Abstract
We explored the neural basis of reversible sentence comprehension in a large group of aphasic patients (N=79). Voxel-based lesion-symptom mapping revealed a significant association between damage in temporoparietal cortex and impaired sentence comprehension. This association remained after we controlled for phonological working memory. We hypothesize that this region plays an important role in the thematic or what-where processing of sentences. In contrast, we detected weak or no association between reversible sentence comprehension and the ventrolateral prefrontal cortex, which includes Broca’s area, even for syntactically complex sentences. This casts doubt on theories that presuppose a critical role for this region in syntactic computations.
Keywords: Broca’s area, Prefrontal, Temporoparietal, Thematic Roles, Syntax
Introduction
Neuroimaging and lesion studies have consistently implicated both anterior and posterior perisylvian areas in sentence comprehension, but the separate functional contributions of these areas still need to be clarified. Sentence comprehension is a complex process involving access to different linguistic representations, the manipulation of these representations in memory, and other possibly non-linguistic, task-sensitive processes such as cognitive control. This complexity leads to difficulty in determining the functional basis of comprehension deficits after brain damage and activation during neuroimaging studies. In this study we attempted to clarify the role of frontal and temporoparietal regions in sentence comprehension using voxel-based lesion symptom mapping (VLSM. Bates et al., 2003) in a large cohort of aphasic patients.
Many neuroimaging studies have found greater activation in ventrolateral prefrontal cortex (VLPFC) for complex sentences compared to their simpler counterparts (Ben-Shachar, Hendler, Kahn, Ben-Bashat, & Grodzinsky, 2003; Caplan, Alpert, & Waters, 1998, 1999; Fiebach, Schlesewsky, Lehmann, Cramon, & Friederici, 2005; Just, Carpenter, Keller, Eddy, & Thulborn, 1996; Stromswold, Caplan, Alpert, & Rauch, 1996). Broadly speaking, this evidence has been interpreted in two different ways. Syntax-oriented theories propose that VLPFC - particularly Brodmann areas 44 and 45 referred to jointly as Broca’s area - supports sentence comprehension via core syntactic computations (e.g., Ben-Shachar et al., 2003; Caplan et al., 1998). As such, VLPFC involvement is necessary for accurate comprehension, especially for more complex sentences. In contrast, resource-oriented theories propose that VLPFC supports sentence comprehension via processes such as working memory and cognitive control (Fiebach et al., 2005; Novick, Trueswell, & Thompson-Schill, 2005). These accounts are consistent with the view that VLPFC plays an indirect role in comprehension via its modulatory function over linguistic representations that reside elsewhere. The need for such modulatory function would depend upon both stimulus and task factors. For example, VLPFC involvement may be most required when a prepotent interpretation must be overridden in favor of a less preferred meaning (Novick et al., 2005). At stake is the accurate characterization of the function of VLPFC, particularly Broca’s area, in language. Here we analyzed the relation between VLPFC damage and comprehension deficits in aphasia to elucidate whether this region plays a necessary role in sentence comprehension.
Posterior perisylvian areas including superior and middle temporal gyri and the inferior parietal lobule are thought to underlie many component processes of sentence comprehension, including storage of semantic representations, verbal working memory and verb argument structure processing (Caplan et al., 2001; Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004; Hickok & Poeppel, 2004; Lau, Phillips, & Poeppel, 2008; Ouden, Fix, Parrish, & Thompson, 2009; Wise, 2003). Here we aimed to identify the specific temporal and parietal areas that are critical for sentence comprehension. Further, we controlled for covariates of sentence comprehension ability such as phonological working memory and asked whether the role of temporoparietal areas in sentence comprehension extends beyond phonological and lexical level processes.
We addressed these questions by performing VLSM analyses on sentence comprehension scores from 79 left-hemisphere stroke patients with aphasia. Sentences were semantically reversible (e.g., The man served the woman) such that accurate comprehension could not be achieved by using semantic knowledge of who typically does what. Such reversible sentences are often used in psycholinguistic and neurolinguistic studies to isolate “syntactic” comprehension processes i.e., the derivation of the correct meaning of a sentence from its syntactic form.
