Abstract
Many believe that the ability to understand the actions of others is made possible by mirror neurons and a network of brain areas known as the action-observation network (AON). Despite nearly two decades of research into mirror neurons and the AON, however, there is little evidence that they enable the inference of the intention of observed actions. Instead, theories of action selection during action execution indicate that a ventral pathway, linking middle temporal gyrus with the anterior inferior frontal gyrus, might encode these abstract features during action observation. Here I propose that action understanding requires more than merely the AON, and might be achieved through interactions between a ventral pathway and the dorsal AON.
Mirror neurons and the action-observation network
Mirror neurons are a class of neuron that was first discovered in the premotor area F5 of the macaque monkey [1]. Subsequent studies have confirmed the presence of mirror neurons in F5 [2-6] and also demonstrated them to be present in a region of the inferior parietal lobule: area PF [7,8]. The defining property of mirror neurons is that they discharge not only when the monkey executes a reach and grasp action but also when the monkey observes a similar action performed by the experimenter. A number of neuroimaging studies have provided evidence that mirror neurons might also exist in humans and that homologous areas in the human brain are activated when observing and executing movements [9-14].
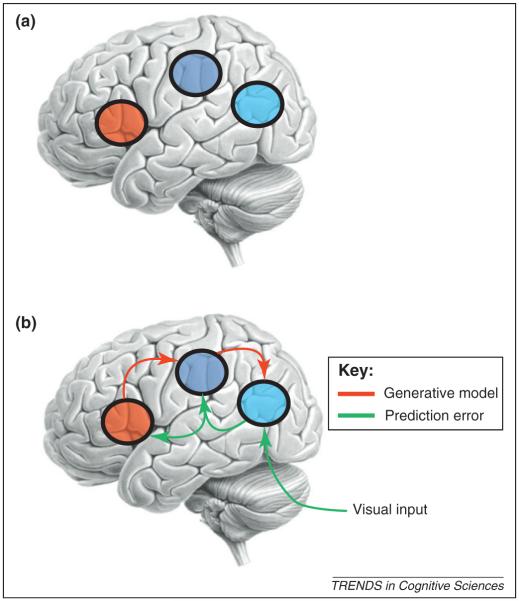
Cortical areas that have been shown to contain mirror neurons are often described as part of an action-observation network (AON) that is generally considered to consist of three bilateral cortical areas that are reciprocally connected: the ventral premotor cortex, inferior parietal lobule and superior temporal sulcus (STS) ([15], Figure 1a). Because some of the areas of the AON contain mirror neurons [16] this network is sometimes referred to as the mirror neuron system.
Figure 1.
The human AON. (a) This schematic shows the three reciprocally connected areas of the human AON. The areas known to contain mirror neurons are the ventral IFG, shown in red, and the inferior parietal area, shown in green. These two areas are reciprocally connected [50] creating a premotor-parietal mirror system. Neurons within the STS, shown in blue, have also been shown to respond selectively to biological movements, both in monkeys [51] and in humans [52-54]. The STS is reciprocally connected to the inferior parietal area [55,56] and therefore provides visual input to the mirror system. (b) This schematic shows the predictive coding model of the AON. Predictive coding is based on minimising prediction error though recurrent or reciprocal interactions among levels of a cortical hierarchy. In the predictive coding framework, each level of a cortical hierarchy employs a generative model to predict representations in the level below. This generative model uses backward connections to convey the prediction to the lower level where it is compared to the representation in this subordinate level to produce a prediction error. This prediction error is then sent back to the higher level, via forward connections, to adjust the neuronal representation of sensory causes, which in turn changes the prediction.
