic phase because of decreasing chemokine levels
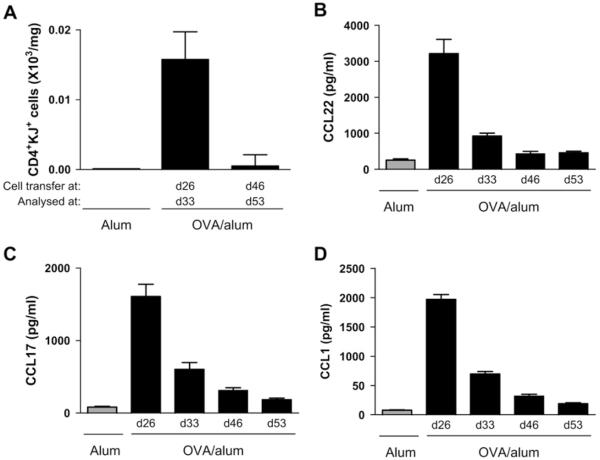
Because CD4+CD25+ regulatory T-cell transfer at day 46 had no effect on airway inflammation or remodeling, we determined whether there were differences in numbers of transferred cells found in the lung tissue at this time point compared with transfer at day 26. We transferred CD4+CD25+ regulatory T cells at day 26 and analyzed numbers of cells in the lung at day 33 and compared these with CD4+CD25+ regulatory T cells administered at day 46 and analyzed the lung at day 53 (therapeutic protocol A vs established remodeling protocol C; see Fig E1). Interestingly, we were able to detect transferred CD4+CD25+ regulatory T cells in the lung at day 33 after transfer at the peak of inflammation at day 26 but not after administration on day 46 (Fig 5, A). It has previously been demonstrated that human CD4+CD25+ regulatory T cells express CCR4 and CCR8 and migrate to these chemokine receptor ligands in vitro.18 We therefore determined expression of CCL22/MDC, CCL17/TARC, and CCL1/TCA-3 in the lung during the chronic model. CCL22, CCL17, and CCL1 levels were all highest at day 26 after acute challenge and had decreased considerably by days 33, 46, and 53 (Fig 5, B-D). It is therefore possible that CD4+CD25+ regulatory T cells can be recruited and retained in the lung at early time points in this model, such as transfer at day 26 or before acute challenge, as shown by our previous study,3 but at later time points, such as day 46, the levels of CCL22, CCL17, and CCL1 are not sufficient to allow recruitment or retention of cells within the lung.
FIG 5.
Transferred CD4+CD25+ regulatory T cells might not be recruited to the lung at later time points because of decreasing levels of proinflammatory chemokines. Transferred CD4+CD25+ regulatory T cells (A) were detected in lung tissue digest by expression of CD4 and the DO11.10 T-cell receptor. CCL22/MDC (B), CCL17/TARC (C), and CCL1/TCA-3 (D) levels were measured in lung tissue.
DISCUSSION
We have demonstrated for the first time that therapeutic transfer of allergen-specific CD4+CD25+ regulatory T cells is effective inresolving established inflammation and preventing the development of airway remodeling. However, CD4+CD25+ regulatory T-cell transfer late in a chronic challenge model had no effect on established inflammation and remodeling.
The ability of CD4+CD25+ regulatory T cells to reverse established airway inflammation and prevent development of airway remodeling is in keeping with the ability of these cells to reverse established inflammation in models of other diseases, such as colitis.6 The increased expression of IL-10 supports our previous data, as well as data from other studies, suggesting an important in vivo suppressor role for IL-10.3,5,19,20 In contrast, there was no increase in TGF-β levels in the airway after transfer of CD4+CD25+ regulatory T cells, suggesting that regulation was not through this cytokine in this setting. Instead, expression of soluble TGF-β in tissues was significantly decreased, which might in part explain inhibition of the development of airway remodeling.16,17
Prophylactic administration of CD4+CD25+ regulatory T cells inhibits development of AHR.3 However, we now demonstrate that therapeutic transfer of these regulatory cells is unable to reverse established AHR, despite suppressive effects on airway eosinophilia and remodeling. Although one feature of the model is that chronic inflammation and AHR wane over time despite establishment of airway remodeling, both inflammation and AHR remain significantly increased during prolonged challenge. IL-13 is considered to be a critical mediator of AHR12,21; however, here we show that although IL-13 expression was decreased after administration of CD4+CD25+ regulatory T cells, AHR was not affected. A previous study has shown that although IL-13 is needed for the initial development of AHR, it is not required for its maintenance, and this might account for the lack of correlation between IL-13 expression and AHR seen here.22 Our findings also suggest that inflammation and AHR can be uncoupled and concur with previous studies that demonstrate that effects on inflammation are not always predictive of changes in AHR.11,17,23 Importantly, this might also be true of human asthma, in which it has been shown that anti-IL-5 antibody treatment reduced peripheral blood and lung eosinophilia and changes of airway remodeling but did not affect AHR.13,24 There are also indications that changes in lung function might even predate the onset of inflammation and remodeling, as shown in a recent biopsy study of wheezy infants.25 Other studies have also found that remodeling and AHR might not necessarily be predictive of each other. Indeed, a recent study has shown that although anti-TGF-β reduces airway remodeling in a mouse model, it actually increases AHR.17 If CD4+CD25+ regulatory T cells prevent airway remodeling through decreased TGF-β expression, as our data suggest, this might explain the lack of effect on AHR. This uncoupling of AHR and remodeling can also occur in asthmatic patients, where at least one study has shown a negative correlation between airway reactivity and airway wall thickness.26 The determinants of AHR in asthma remain controversial but might include factors other than, or in addition to, airway inflammation and remodeling. Recent data from human studies suggest that eosinophilic inflammation might predict asthma exacerbations, although remodeling can lead to diminished airway function over time.27,28 The effect of therapy to alter CD4+CD25+ cell function on these features in patients would be of great interest.