VLSM is a technique used to investigate voxel-wise correlations between lesion status and behavior. A test statistic (e.g., t-value) is computed based on the difference in behavioral score between participants who have damage to a particular voxel and those who do not. Effects across the whole brain are then evaluated for significance after correcting for multiple comparisons. Differences between VLSM and traditional methods of overlaying lesions have been discussed extensively elsewhere (Bates et al., 2003; Kimberg, Coslett & Schwartz, 2007; Rorden & Karnath, 2004). Among other things, VLSM uses inferential and not merely descriptive statistics; it can be applied to continuous (i.e., non-dichotomized) behavioral data from a variety of patients with different behavioral and lesion profiles; and it avoids having to choose pre-determined regions of interest. Of these features, the last is less clearly an advantage for the current study, since previous studies of sentence processing provide strong rationale for focusing on particular anterior and posterior brain regions. With that in mind, and to see whether evidence from regional analyses would converge with the VLSM results, we additionally computed the association between behavioral scores and lesion extent in several a priori Brodmann areas.
Methods
Subjects
We analyzed data from 79 patients recruited from the patient registry at the Moss Rehabilitation Research Institute (Schwartz, Brecher, Whyte, & Klein, 2005). All patients had aphasia following left hemisphere stroke, and met the following criteria: English as primary language, adequate vision and hearing, pre-morbid right handedness, no major psychiatric or neurologic co-morbidities, and a left-hemisphere cortical lesion confirmed via MRI or CT. All patients scored 80% or higher on a lexical comprehension test (see below) allowing us to evaluate variability in syntactic comprehension independent of lexical deficits. The patient group was diverse. On the Western Aphasia Battery (Kertesz, 1982), the Aphasia Quotient range was 38 to 97.6, mean=78.8, and the aphasia subtype breakdown was 34 Anomic, 21 Broca’s, 15 Conduction, 1 Transcortical Motor and 8 “recovered” with aphasia quotient>93.8.
Patients gave written permission to participate under a protocol approved by the Albert Einstein Medical Center Institutional Review Board. Structural images were obtained under a protocol at the University of Pennsylvania School of Medicine.
Sentence Comprehension Behavioral Measure
Sentence comprehension scores were obtained from a 2 alternative forced choice sentence-to-picture-matching task that is part of the Philadelphia Comprehension Battery (Saffran, Schwartz, Linebarger, Martin, & Bochetto, 1998). Patients were tested on 30 sentences, 5 each of actives, actives with prepositional phrases1, passives, locatives, subject relative clauses and object relative clauses.
For each sentence type, the lexical content in the target and distractor pictures was the same. Participants could succeed only by assigning thematic roles in accordance with the syntax. For example, for the sentence “The man serves the woman”, one picture showed a man serving a woman and the other showed a woman serving a man. See Table 1 for example stimuli. Our primary behavioral measure was percent correct averaged across all sentence types (SentComp). We also analyzed percent correct on a subset of sentence types where necessary.
Table 1.
Example stimuli in sentence comprehension test
| Type | Sentence | Target picture | Distractor picture |
|---|---|---|---|
| Active | The girl washes the boy. | girl washing boy | boy washing girl |
| Active- prep | The policeman shoots the robber in the alley. | policeman shooting robber | robber shooting policeman |
| Loc | The plate is under the napkin. | plate under napkin | napkin under plate |
| Passive | The man is served by the woman. | woman serving man | man serving woman |
| Subj-rel | The dog that followed the hunter was alert. | dog following hunter | hunter following dog |
| Obj-rel | The girl that the boy washed was talkative. | boy washing girl | girl washing boy |
Lexical Comprehension Screen
We only included those patients who scored 80% or higher on a lexical version of the sentence comprehension test. These “lexical” trials were interspersed with the critical trials described above and followed the same procedure. For each sentence type, the lexical trials could be answered by paying attention to lexical content alone. For example, for the sentence “The dog chases the boy”, the two accompanying pictures were of a dog chasing a boy (target) and of a dog chasing a rabbit (distractor). By including only patients who passed the lexical screen, we could be confident that the variance in the sentence comprehension scores was due to syntactic and not lexical comprehension.
Other Behavioral Measures
In secondary analyses, we controlled for phonological working memory as measured by rhyme probe span2 and non-word repetition. The rhyme probe span (Rhyme) measures the list length for which participants can correctly judge whether a probe word rhymes with one of the items in the preceding list. Participants listened to a list and an immediately following probe word, and responded “Yes” or “No” (e.g., List: black-more-some, Probe: plum, Response: yes; List: fright-chill-threw; Probe: steep; Response: no). The test starts with list length 1 and advances to the next length if the participant scores at least 75% correct. The maximum score is 9. In the non-word repetition (NWRep) test, participants were asked to repeat single non-words of 1, 2 or 3 syllables (e.g., fos; tayson; dunapour) immediately after hearing them. The score is a simple % correct out of 60 items.