Ever since their discovery, it has been proposed that mirror neurons and the AON underlie our ability to understand actions ‘transforming visual information into knowledge’ ([16], p. 172; see also [17]). However, despite nearly two decades of research there is little empirical support for this proposed role of mirror neurons in action understanding and this has led some to speculate that mirror neurons might not even have any functional role in observed action understanding [18]. One source of confusion when considering any potential functional role of the AON is what is meant by action understanding. Actions can be described at multiple levels and therefore there are multiple levels at which an observed action can be ‘understood’. For simplicity, in this article I will consider that actions can be described at four levels only: (i) the kinematic level: the trajectory and the velocity profile of the action, including both the reach and grasp phase of a goal directed action; (ii) the motor level: the processing and pattern of muscle activity required to produce the kinematics; (iii) the goal level: the immediate purpose of the action, for example to grasp an object and (iv) the intention level: the overall reason for executing the action. These levels are clearly nonindependent and can be organised hierarchically, with the kinematics being dependent on the motor level, the motor level being dependent on the goal level and the goal level being dependent on the intention level. As one moves up the hierarchy, the action is described in more and more abstract terms. Here I will propose that the dorsal AON is unlikely to encode to the more abstract levels of understanding such as the intention and the goal of the action. Instead, the ability to understand an action at these abstract levels is more likely to be dependent on the interaction between the AON and a ventral pathway linking middle temporal gyrus (MTG) with the anterior inferior frontal gyrus (IFG).
How do mirror neurons and the AON enable action understanding?
Virtually all accounts of the role of mirror neurons in action understanding assume that this process occurs exclusively within the three reciprocally connected areas of the AON. Initial accounts proposed that visual information was transformed as it was passed by forward connections from visual areas in the temporal lobe, via inferior parietal areas, until mirror neurons in the premotor area F5 were activated [17]. The idea was that, because we know what our intention is when we activate our F5 neurons during action execution, when the same neurons are active during action observation, we can infer the intention of the observed action. However, there are a number of problems with this model. The first is that for this model to be able to infer the goal or intention of an observed action from observing the kinematics of that action there would have to be a one-to-one mapping between the goal and the kinematics. This is not the case because the same goal can be achieved with many actions and, more problematically, the same action can be used for many different goals and intentions [19-21]. A second problem is that patients with damage to regions of BA44/BA6 are still able to infer the goal and intention of an observed action (see [18]). If regions of BA44/BA6 encode the goal and intention of the action then one would predict that in patients with damage to these areas there would be a deficit in their ability to infer the goal and intention of an observed action [18]. The fact that regions believed to contain mirror neurons are not essential for inferring the goal or intention of an observed action suggests that either mirror neurons do not encode the goal/intention of an observed action or that they do not do so uniquely.
It has previously been argued that the first of these problems can be resolved if we consider that mirror neurons discharge during action observation not because they are driven by the visual input but because they are part of a generative model that is predicting the sensory input ([20,21]; see Figure 1b). In this predictive coding model, the motor system is active when observing an action because it is the best model of the observed action. Within this framework, the generative model starts with a prior prediction of the goal or intention of an observed action. Given this prior the AON generates a prediction of what the sensory consequences would be of the most likely action that would be needed to be executed to achieve that goal or intention: the kinematics of the action. By comparing the predicted sensory information with the actual sensory information the system can assess the likelihood of the prior goal or intention. If the prediction is correct we are able to infer the goal and intention of the observed action. Several recent studies have now found evidence in favour of this type of recognition model during action observation [22-25]. One problem with the predictive coding model is that it requires a prior expectation about the goal and the intention of the observed action. So although predictive coding can resolve the one-to-many mapping problem it creates a new problem: where and how are the goal and intention priors generated? In this article I will argue that the goals and intentions of an observed action are encoded in a network different from the AON, one that recently has been proposed to be involved in the process of action control during action execution [26].
Two-pathway model of action understanding
Recent theories of action control in action execution propose that the ventral IFG is organised along its rostralcaudal axis to represent the different levels of abstraction of an action with the most anterior regions (BA47) encoding the most abstract semantic representations (see Glossary) and the most posterior regions (BA44/BA6) encoding the more concrete representations [26] (red line, Figure 2).
