Abstract
The recent development of new gold(I) catalysis methodologies has opened the door to new disconnections for the total synthesis of bioactive complex molecules. Below is described the application of a gold(I)-catalyzed hydroarylation of an allene with indole toward the total synthesis of flinderoles B–C, members of a new class of antimalarial bisindole alkaloids isolated from plants of the Flindersia genus. The key gold(I) step establishes both the pyrrolidine and isobutenyl functionalities unique to these compounds. Other important steps of the synthesis include a convergent Horner–Wadsworth–Emmons olefination to construct the bridging alkene and a new strategy for α-indole enolate alkylations.
Introduction
Malaria presents a significant disease burden in the developing world, causing one in five childhood deaths as well as decreasing economic growth in countries with high rates of transmission.1 As such, malaria prevention and treatment are an integral part of alleviating poverty worldwide. The Plasmodium parasites which cause this disease have begun exhibiting multi-drug resistance; thus, the majority of these drugs are now dosed as combination therapies, leading to greater treatment costs and lower patient compliance.2 Because of this emerging resistance, there is great demand for antimalarial therapeutics that act orthogonally to existing drugs.
Given the historical precedent of using natural products as antimalarial drugs, the scientific community has again turned to nature in search for new therapies. In the process of such a screening campaign, novel bisindole alkaloid flinderole A (1), isolated from the Papua New Guinean plant Flindersia acuminata, and related molecules flinderoles B (2) and C (3) from F. ambionensis, were identified as having antimalarial activity. These compounds demonstrated selective growth inhibition against Dd2 (choroquine-resistant) P. falciparum malaria strain with IC50 values between 0.15–1.42 μM.3
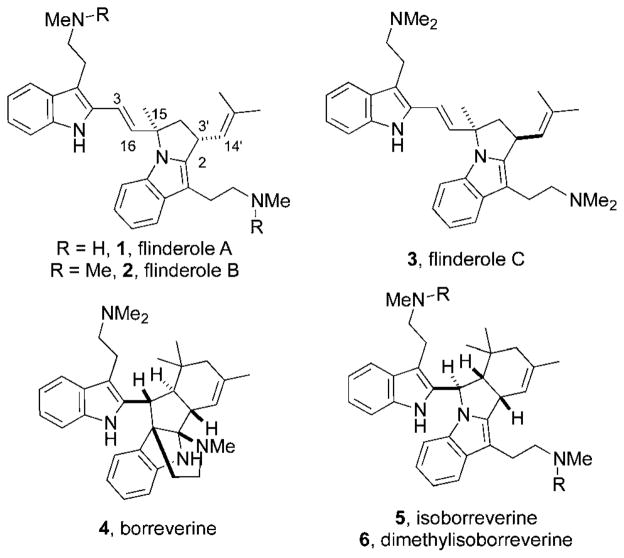
We saw the flinderoles as an attractive target for total synthesis not only for their impressive biological activity but also because of their structural novelty. The flinderoles are distinguished from the isomerically related borreverines (4–6, Fig. 1)4 by three unique features: the C-3/C-16 trans-disubstituted olefin linking the two tryptamine subunits, the pyrrolidine ring featuring an isobutenyl side-chain on the eastern portion of the molecule, and the methyl quarternary center at C-15.5 We believed that the key flinderole functionalities could all be set by gold(I)-catalyzed hydroarylation of an allene electrophile with the C-2 position of indole. Total synthesis of flinderole through this type of disconnection serves as a platform to showcase our understanding of allene reactivity in gold(I) catalysis.6
Fig. 1.
Flinderoles A–C (1–3) are structurally distinct from the related bisindole alkaloids borreverine (4) and the isoborreverines (5–6).