VLSM Analysis
We analyzed structural images acquired using MRI (n=43) or CT (n=36). Details of the imaging, segmentation, and registration procedures have previously been reported (Schwartz et al., 2009). For patients with MRI scans, lesions were first drawn manually on a 1×1×1 mm T1-weighted structural image. Prior to warping, lesions were masked, and the structural scans and lesion maps were registered to a common template constructed from images acquired on the same scanner, using a symmetric diffeomorphic registration algorithm (Avants, Schoenemann & Gee, 2006; see also http://www.picsl.upenn.edu/ANTS/). A single mapping from this intermediate template to the 1 mm MNI-space “Colin27” volume (Holmes et al., 1998) was used to complete the mapping from subject space to MNI space. The final lesion map was quantized to produce a 0/1 map using 0.5 as the cut-off. An experienced neurologist who was blind to the behavioral data verified depictions of the lesions. For patients with CT scans, the same neurologist drew lesion maps directly onto the Colin27 volume.
We excluded voxels in which fewer than five patients had lesions.3 All analyses were done using VoxBo (www.voxbo.org). We computed t-statistics comparing patients with and without lesions in each voxel. The resulting t-map was thresholded to control for false discovery rate (FDR: Genovese, Lazar, & Nichols, 2002) at q=0.01 where q is the expected proportion of false positives among supra-threshold voxels. Although our 0.01 threshold is the most stringent of FDR thresholds used in the literature, it should be borne in mind that the FDR correction is relatively relaxed, in that it controls for expected proportion of false positives amongst suprathreshold voxels and not for likelihood of any false positives, as is the case with Bonferroni correction or other methods for control of the family-wise error rate (Kimberg, et al. 2007).
Regional Analysis
As noted, we supplemented the voxel-based analysis with regional analyses computing the association between behavioral scores and percent damage in 10 a priori Brodmann areas (BA 7, 20, 21, 22, 37, 38, 39, 40, 44 and 45). This list, while non-exhaustive, includes areas frequently implicated in language processing such as those in the inferior frontal region (BA 44, 45), inferior, middle and anterior temporal region (BA 20, 21, 22, 37, 38), the junction of temporal and parietal cortices (BA 39, 40) and the superior parietal lobe (BA 7). These percent damage scores were obtained using a modified left hemisphere-only version of the Brodmann atlas available with MRIcron. Each lesion mask was overlaid on the atlas, and using a feature in VoxBo (www.voxbo.org), the proportion of overlapping voxels between the lesion mask and our region of interest was calculated.
The two methods – VLSM and regional analysis – differ in spatial scale, and have complementary strengths and weaknesses. The regional analysis has reduced power to detect patterns that are only present in a subset of voxels within the region. The advantage that VLSM holds in this regard is one reason why we chose to use it in the main analysis. On the other hand, VLSM requires a much greater number of statistical tests and therefore incurs a steeper correction for multiple comparisons in order to maintain the same overall false positive rate. Therefore, associations with sentence comprehension that are represented among many temporoparietal or frontal voxels but are weak in magnitude could be missed by VLSM but detected by regional analysis. In the ideal case, negative findings obtained with VLSM for temporoparietal or frontal areas would be confirmed by the regional analysis, lending confidence that these were not method-dependent Type-2 errors. Convergence onto the same positive findings would assuage concerns about the false positive rate tolerated by the FDR correction method.
Results
Behavioral Measures
Results from the behavioral tests are shown in Table 2. Sentence comprehension accuracy ranged between 37% and 100% with a mean of 74.8%. Scores grouped by subsets of sentence types showed the expected effects of canonicity and number of propositions. In canonical English structures, the syntactic subject is the agent of the action being described; in non-canonical structures the subject is the patient. Under many linguistic theories, non-canonical structures are derived by the movement of a syntactic constituent from its canonical structural position to a different one (see e.g., Grodzinsky & Santi, 2008). Thus, non-canonical structures are often harder to process than their canonical counterparts by virtue of their being less frequent and/or more syntactically complex. Performance in our task was consistent with this general pattern: accuracy on non-canonical sentence types (passives, object relatives) was significantly lower than that on canonical sentence types (actives, subject relatives. Mean=62.2% vs. 84.6%. F(1,78)=82.2; p<.001)4.
Table 2.