Glossary.
Abstract representation of an action: the levels at which the action can be described where there is no one-to-one mapping between the description and the actual action performed. These could be the goal and intention levels of an action. For example, the goal of picking up a cup can be achieved with many different actions.
Concrete representation of an action: the unique description of one component of an action, where there is a one-to-one mapping between the description and the action. For example, the description of a particular type of grip.
Figure 2.
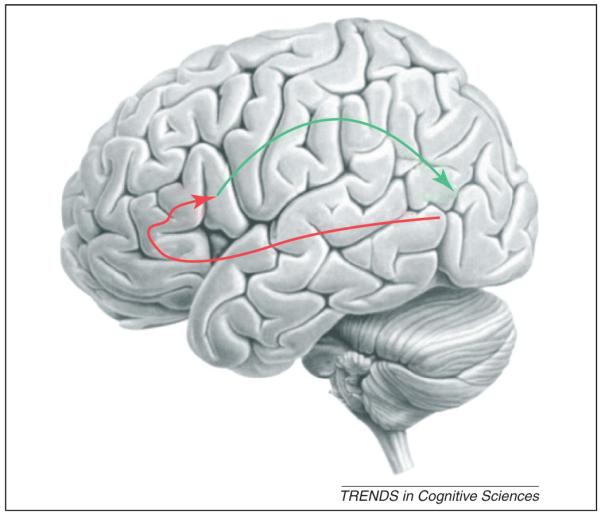
A schematic of the two-pathway framework. In this schematic the ventral pathway of the connected areas MTG, BA47, BA45 and BA44/BA6 is shown in red and the dorsal AON pathway is shown in green.
If we consider, for example, the actions involved in drinking a cup of tea (Figure 3), the overall intention would be to drink a cup of tea. To achieve this we would have to reach and grasp the teacup. Although there are many ways we could reach and grasp the cup, some of these actions would be incompatible with the overall intention of the action: to drink a cup of tea. Therefore, in order to be able to achieve this overall intention we have to select the most appropriate reach and grasp from all possible reaches and grasps to the cup.
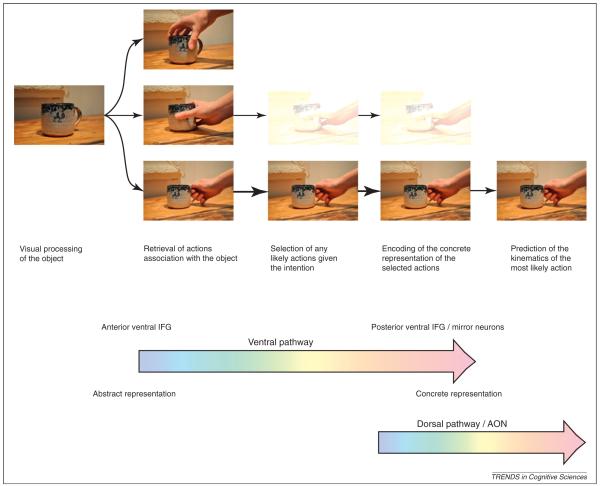
Figure 3.
An example of action understanding in the two-pathway framework. In this example the intention is to drink a cup of tea. The first step is visual processing and identification of the object as a cup. The second step is the retrieval of actions that we have learned to be associated with that object. The third step is the selection of the most probable actions given the intention. Note here that more than one action can be selected but that the likelihood of the action can be signalled through the strength of that action’s representation, indicated here by the transparency of the picture. The top action is not probable and is not selected. The fourth step is the encoding of the motor parameters to generate a prediction of the sensory consequences of the observed action. Again multiple actions can be encoded as before. The fifth step is the prediction of the sensory consequences of the most probable action. Here only the most probable action is encoded. In this schematic, steps 2–4 would be encoded in the ventral pathway of the connected areas MTG, BA47, BA45 and BA44/BA6 with the representation of the action changing from the abstract to the concrete through these steps. Steps 4–5 would represent the generation of the predicted sensory consequences of the action encoded in the dorsal AON pathway.