The reduction in TGF-β expression in lung tissue after transfer of CD4+CD25+ T cells might be responsible for the decrease in airway remodeling seen later at day 53. In addition to having antiinflammatory actions, TGF-β has been shown to be an important profibrotic mediator.16,17,29 Because TGF-β can be produced by eosinophils,30 transfer of CD4+CD25+ regulatory T cells can result in a reduction in TGF-β levels caused by decreasing lung eosinophilia. It has also been shown that IL-13 can induce TGF-β–mediated fibrosis.31 Transferred CD4+CD25+ regulatory T cells reduced IL-13 expression in the lung, and thus this might also result in decreased TGF-β levels. There might be beneficial effects on remodeling when the cells are transferred at day 26 because they reduce the degree of inflammation at day 33, decreasing expression of TGF-β in the lung and having subsequent downstream effects on remodeling at day 53.
Transfer of CD4+CD25+ regulatory T cells at day 46 had no effect on established airway remodeling changes, and this might be due to a lack of selective recruitment of these cells to the lung. It has previously been shown that CD4+CD25+ regulatory T cells express CCR4 and CCR8.18,32 Because we observed dramatic decreases in levels of CCL22/MDC, CCL17/TARC, and CCL1/TCA-3 as the chronic challenge phase of the model progresses, this suggests that the CD4+CD25+ regulatory T cells transferred at day 46 during chronic allergen challenge might not remain in the lung because of the absence of gradients of these chemokines. This could represent a mechanism for recruiting regulatory T cells specifically to sites of inflammation and is in keeping with our previous study showing that CD4+CD25+ regulatory T cells were not recruited to the lung in unsensitized mice in which there is no induction of proinflammatory chemokines.3 The amount of inflammation in the lung at day 46 is much lower than at day 26 and therefore might not trigger recruitment of CD4+CD25+ regulatory T cells (see Fig E6). This suggests that there could be a threshold of inflammation necessary for the recruitment of CD4+CD25+ regulatory T cells in vivo, which might be an important mechanism by which excessive immune responses are suppressed, although appropriate responses might still occur. The precise signals by which CD4+CD25+ T cells migrate to sites of inflammation in vivo are not yet established, and this identifies an important avenue of investigation to maximize their use as a potential therapy.
We attempted to address whether transfer of regulatory T cells could reverse established remodeling changes by transferring cells late during disease. However, although we could not detect any effect on remodeling when the cells were transferred at day 46, this might be because the transferred regulatory cells were not recruited to the lung. It is therefore difficult to comment on whether CD4+CD25+ regulatory T cells can reverse established remodeling. In chronic human asthma remodeling of the airway is accompanied by persistent eosinophilic inflammation together with increased expression of CCL1, CCL17, and CCL228 so that there might be potential for recruitment of CD4+CD25+ T cells to the airways in human asthmatic subjects, which is not accurately reflected in the chronic challenge model in mice.
The data presented here strengthen the concept of regulatory T cells as suppressors of allergic inflammation in vivo. In support of this, one study has shown that the in vitro derivation of IL-10–producing regulatory T cells induced by culture in dexamethasone and vitamin D3 can be extrapolated to the treatment of human asthma.33 We have shown for the first time that therapeutic administration of allergen-specific CD4+CD25+ regulatory T cells can indeed reverse many of the features of chronic allergen-induced airway inflammation and suggest that this might occur through IL-10. Importantly, CD4+CD25+ regulatory T cells also prevent the development of airway remodeling, and our data suggest that this might occur through an early decrease in lung expression of the profibrotic cytokine TGF-β. In contrast, CD4+CD25+ regulatory T cells did not change established remodeling, although this might be due to the lack of recruitment of the transferred cells to the lung tissue. Together, this study implies that enhancing the activity of CD4+CD25+ regulatory T cells might be of therapeutic benefit in suppressing established inflammation and might decrease airway remodeling changes through a reduction in TGF-β levels. Further studies are necessary to develop strategies that enhance the specific activity of CD4+CD25+ regulatory T cells within the lung during chronic allergic disease.