Behavioral scores
| Test/Measure | Mean (SD) | Median | Min | Max |
|---|---|---|---|---|
| SentComp (All) (% correct) | 74.8 (16.5) | 73.3 | 37 | 100 |
| SentComp-Canon (% correct) | 84.6 (18.1) | 90 | 20 | 100 |
| SentComp-NonCanon (% correct) | 62.2 (24.2) | 60 | 10 | 100 |
| SentComp-1P (% correct) | 78.2 (19.8) | 80 | 30 | 100 |
| SentComp-2P (% correct) | 68.5 (21.3) | 70 | 20 | 100 |
| Rhyme (max. list length) | 3.2 (1.7) | 3 | 0.5 | 7.3 |
| NWRep* (% correct) | 53 (25.7) | 53 | 0 | 98 |
Two patients did not complete this test. Statistics are computed from 77 patients.
We also split sentences according to the number of propositions. Two-proposition sentences (subject relatives, object relatives) require maintaining and matching multiple propositions to the corresponding picture and could consequently be harder than one-proposition sentences (actives, passives). Consistent with this idea, accuracy in sentence-to-picture matching was significantly lower for the former compared to the latter (Mean=68.5% vs. 78.2%. F(1,78)=21.7;p<.001).
Anatomical Findings
Comparing Lesions in Frontal and Temporoparietal Areas
The focus of the current study is to evaluate the relationship between reversible sentence comprehension and damage in frontal and temporoparietal areas. In VLSM, the power to detect brain-behavior relationships at a given voxel depends on the number of patients in the lesioned and unlesioned groups. An optimal distribution is half and half (in the current dataset, ~39 lesioned and unlesioned in any voxel). Figure 1 shows the lesion overlap map for all 79 patients. Coverage was good in both regions. The maximum number of lesions in a BA 44/45 voxel was 37; the maximum for BA 22/39/40 was 35. Thus, a priori power to detect a given effect size should be roughly comparable for voxels in the two regions.
Figure 1.
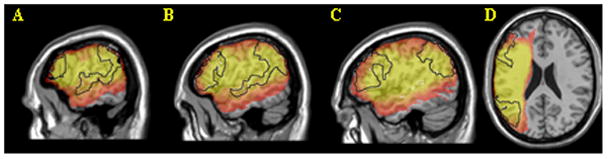
Lesion overlap map for 79 patients. Regions of interest (BA 44/45, BA 22/39/40) are indicated by black outlines. Maps A-C are at MNI x coordinates -60, -54 and -48. Map D is a single axial slice at z=22. Number of lesions in each voxel is rendered in a red (5) to yellow (20 and above) scale.
Overall Sentence Comprehension
First, we explored the voxelwise associations between lesion status and comprehension of all sentence types. We entered SentComp scores into the VLSM analysis. Results showed a large supra-threshold cluster in the temporoparietal region and no suprathreshold voxels in VLPFC (Figure 2. 12661 suprathreshold voxels spanning BA 21, 22, 39 and 40).
Figure 2.
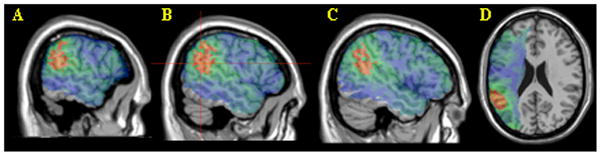
Sentence comprehension scores. Voxels exceeding the FDR threshold (q=0.01) are rendered in a red (t=3.61) to yellow (t>5) scale. Non-significant values are rendered on a green (t just below threshold) to blue (t=0 or below) scale. Maps A-C are at MNI x coordinates -60, -54 and -48. Map D is a single axial slice at z=22. The peak t value of 5.42 was centered on MNI coordinates -54, -55, 22 (3 voxels) which is indicated by crosshairs in panel B.
This finding was corroborated by regional analyses. We explored the regionwise correlations between sentence comprehension scores and percent damage in 10 Brodmann areas (see Methods). In bivariate analyses, SentComp scores correlated significantly with percent damage in BA 7 (r=−.25), 21 (r=−.25), 22 (r=−.37), 39 (r=−.43) and 40 (r=−.41) (all p’s<.05). There was also a marginal correlation with BA 44 (r=−.21, p=.06). Correlations between SentComp and damage in BA 20, 37, 38 and 45 were not significant (p>.1). We entered overall lesion volume and percent damage in those areas that marginally or significantly correlated with the behavioral scores (BA 7, 21, 22, 39, 40, 44) into a simultaneous regression. The overall model was significant (R2=.29, p<.002) with marginally significant independent contributions from percent damage in BA 22 (β=−.45, p=.06) and BA 39 (β=−.26, p=.1) only. All the other predictors, including lesion volume, were not significant (p>.1).