Retrosynthetic strategy
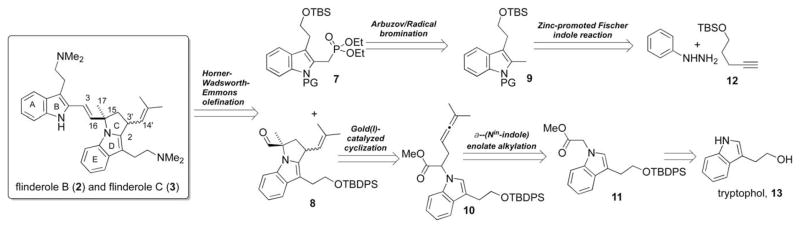
We designed a convergent retrosynthetic strategy to best take advantage of this disconnection. We planned to arrive at the natural products 2 and 3 through Horner–Wadsworth–Emmons olefination of phosphonate 7 and aldehyde 8, followed by deprotection and functional group manipulation to afford the N-dimethyltryptamine side chain. Desired phosphonate 7 arises from radical bromination and Arbuzov reaction of protected 2-methyltryptophol 9. The 2,3-disubstitutued indole is assembled through zinc-promoted hydrohydrazination/Fischer indole cascade of protected pentynol 12.7 The pyrrolidine 8 is formed through gold(I)-catalyzed cyclization of indole-allene 10 followed by enolate alkylation to install the necessary quarternary center at C-15. Indole-allene substrate 10 is constructed through two sequential alkylation steps: Nα-alkylation to introduce the ester, forming 11, followed by α-Nin-indole enolate alkylation to introduce the allene (Fig. 2).We believe that the convergent nature of this synthesis allows for rapid assembly of this complex molecule as well as lending easy access to a broad array of flinderole analogs.
Fig. 2.
Retrosynthetic strategy toward flinderoles B(2) and C (3).
In the course of our investigation, Dethe and coworkers reported the biomimetic total synthesis of flinderoles B and C using a Lewis-acid catalyzed [3 + 2] reaction to assemble the pyrrolidine C ring.8 In their synthesis, they introduce the amine side-chains by reductive amination, a possibility that we had also considered. Given their success with this tactic, we also planned to employ a reductive amination to complete the total synthesis of these two compounds.
Synthesis of aldehyde 8
By far the more complicated portion of flinderole, the eastern fragment 8 posed two key problems: installation of the allene moiety and diastereoselective formation of the quarternary center at C-15.
Preparation of indole-allene 10
We built our strategy toward the substrate for the key gold(I) step around enolate alkylation of an Nin-indole acetate. From a retrosynthetic standpoint, we took this approach for two reasons: a) it provides a handle for later installation of the C-17 methyl group at C-15, and b) the ester could later be transformed in to the aldehyde necessary for the Horner–Wadsworth–Emmons olefination.
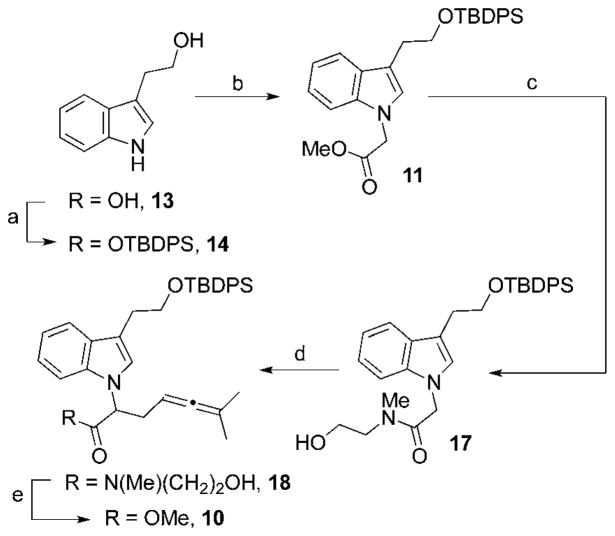
We elected to start by protecting commercially available tryptophol9 (13) as the tert-butyldiphenylsilyl (TBDPS) ether (Scheme 1); the TBDPS group is less prone to hydrolysis by alkoxide bases than other commonly used silyl ethers. N-alkylation with methyl bromoacetate in the presence of potassium tert-butoxide afforded a 3 : 1 mixture of N-indole acetate 11 and starting material, giving the desired product in 50% yield (72% BORSM). The product distribution obtained proved invulnerable to changes in base, solvent or temperature.10
Scheme 1.
(a) TBDPSCl, Et3N, DMAP, CH2Cl2, 18 h, rt; 89%. (b) KOt-Bu, 0 °C, 1h; methyl bromoacetate, rt, 18 h; 50% (72% BORSM). (c) N-methylaminoethanol, NaOMe, THF, rt, 2 h; 73%. (d) LDA, LiCl, THF, −78 °C, 1 h; 0 °C, 15 min; rt, 5 min; Me2C=C=CHCH2Br (15), 0 °C, 2 h; 93%. (e) dimethyl carbonate, NaOMe, CH2Cl2, rt, 18 h; 96%.