It has been proposed that these processes of retrieval and selection might functionally dissociate in the IFG. The process of retrieving actions that are semantically related to the object has been associated with regions of the anterior IFG (BA47), as well as with the connected regions of the MTG [26-29]. The process of selection of the most appropriate action occurs in the medial regions of the IFG (BA45 and anterior regions of BA44) [26,27,30]. Once the most appropriate action is selected, the motor parameters required to enact that action are encoded in the most posterior parts of the IFG (BA44/BA6) [26]. In this way, there is a gradient of the representation of the action from the semantic level through the goal level to the concrete level along the rostral–caudal axis of the IFG, forming a ventral pathway from the MTG to the posterior parts of the IFG via the anterior regions of the IFG.
It has previously been suggested that a similar functional dissociation along the rostro–caudal axis of the ventral IFG might exist for observed actions [31-33]. In this model the properties of the observed action that pertain to the more abstract levels of an observed action, such as the goal and intention, would reside in the anterior portion of the IFG (BA45, BA47 as well as MTG) and the properties of the observed action that encode the more concrete, motor representations would be in the posterior portion [31-33]. Mirror neurons in the posterior IFG (area F5c in monkeys and BA44/BA6 in humans) would encode the concrete representations of observed actions and not the more abstract goals and intentions of the action. The proposal here is that the prior prediction of the intention and goal of an observed action required in the predictive coding model is generated through the ventral pathway. Once the most probable goal is estimated, then a prediction of the sensory consequences of this action can be generated by a more dorsal pathway through the classical areas of the AON, as has already been described [20,21] (Figure 2). In this framework, the most probable prediction of the intention of the observed action would be estimated from the context in which the action was observed in areas outside of the AON [34,35]. This prior prediction of the intention will impact on the process of action selection within the IFG.
Of note is that a similar gradient has also been proposed in more dorsal regions of the prefrontal cortex [26]. It has been proposed that this pathway could encode simple associations between a stimulus and a motor act with these associations represented in the dorsal premotor cortex [36,37]. This is of interest because several studies investigating whether mirror neurons are a result of learned visual motor associations have found effects in dorsal as well as ventral premotor cortex [38,39]. One possibility is that simple automatic association might be encoded differently from more complex associations.
Differences in spatial scale of activations in humans and monkey
The two-pathway model of action understanding proposed above can explain some of the anomalies of the human neuroimaging work. Although human neuroimaging studies have provided evidence that similar cortical areas are activated during action observation as those areas in macaque monkeys reported to contain mirror neurons [9-14], closer inspection reveals that there is a large difference in the spatial scale of activations reported in humans compared with the macaque monkey. The macaque monkey area F5 has been shown to be subdivided into at least three cytoarchitecturally different regions: F5a, F5p and F5c [40,41]. Neurons in each of these subdivisions are activated during observation and execution of actions but mirror neurons have been demonstrated predominantly in area F5c [17,41]. By contrast, human neuroimaging studies have reported activations throughout the IFG including BA45, BA44, ventral BA6 (see [42,43]) and even dorsal BA6 [38,44]. Such activations are commonly interpreted as reflecting mirror neuron activity [38,42-44]. Such a vast difference in spatial scale can only have two explanations: (i) mirror neurons in humans are more widespread than in the macaque or (ii) the blood-oxygenation-level-dependent (BOLD) activations do not reflect mirror neuron activity but neural activity correlated with the observation of an action.