Our results thus far lend support to a statistically significant relationship between temporoparietal regions and sentence comprehension, and offer weak or no support for a similar relationship between frontal regions and sentence comprehension. Additionally, we could ask if there is a meaningful difference between the effects of temporoparietal versus frontal lesions on reversible sentence comprehension. We evaluated this question using a post-hoc regional analysis. We compared the correlations between comprehension accuracy and percent damage in BA 22/39/40 (combined together) and BA 44/45 (combined together). The correlation in the frontal region was −.206, while the correlation in the temporoparietal region was −.495. The difference between these two correlations was significant by the Hotelling-Williams test (tHW = 2.19, p<.05. Steiger, 1980). This suggests a stronger relationship between comprehension accuracy and temporoparietal compared to frontal lesions.
Effect of Canonicity
Broca’s area within VLPFC has been linked in particular to the comprehension of non-canonical sentences. It has been theorized that this might reflect either increased syntactic working memory demands or the specific processing of syntactic movement (Ben-Shachar et al., 2003). To investigate possible differences between canonical and non-canonical sentence comprehension, we performed separate analyses for comprehension scores on actives and subject relatives (canonical) and passives and object relatives (non-canonical). The results are shown in Figure 3. Significant results were found in temporoparietal voxels for both sentence groups (canonical: 3009 suprathreshold voxels in BA 39 and 40. Non-canonical: 10439 suprathreshold voxels in BA 22, 39 and 40). There were no suprathreshold voxels in VLPFC for either sentence group.
Figure 3.
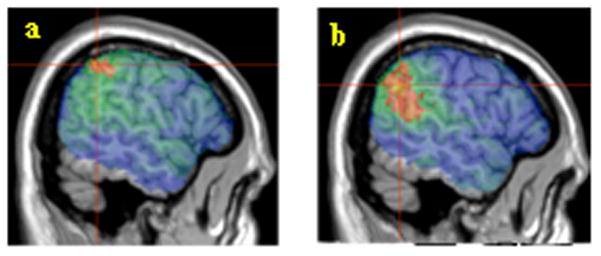
a) Canonical comprehension scores. Voxels exceeding the FDR threshold (q=0.01) are rendered in a red (t=3.96) to yellow (t>5) scale. The peak t value of 5.31 was centered on MNI coordinates -57, -47, 46 (11 voxels) indicated by crosshairs. b) Non-canonical comprehension scores. Voxels exceeding the FDR threshold (q=0.01) are rendered in a red (t=3.66) to yellow (t>5) scale. The peak t value of 5.57 was centered on MNI coordinates -56, -54, 32 (8 voxels) indicated by crosshairs.
In regional analyses, we examined bivariate correlations between canonical and non-canonical comprehension scores and the 10 chosen Brodmann areas. Both canonical and non-canonical scores were significantly correlated with percent damage in BA 7, 22, 39, and 40 (p’s<.05). In addition, there was a marginal correlation between canonical scores and damage in BA 44 (p=.07).
We also analyzed the difference between non-canonical and canonical scores. For the VLSM analysis, we were unable to obtain a threshold for FDR=0.01. In the regional analysis, the difference score was significantly correlated with damage in BA 39 (p<.05) and no other region.
Phonological Working Memory
What functional interpretation should we assign to the temporoparietal effects? Areas near the posterior tip of the Sylvian fissure, at the junction of temporal and parietal cortices, have been hypothesized to underlie phonological working memory and/or sensorimotor integration (Hickok & Poeppel, 2004; Narain et al., 2003). We evaluated whether the role of temporoparietal areas in sentence comprehension extends beyond these functions.
To control for phonological working memory, we entered patients’ rhyme probe spans as covariates in the VLSM analysis of SentComp scores. This analysis yielded suprathreshold voxels in temporoparietal areas only (Figure 4a. 3405 voxels in BA 21, 22, 39 and 40).
Figure 4.
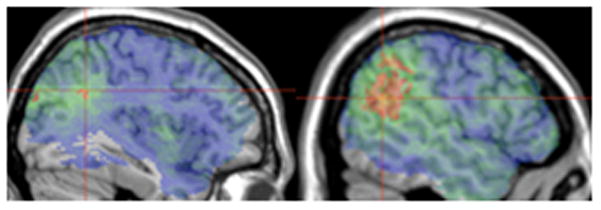
a) Sentence comprehension scores with rhyme probe spans as covariates. Voxels exceeding the FDR threshold (q=0.01) are rendered in a red (t=4) to yellow (t>5) scale. The peak t value of 5.15 was found at MNI coordinates -38, -53, 31 (1 voxel) indicated by crosshairs. b) Sentence comprehension scores with non-word repetition scores as covariates. Voxels exceeding the FDR threshold (q=0.01) are rendered in a red (t=3.62) to yellow (t>5) scale. The peak t value of 5.35 was centered at MNI coordinates -53, -55, 22 (3 voxels) indicated by crosshairs.