Initial attempts at alkylating the lithium enolate of 11 with allenyl bromide 15 led to low yields and a complex mixture of products. Further analysis of the products formed indicated that this compound can react through two competing pathways: attack by C-15 to afford desired indole-allene 10, and elimination to yield the carboxylic acid upon aqueous workup. We hypothesized that the undesired pathway arose due to the destabilizing influence of the α-indole nitrogen. We felt that we could bias the reaction toward the alkylation pathway by changing the alkoxy leaving group such that elimination was disfavored. The successful use of doubly anionic amide enolates for making unnatural amino acids inspired us to try alkylation of an N-methylamidoethanol functionality; to test this hypothesis, we prepared N-methyl-N-ethanolamide 17.11 Gratifyingly, alkylation of this compound using LDA and LiCl gave the desired allene 18 in 92% yield. The amide auxiliary is readily removed using an excess of dimethyl carbonate and sodium methoxide to give methyl ester 10 in 95% yield.12
Gold(I)-catalyzed hydroarylation of allene with indole
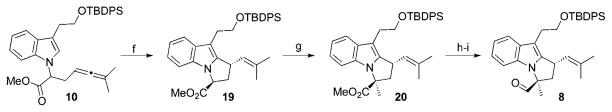
Initial attempts using 5 mol% triphenylphosphinegold(I) as the metal catalyst failed to elicit cyclization of allene 10. By moving to more electropositive N-heterocycliccarbene catalyst IPrAuSbF6, we were able to obtain the desired pyrrolidine 19 as a single diastereomer in 91% yield (Scheme 2).While we found that we could still see reactivity with a catalyst loading of 2 mol%, we obtained substantially less desired product (81% yield) and also observed unwanted diene side products. Due to the decreased yield with lower catalyst loading, we opted to use 5 mol% catalyst loading in this step.
Scheme 2.
(f) 5 mol% IPrAuCl, 5 mol% AgSbF6, DCE, 45 °C; 88%. (g) LiHMDS, THF, −78 °C, 1 h; MeI, 1 h; 94% (2 : 1 dr). (h) DIBAL-H, toluene, − 78 °C, 6 h; 90%. (i) SO3·pyr, Et3N, 1 : 1 DMSO/CH2Cl2, 0 °C, 1 h; 75% (2 : 1 dr).
With the pyrrolidine established, we were poised to introduce the C-17 methyl group. A base screen revealed that the enolate of 19 exhibited the same type of ambident reactivity as was observed in alkylation of indole acetate 11. Fortunately, selectivity was achieved by using LiHMDS, generating solely the desired methylated compound 20 in 94% yield as a 2 : 1 mixture of diastereomers 20 and 3′-epi-20. While modest facial selectivity was observed in the methylation reaction, both isomers were en route to natural products: 20 gives flinderoles A and B, whereas 3′-epi-20 leads to flinderole C. Reduction of the methyl ester with LiAlH4 and subsequent Parikh–Doering oxidation yielded aldehyde 8 in 68% yield over two steps.
Total synthesis of flinderoles B and C
Our strategy toward the flinderoles hinges on uniting the two flinderole fragments by a Horner–Wadsworth–Emmons olefination, requiring synthesis of a tryptamine derivative possessing a benzylic phosphonate at the C-2 position. Successful formation of the bisindole adduct would allow us to intercept to the Dethe intermediate and finish the total synthesis.
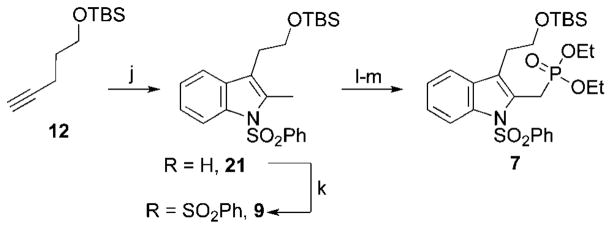
Exposing phenylhydrazine and TBS-protected pentyn-4-ol 12 to an excess of zinc(II) chloride in THF at 105 °C gave desired TBS-protected 2-methyltryptophol 21 in 76% yield in a modification of the conditions reported by Beller (Scheme 3).7 Phenylsulfonamide protection of the Nin-position occured cleanly using phenylsulfonyl chloride and potassium hydroxide to yield 9. Performing a radical bromination/Arbuzov sequence on 2-methyltryptophol 9 afforded phosphonate 7 in 77% yield.