In line with this second explanation, it has recently been argued that the fact that a volume of cortex in IFG has an increased BOLD signal during observation and execution of an action does not necessarily mean that the same neurons are active in both conditions [42,45]. These authors proposed that the best approach to attribute the functional magnetic resonance imaging (fMRI) response to a single neuronal population is fMRI adaptation, or repetition suppression (Box 1). The logic of this approach is that as stimuli that evoke activity in a specific neuronal population are repeated, the magnitude of the BOLD response decreases or adapts [42,45,46]. Areas of the cortex that contain mirror neurons should show adaptation both when an action is executed and subsequently observed and when an action is observed and subsequently executed. Using such an fMRI adaptation paradigm, a recent study showed significant effects in human IFG that are consistent with the presence of mirror neurons [47]. Interestingly, these adaptation effects were not observed throughout the IFG but only in the most posterior part at the border of BA44 and BA6. This is consistent with the dissociation of abstract and concrete representations of the observed action along the rostral–caudal axis of the IFG. Whereas one would predict that there should be regions active throughout the IFG, mirror neurons encoding the concrete representations should be found only in the most posterior regions (Box 2).
Box 1. fMRI adaptation and mirror neurons.
fMRI adaptation, or repetition suppression, is a neuroimaging tool that has been adopted to identify neuronal populations that encode a particular stimulus feature [46]. Dinstein and colleagues [42] argued that areas of the cortex that contain mirror neurons should show fMRI adaptation both when an action is executed and subsequently observed and when an action is observed and subsequently executed, because mirror neurons are neurons that discharge both to the observation and execution of the same action. In other words, the stimulus feature encoded in mirror neurons is repeated irrespective of whether the action is observed or executed. However, the results of such studies have produced mixed results. Of the four studies published to date using this technique, only one has demonstrated significant fMRI adaptation consistent with the presence of human mirror neurons [47]. These authors argued that the reason that they found an effect whereas the others did not [42,57,58] could be explained by the fact that their task was a goal-directed action and not a pantomimed action. It has previously been shown that mirror neurons in the macaque monkey only respond to goal-directed actions [4]. However, it should be noted that there is no empirical evidence to date demonstrating that the firing pattern of mirror neurons is modulated in any way by the repetition of the observation of the same action. This has led some to question the validity of using the fMRI adaptation paradigm as a tool to isolate mirror neurons in humans [48].
Box 2. Questions for future research.
Is the IFG functionally dissociable, with abstract features of the observed action represented in more anterior regions and concrete features represented in the posterior regions?
Are mirror neurons and the AON involved in the concrete representations of the action that lead to a prediction of the sensory consequences of that action?
Are inferences based on low-level features, such as the kinematics, dissociable from inferences based on high-level features, such as intentions and goals?
What is the functional interaction of mirror neurons in posterior IFG and inferior parietal lobule?
Are the time courses of the representations of the goals and kinematics different? If the goals are represented hierarchically, as predicted, they should be represented for longer.
What is the interaction between ventral parts of the IFG proposed to be involved in cognitive retrieval and more dorsal parts such as the dorsal premotor cortex that have been shown to be more active in simple learned associations?
The role of mirror neurons in the two-pathway model
One consequence of this two-pathway framework is that it requires that mirror neurons do not encode the semantic representations of the action associated with the abstract goals and intentions, but rather encode the concrete representations of the action. Since their discovery, it has been proposed that the properties of mirror neurons in area F5 of the macaque monkey are consistent with these neurons encoding the ‘goal’ of an observed action [16,17,48]. The reason that mirror neurons are thought to encode these more abstract features of the observed action was initially driven by the observation that in area F5 there was a subclass of broadly congruent mirror neurons that discharged during action execution when a particular action was performed only with the hand but discharged during action observation when the action was performed with either the hand or the mouth. The authors argued that these mirror neurons showed activity ‘if the end-goal was the same’ [2], regardless of how it was achieved. In the two-pathway framework proposed here such a result could be explained by parallel action selection [49]. In other words during action observation multiple possible actions are selected and processed but only one is represented more frequently or strongly than the others (Figure 3). This is precisely what was reported in [2]. For any one observed action, the largest population of mirror neurons encoded this particular action.