Repetition tasks are a widely accepted means of measuring sensorimotor integration. We entered patients’ NWRep scores as covariates in the VLSM analysis of SentComp scores. Two patients did not complete the NWRep task, so this analysis was performed on measures from the remaining 77 patients. Figure 4b shows the suprathreshold voxels, which were in temporoparietal areas only (12170 voxels in BA 21, 22, 39 and 40).
These results suggest that temporoparietal regions play an additional role in sentence comprehension that cannot be reduced to phonological working memory.5 In regional analyses, we entered percent damage in BA 21, 22, 39 and 40 in a simultaneous regression with rhyme probe span. This revealed significant independent contributions from rhyme probe span (β=.4) and BA 39 damage (β=−.28). A similar simultaneous regression with non-word repetition scores replacing rhyme probe span revealed that NWRep was a significant predictor of SentComp scores (β=.26) and BA 39 damage made a marginal independent contribution (β=−.24; p=.07).
Task Demands
Sentence comprehension as measured by the sentence-to-picture matching task involves task-related resources that may be different from core comprehension processes. Participants need to hold the linguistically derived sentence interpretation in memory and compare it to their analyses of the two pictures. The demands of this matching process may be increased when participants have to maintain two propositions as opposed to one proposition in mind. Thus, subject and object relative sentences (e.g., The girl that washed the boy was talkative), which contain two propositions, may recruit more task-related resources than single proposition active and passive sentences (Waters, Rochon, & Caplan, 1998). In our task, successful selection of the target picture did not require processing of the second proposition (e.g., that the girl was talkative). Nevertheless, we evaluated whether different brain regions would show differential sensitivity to the number of propositions.
We were unable to obtain FDR thresholds for the VLSM analysis of two-proposition minus one-proposition scores (subject + object relatives minus actives + passives). Figure 5 therefore shows the uncorrected t map (t>1.67) from this analysis. Importantly for our purposes, there was no suggestion of a preferential relation between number of propositions and temporoparietal areas. In fact, the highest t values for this analysis were in the frontal cortex - in BA 44. This is compatible with the interpretation that this region subserves the task-related resources (e.g., working memory) that are additionally required for two-proposition compared to one-proposition sentences. However, any such interpretation would have to be tentative due to the lack of inferential statistics.
Figure 5.
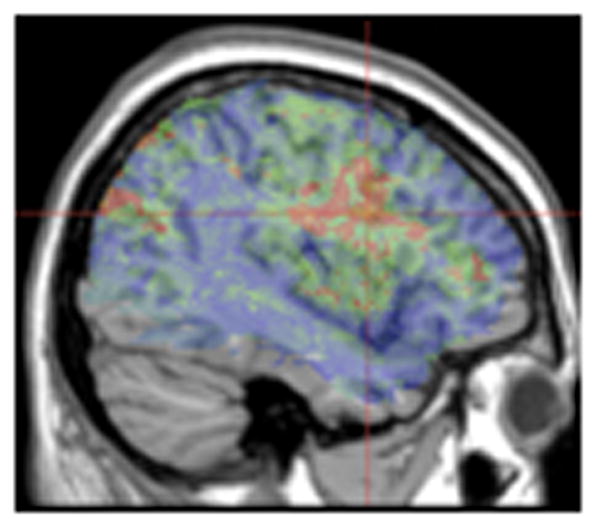
Two-proposition minus one-proposition comprehension scores. Voxels exceeding an uncorrected t-test threshold are rendered in a red (t=1.67) to yellow (t>5) scale. The peak t value of 4.03 was centered at MNI coordinates -42, 5, 26 (2 voxels) indicated by crosshairs.
Broca’s Aphasia
Evidence from Broca’s aphasia is often cited in favor of a critical role for Broca’s area in syntactic comprehension. The underlying assumptions behind this inference are problematic (see Discussion). In our sample, the comprehension abilities of the 21 Broca’s aphasics covered the spectrum: 10 performed poorly (<=65%), 6 performed well above chance (>=80%), and 5 scored somewhere in between, suggesting no special relationship between Broca’s aphasia and impaired syntactic comprehension. Lesion comparisons also throw doubt on the presumed link between impaired comprehension and damage in Broca’s area. Figure 6 shows percent BA 39 and BA 44 damage for two roughly matched groups of Broca’s aphasics (4 poor and 4 good comprehenders. Table 3). Most good and poor comprehenders had extensive BA 44 damage (> 40%), but the poor comprehenders tended to have more damage in BA 39.