Scheme 3.
(j) phenylhydrazine, 12, ZnCl2, THF, 105 °C, 18 h; 76%. (k) PhSO2Cl (3.0 equiv), KOH (5.0 equiv), THF, 0 °C to rt, 18 h; 68%. (l) NBS, AIBN, CCl4, 75 °C, 18 h. (m) P(OEt)3 55 °C, 48 h; 77%.
Horner–Wadsworth–Emmons olefination: formation of the bisindole adducts
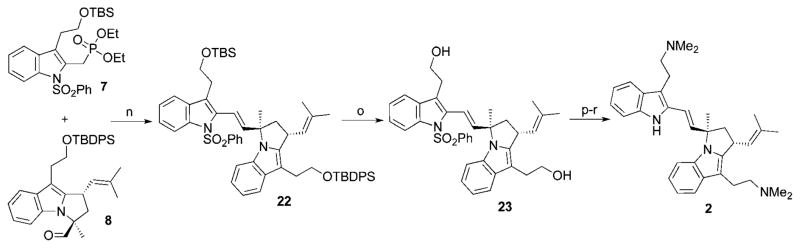
Olefination reactions of 2-(methylindole)phosphonates with aromatic aldehydes have been reported by Srinivasan and coworkers; the authors use sodium hydride as the base, and require elevated temperature and long reaction times to obtain full conversion to the alkene product.13 Reaction of phenyl-sulfonamide 7 and aldehydes 8 and 3′-epi-8 using the Srinivasan conditions gave desired olefin products 22 and 3′-epi–22 in modest yields (36% and 33%, respectively).
Cleavage of the silyl ethers using TBAF allowed us access to alcohols 23 and 3′-epi-23. Following the sequence reported by Dethe and coworkers,8 we were able to access the natural products flinderole B (2) and flinderole C (3) in 62% and 66% yields, respectively (Scheme 4).
Scheme 4.
(n) 7, 8, NaH, THF, 0 °C, 1 h; 22: 36%, 3′-epi-22: 33%. (o) TBAF, THF, rt, 7h; 23: 79%, 3′epi-23: 74%. (p) IBX, EtOAc, 80 °C, 1 h. (q) Me2NH, NaCNBH3, AcOH/MeOH, rt, 18 h; 68%, 3′-epi: 73%. (r) Na/Hg, Na2HPO4, MeOH, rt, 3h; 2: 95%; 3: 91%.
Conclusions
The total synthesis of antimalarial bisindole alkaloids flinderole B (2) and C (3) was successfully completed with 18 steps (14 longest linear sequence) in 4% overall yield from the commercially available tryptophol 13.14 The three unique structural features of the flinderole molecular scaffold—the pyrrolidine C ring, the C-3′ isobutenyl functionality, and the unsaturation at C-14′—are formed through a gold(I)-catalyzed intramolecular hydroarylation of a pendant allene. The application of gold(I)-catalyzed indole hydroarylation reactions to the synthesis of related bisindole natural products is ongoing and shall be reported in due course.
Supplementary Material
Acknowledgments
We thank Stephen T. Heller for help with synthesis strategy and Hunter P. Shunatona for providing essential material. We gratefully acknowledge NIHGMS (RO1 GM073932-05) for financial support and Johnson-Matthey for a generous gift of AuCl3.