Supplementary Material
Key messages.
Therapeutic administration of CD4+CD25+ regulatory T cells during existing allergic airways disease reverses inflammation.
CD4+CD25+ regulatory T cells prevent the development of airway remodeling.
Acknowledgments
Supported by the Wellcome Trust (reference no. 057704). D.S.R. was supported by aWellcome Trust Research Leave Award for Clinical Academics, and C.M.L. was supported by a Wellcome Senior Fellowship in Basic Biomedical Sciences.
We thank Lorraine Lawrence for histologic sectioning and staining, Dr Harsha Kariyawasam for critical reading of the manuscript, and Dr Kasia Hawrylowicz for helpful discussion.
Abbreviations used
- AHR
Airway hyperresponsiveness
- BAL
Bronchoalveolar lavage
- MDC
Monocyte-derived chemokine
- OVA
Ovalbumin
- TARC
Thymus and activation-regulated chemokine
- TCA-3
T-cell activator 3
Footnotes
Disclosure of potential conflict of interest: J. Kearley is employed by MedImmune, Inc. D. S. Robinson has consulting arrangements with MedImmune and Leti and is on the speakers’ bureau for GlaxoSmithKline. C. M. Lloyd has declared that she has no conflict of interest.
REFERENCES
- 1.Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116:949–59. doi: 10.1016/j.jaci.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman PM, Arbery J, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 3.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–47. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–61. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joetham A, Takada K, Taube C, Miyahara N, Matsubara S, Koya T, et al. Naturally occurring lung CD4+CD25+ T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J Immunol. 2007;178:1433–42. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 6.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 7.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–21. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Jeffery PK, Busse WW, Johnson M, Asthma Vignola AM. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–45. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 9.Ward C, Walters H. Airway wall remodelling: the influence of corticosteroids. Curr Opin Allergy Clin Immunol. 2005;5:43–8. doi: 10.1097/00130832-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 10.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy. 2004;34:497–507. doi: 10.1111/j.1365-2222.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna E, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–9. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 12.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd CM, Gonzalo JA, Nguyen T, Delaney T, Tian J, Oettgen H, et al. Resolution of bronchial hyperresponsiveness and pulmonary inflammation is associated with IL-3 and tissue leukocyte apoptosis. J Immunol. 2001;166:2033–40. doi: 10.4049/jimmunol.166.3.2033. [DOI] [PubMed] [Google Scholar]
- 15.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J Immunol. 2005;174:5774–80. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 17.Alcorn JF, Rinaldi LM, Jaffe EF, van Loon M, Bates JHT, Janssen-Heininger YMW, et al. Transforming growth factor-beta1 suppresses airway hyperresponsiveness in allergic airway disease. Am J Respir Crit Care Med. 2007;176:974–82. doi: 10.1164/rccm.200702-334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J Exp Med. 2001;194:847–54. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of auto-immune gastritis and colitis by CD4+CD25+T cells. J Autoimmun. 2001;16:115–23. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 21.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 22.Leigh R, Ellis R, Wattie J, Donaldson DD, Inman MD. Is interleukin-13 critical in maintaining airway hyperresponsiveness in allergen-challenged mice? Am J Respir Crit Care Med. 2004;170:851–6. doi: 10.1164/rccm.200311-1488OC. [DOI] [PubMed] [Google Scholar]
- 23.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–17. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leckie MJ, Brinke, Khan J, Diamant Z, O’Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–8. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 25.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171:722–7. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 26.Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med. 2003;168:983–8. doi: 10.1164/rccm.200211-1268OC. [DOI] [PubMed] [Google Scholar]
- 27.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 28.Bumbacea D, Campbell D, Nguyen L, Carr D, Barnes PJ, Robinson D, et al. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur Respir J. 2004;24:122–8. doi: 10.1183/09031936.04.00077803. [DOI] [PubMed] [Google Scholar]
- 29.Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-β and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol. 2005;175:5390–5. doi: 10.4049/jimmunol.175.8.5390. [DOI] [PubMed] [Google Scholar]
- 30.Munitz A, Levi-Schaffer F. Eosinophils: “new” roles for “old” cells. Allergy. 2004;59:268–75. doi: 10.1111/j.1398-9995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta-1. J Exp Med. 2001;194:809–22. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soler D, Chapman TR, Poisson LR, Wang L, Cote-Sierra J, Ryan M, et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP31 regulatory and Th2 effector lymphocytes. J Immunol. 2006;177:6940–51. doi: 10.4049/jimmunol.177.10.6940. [DOI] [PubMed] [Google Scholar]
- 33.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2005;116:146–55. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.