Figure 6.
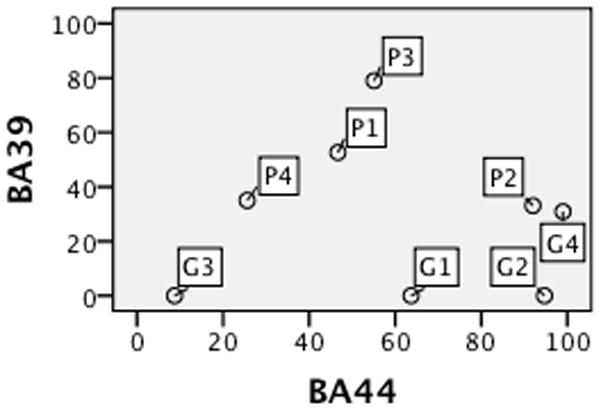
Percent damage in BA 39 and BA 44 for 8 Broca’s aphasic poor (P) and good (G) comprehenders.
Table 3.
Two groups of roughly matched Broca’s aphasics
| Patient group | Age at testing | Education (years) | WAB fluency | Lesion volume (cc) | SentComp accuracy |
|---|---|---|---|---|---|
| Poor comprehenders | |||||
| MR0047 | 64 | 13 | 4–5 | 171.6 | 53% |
| MR0190 | 59 | 16 | 4 | 205.3 | 47% |
| MR1737 | 43 | 12 | 4 | 92.6 | 63% |
| MR0057 | 63 | 16 | 4 | 148.8 | 60% |
| Average | 57.3 | 14.3 | 4.3 | 154.6 | 55.8 |
| Good comprehenders | |||||
| MR1238 | 49 | 14 | 4 | 172.2 | 100% |
| MR0046 | 68 | 12 | 4 | 197.3 | 93% |
| MR0865 | 56 | 12 | 4 | 71.8 | 93% |
| MR1283 | 54 | 16 | 4–5 | 307.9 | 80% |
| Average | 56.8 | 13.5 | 4.3 | 187.3 | 91.5 |
Discussion
The neural basis of comprehension has long been an object of study (Wernicke, 1874). Early studies relied on small patient samples and imprecise neuroanatomical data. State of the art imaging technology now makes it possible to analyze lesion-deficit relations more precisely. Recent studies using functional connectivity analysis and voxel-based morphometry have begun to elucidate how damage or atrophy in anterior and posterior brain regions might impact component processes of sentence comprehension (Amici et al., 2007; Warren, Crinion, Ralph, & Wise, 2009). We used VLSM to address similar questions (see also Bates et al., 2003; Dronkers et al., 2004). We explored the role of VLPFC and temporoparietal areas in reversible sentence comprehension as evaluated by a sentence-to-picture matching task. Lesion coverage was roughly comparable in the two areas, yet voxels carrying an association between comprehension accuracy and lesion status were identified in temporoparietal areas but not VLPFC. Results of the regional analyses generally corroborated what we found with VLSM. Correlations between comprehension scores and percent damage for a priori Brodmann areas were significant for one or more temporoparietal areas (BA 22/39/40), whereas in frontal regions they were non-significant (BA 45) or marginally significant (BA 44). A post-hoc analysis also showed that the correlation between SentComp and percent damage was significantly greater in the temporoparietal region than in the frontal region.
Temporoparietal cortex
Across different analyses, we found a robust association between damage in posterior perisylvian areas and impaired reversible sentence comprehension. VLSM results consistently implicated voxels at the junction of temporal and parietal lobes (BA 21/22 and BA 39/40). These results are consistent with other VLSM studies, which have also found an association between auditory comprehension and posterior temporal and inferior parietal areas (Bates et al., 2003; Dronkers et al., 2004). Our regional analyses corroborate the VLSM results – both overall sentence comprehension scores and separate canonical and non-canonical sentence comprehension scores were significantly correlated with percent damage in BA 22, 39 and 40. BA 22 and 39 continued to show a marginal correlation with sentence comprehension scores even after controlling for lesion volume. As described below, our results extend previous VLSM findings in two other important ways.