Footnotes
Electronic supplementary information (ESI) available: Data for new compounds and experimental procedures. See DOI: 10.1039/c1sc00290b
Notes and references
- 1.(a) Sachs J. Nature. 2002;415:680. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]; (b) Ioset JR. Curr Org Chem. 2008;12:643. [Google Scholar]
- 2.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Lancet Infect Dis. 2002;2:209. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez LS, Buchanan MS, Carroll AR, Feng YJ, Quinn RJ, Avery VM. Org Lett. 2009;11:329. doi: 10.1021/ol802506n. [DOI] [PubMed] [Google Scholar]
- 4.(a) Pousset JL, Cavé A, Chiaroni A, Riche C. J Chem Soc, Chem Commun. 1977:261. [Google Scholar]; (b) Tillequin F, Koch M, Pousset JL, Cavé A. J Chem Soc, Chem Commun. 1978:826. [Google Scholar]
- 5.Raputindoles A-D, bisindole alkaloids which feature a similar trans-olefin linkage and pyrrolidine functionalization as the flinderoles, were discovered subsequently. See: Vougogiannopoulou K, Fokialakis N, Aligiannis N, Cantrell C, Skaltsounis AL. Org Lett. 2010;12:1908. doi: 10.1021/ol100584w.
- 6.For other examples of gold(I)-catalyzed hydroarylations of allenes, see (with pyrrole): Liu Z, Wasmuth AS, Nelson SG. J Am Chem Soc. 2006;128:10352. doi: 10.1021/ja0629110.(with indole): Liu C, Widenhoefer RA. Org Lett. 2007;9:1935. doi: 10.1021/ol070483c.(with electron-rich benzene derivatives): Tarselli MA, Gagné MR. J Org Chem. 2008;73:2439. doi: 10.1021/jo7024948.For review see: Krause N, Winter C. Chem Rev. 2011;111:1994. doi: 10.1021/cr1004088.Shapiro ND, Toste FD. Synlett. 2010:675. doi: 10.1055/s-0029-1219369.
- 7.Alex K, Tillack A, Schwarz N, Beller M. Angew Chem, Int Ed. 2008;47:2304. doi: 10.1002/anie.200703823. [DOI] [PubMed] [Google Scholar]
- 8.Dethe DH, Erande RD, Ranjan A. J Am Chem Soc. 2011;133:2864. doi: 10.1021/ja1116974. [DOI] [PubMed] [Google Scholar]
- 9.Tryptophol is also readily prepared by reduction of 3-indoleacetic acid with LiAlH4. See: Nystrom RF, Brown WG. J Am Chem Soc. 1947;69:1197.
- 10.A similar product distribution was observed in the synthesis of apicidin-derived quinolone derivatives: Meinke PT, Colletti SL, Doss G, Myers RW, Gurnett AM, Dulski PM, Darkin-Rattray SJ, Allocco JJ, Galuska S, Schmatz DM, Wyvratt MJ, Fisher SH. J Med Chem. 2000;43:4919. doi: 10.1021/jm0001976.
- 11.(a) Myers AG, Yang BH, Chen H, Gleason JL. J Am Chem Soc. 1994;116:9361. [Google Scholar]; (b) Seebach D. Angew Chem, Int Ed Engl. 1988;27:1624. [Google Scholar]
- 12.Etxebarria J, Vicario JL, Badia D, Carrillo L. J Org Chem. 2004;69:2588. doi: 10.1021/jo0357768. [DOI] [PubMed] [Google Scholar]
- 13.(a) Kannadasan S, Srinivasan PC. Tetrahedron Lett. 2002;43:3149. [Google Scholar]; (b) Mohan B, Nagarathnam D, Vedachalam M, Srinivasan PC. Synthesis. 1985;2:188. [Google Scholar]; (c) Nagarathnam D, Srinivasan PC. Synthesis. 1982;11:926. [Google Scholar]
- 14.For additional examples of total synthesis using gold catalysis from our laboratory, see: Staben ST, Kennedy-Smith JJ, Huang D, Corkey BK, Lalonde RL, Toste FD. Angew Chem, Int Ed. 2006;45:5991. doi: 10.1002/anie.200602035.Linghu X, Kennedy-Smith JJ, Toste FD. Angew Chem, Int Ed. 2007;46:7671. doi: 10.1002/anie.200702695.Sethofer SG, Staben ST, Hung OY, Toste FD. Org Lett. 2008;10:4315. doi: 10.1021/ol801760w.Gonzalez AZ, Benitez D, Tkatchouk E, Goddard WA, III, Toste FD. J Am Chem Soc. 2011;133:5500. doi: 10.1021/ja200084a.For a review see: Hashmi ASK, Rudolph M. Chem Soc Rev. 2008;37:1766. doi: 10.1039/b615629k.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.