Concluding remarks
When we observe actions performed by others we are able to infer the goal and intentions of the observed action. Ever since the discovery of mirror neurons, it has been proposed that our ability to understand actions at these levels is made possible by these neurons and the cortical regions of the AON. However, there is little empirical support for this proposed function. I have argued, instead, that the ability to understand actions at these abstract levels is encoded in the MTG and the more anterior regions of the IFG in a ventral pathway (Figure 2, red arrow). This ventral pathway predicts the most probable intentions and goals of the observed actions through a process of semantic retrieval and selection resulting in the encoding of the representation of the most probable action required to achieve the most probable goal. In this two-pathway model it is this concrete representation of the action that is encoded by mirror neurons and that acts as a prior to predict the sensory consequences of this action through the dorsal AON pathway (Figure 2, green arrow).
Acknowledgements
This work was funded by the Wellcome Trust, UK.
References
- 1.Di Pellegrino G, et al. Understanding motor events: a neurophysiological study. Exp. Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 2.Gallese V, et al. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 3.Rizzolatti G, et al. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 4.Umilta MA, et al. I know what you are doing. A neurophsyiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- 5.Kraskov A, et al. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression? Neuron. 2009;64:922–930. doi: 10.1016/j.neuron.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caggiano V, et al. View-based encoding of actions in mirror neurons of area f5 in macaque premotor cortex. Curr. Biol. 2011;21:144–148. doi: 10.1016/j.cub.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Gallese V, et al. Action representation and the inferior parietal lobule. In: Prinz W, Hommel B, editors. Attention and Performance XIX. Common Mechanisms in Perception and Action. Oxford University Press; 2002. pp. 247–266. [Google Scholar]
- 8.Fogassi L, et al. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 9.Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb. Cortex. 2009;19:1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buccino G, et al. Action observation activates premotor and parietal areas in somatotopic manner: an fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- 11.Decety J, et al. Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain. 1997;120:1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- 12.Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum. Brain Mapp. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. J. Neurosci. 2006;26:1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzolatti G, et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp. Brain Res. 1996;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- 15.Keysers C, Perrett DI. Demystifying social cognition: a Hebbian perspective. Trends Cogn. Sci. 2004;8:501–507. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Gallese V, Goldman A. Mirror neurons and the simulation theory of mind reading. Trends Cogn. Sci. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- 17.Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 18.Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J. Cogn. Neurosci. 2009;21:1229–1243. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob P, Jeannerod M. The motor theory of social cognition: a critique. Trends Cogn. Sci. 2005;9:21–25. doi: 10.1016/j.tics.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Kilner JM, et al. Predictive coding: an account of the mirror neuron system. Cogn. Process. 2007;8:159–166. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilner JM, et al. The mirror-neuron system: a Bayesian perspective. Neuroreport. 2007;18:619–623. doi: 10.1097/WNR.0b013e3281139ed0. [DOI] [PubMed] [Google Scholar]
- 22.Kokal I, Keysers C. Granger causality mapping during joint actions reveals evidence for forward models that could overcome sensory-motor delays. PLoS ONE. 2010;5:e13507. doi: 10.1371/journal.pone.0013507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schippers MB, Keysers C. Mapping the flow of information within the putative mirror neuron system during gesture observation. Neuroimage. 2011;57:37–44. doi: 10.1016/j.neuroimage.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Neal A, Kilner JM. What is simulated in the action observation network when we observe actions? Eur. J. Neurosci. 2010;32:1765–1770. doi: 10.1111/j.1460-9568.2010.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambon V, et al. What are they up to? The role of sensory evidence and prior knowledge in action understanding. PLoS ONE. 2011;6:e17133. doi: 10.1371/journal.pone.0017133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner AD, et al. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- 28.Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binder JR, et al. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson-Schill SL, et al. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeannerod M. The representing brain. Neural correlates of motor intention and imagery. Behav. Brain Sci. 1994;17:187–245. [Google Scholar]
- 32.de Vignemont F, Haggard P. Action observation and execution: what is shared? Soc. Neurosci. 2008;3:421–433. doi: 10.1080/17470910802045109. [DOI] [PubMed] [Google Scholar]
- 33.Hickok G, Hauser M. (Mis)understanding mirror neurons. Curr. Biol. 2010;20:R593–R594. doi: 10.1016/j.cub.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brass M, et al. Investigating action understanding: inferential processes versus action simulation. Curr. Biol. 2007;17:2117–2121. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 35.Kilner JM, Frith CD. Action observation: inferring intentions without mirror neurons. Curr. Biol. 2008;18:R32–R33. doi: 10.1016/j.cub.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Mitz AR, et al. Learning-dependent neuronal activity in the premotor cortex: activity during the acquisition of conditional motor associations. J. Neurosci. 1991;11:1855–1872. doi: 10.1523/JNEUROSCI.11-06-01855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr. Opin. Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Catmur C, et al. Through the looking glass: counter-mirror activation following incompatible sensorimotor learning. Eur. J. Neurosci. 2008;6:1208–1215. doi: 10.1111/j.1460-9568.2008.06419.x. [DOI] [PubMed] [Google Scholar]
- 39.Catmur C, et al. Making mirrors: premotor cortex stimulation enhances mirror and counter-mirror motor facilitation. J. Cogn. Neurosci. 2010 doi: 10.1162/jocn.2010.21590. DOI: 10.1162/jocn.2010.21590. [DOI] [PubMed] [Google Scholar]
- 40.Belmalih A, et al. Cortical connections of the visuomotor parietooccipital area V6Ad of the macaque monkey. J. Comp. Neurol. 2009;512:183–217. doi: 10.1002/cne.21980. [DOI] [PubMed] [Google Scholar]
- 41.Nelissen K, et al. Observing others: multiple action representation in the frontal lobe. Science. 2005;310:332–336. doi: 10.1126/science.1115593. [DOI] [PubMed] [Google Scholar]
- 42.Morin O, Grèzes J. What is “mirror” in the premotor cortex? A review. Neurophysiol. Clin. 2008;38:189–195. doi: 10.1016/j.neucli.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Dinstein I, et al. Brain areas selective for both observed and executed movements. J. Neurophysiol. 2007;98:1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calvo-Merino B, et al. Action observation and acquired motor skills: an FMRI study with expert dancers. Cereb. Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- 45.Dinstein I, et al. A mirror up to nature. Curr. Biol. 2008;18:R13–R18. doi: 10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grill-Spector K, et al. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Kilner JM, et al. Evidence of mirror neurons in human inferior frontal gyrus. J. Neurosci. 2009;29:10153–10159. doi: 10.1523/JNEUROSCI.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat. Rev. Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- 49.Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu. Rev. Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- 50.Luppino G, et al. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp. Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- 51.Oram MW, Perrett DI. Responses of anterior superior temporal polysensory (STPa) neurons to biological motion stimuli. J. Cogn. Neurosci. 1994;6:99–116. doi: 10.1162/jocn.1994.6.2.99. [DOI] [PubMed] [Google Scholar]
- 52.Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- 53.Allison T, et al. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 54.Grossman E, et al. Brain areas involved in perception of biological motion. J. Cogn. Neurosci. 2000;12:711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- 55.Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesusmonkey: a retrograde tracer study. J. Comp. Neurol. 1994;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- 56.Harries MH, Perrett DI. Visual processing of faces in temporal cortex: physiological evidence for a modular organization and possible anatomical correlates. J. Cogn. Neurosci. 1991;3:9–24. doi: 10.1162/jocn.1991.3.1.9. [DOI] [PubMed] [Google Scholar]
- 57.Lingnau A, et al. Asymmetric fMRI adaptation reveals no evidence for mirror neurons in humans. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9925–9930. doi: 10.1073/pnas.0902262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chong TT, et al. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr. Biol. 2008;18:1576–1580. doi: 10.1016/j.cub.2008.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]