First, our task was specifically designed to test the assignment of thematic roles based on syntax. We isolated syntactic comprehension processes by screening participants on a lexical version of our task so that failure in sentence comprehension was unlikely to be due to impaired lexical comprehension. Previous VLSM studies (Bates, et al., 2003; Dronkers et al., 2004) sought to identify areas related to all aspects of auditory comprehension. Thus, they used tests like WAB or CYCLE-R (Curtiss & Yamada, 1988) and tested aphasic patients across the spectrum, including those with lexical comprehension deficits. The results from these studies identified the middle temporal gyrus as a critical area for some aspect of auditory comprehension, likely lexical processing (Dronkers et al., 2004). Interestingly, these studies also reported findings in BA 39, consistent with our own results.
We also extend previous findings in showing that the role of temporoparietal areas in sentence comprehension is not limited to their role in phonological working memory and sensori-motor integration. Dronkers et al. (2004) interpreted their temporoparietal results in such terms. This is in line with recent proposals regarding the roles of different posterior perisylvian areas. Hickok and Poeppel (2004) have proposed that bilateral superior temporal gyrus (STG) is responsible for acoustic-phonetic processing, left inferior temporal cortex (ITC) for the sound-meaning interface, and the boundary between parietal and temporal lobes near the Sylvian fissure (Sylvian-parietal-temporal or Spt) for the sensori-motor interface. They propose that the role of the sensori-motor interface is to support verbal working memory – in their view, the ability to use articulatory processes (rehearsal) to keep auditory representations active. Similarly, Wise and colleagues have reported several results that implicate the temporoparietal region in sentence comprehension. They interpret these results as reflecting either working memory and/or sensori-motor processing (Crinion, Lambon-Ralph, Warburton, Howard, & Wise, 2003; Narain et al., 2003; Scott, Blank, Rosen, & Wise, 2000; Wise, 2003). The voxels where we found a significant association with comprehension scores include those in the temporoparietal cortex. These associations remained even after controlling for rhyme probe span and non-word repetition. Thus, while we do not argue against a role for temporoparietal cortex in the above-mentioned working memory and sensori-motor processes, we have evidence that this region plays some other additional role in sentence comprehension.
Our results are consistent with neuroimaging studies that have used different paradigms than sentence-picture matching. It is notable that some studies whose main focus was VLPFC have nevertheless found significant results in left posterior temporal and/or inferior parietal cortices (e.g., Ben-Shachar, Palti, & Grodzinsky, 2004: BA 39 bordering BA 22 and 37). Two recent neuroimaging studies emphasized the involvement of left temporal and parietal regions to the exclusion of VLPFC. The first study compared German canonical and non-canonical sentences equated for accuracy and reaction times (i.e., difficulty) and found increased activation for non-canonical sentences in superior and posterior temporal cortex (BA 21/22, 41/42. Wartenburger et al., 2004). The second study compared Japanese passive and active sentences found increased activation for the passives in the inferior parietal lobule (BA 39. Yokoyama et al., 2007).
Our results are also consistent with a study that found significant correlations between syntactic comprehension and lesion extent/PET metabolism in temporoparietal areas in aphasic patients (Caplan et al., 2007). For example, accuracy in their sentence-to-picture matching task was significantly correlated with percent lesion volume in Wernicke’s area and PET activity in the inferior parietal lobe. Performance in an object manipulation task was correlated with percent lesion volume in the inferior and superior parietal lobes.
Collective evidence thus favors a role for temporoparietal regions in sentence comprehension. We have already argued that this role may not be restricted to working memory functions. Reversible sentence comprehension requires the computation of thematic relations between sentence constitutents (who did what to whom). This might require the dynamic binding of “what” (a particular entity) and “where” (the role that an entity plays in an event) (Chang, Dell, & Bock, 2006). The auditory system may contain a dorsal-ventral partitioning similar to the one proposed for vision (Rauschecker, 1998). Areas at the junction of superior temporal and inferior parietal cortices may be well-situated anatomically for binding the two streams. Animal models suggest that suprasylvian and infrasylvian regions are massively interconnected (Petrides & Pandya, 2009). It is possible that the spatial processing in the dorsal stream is sufficiently abstract so as to play a role in different domains. Luria (1970) described patients with lesions near the junction(s) of temporal, parietal and occipital lobes who showed a variety of deficits including an inability to distinguish between phrases like “brother’s father” and “father’s brother”, poor comprehension of complex sentences, as well as deficits in mathematical calculation and spatial orientation. More recently, BA 39 has been implicated as a possible common substrate for arithmetic, relational reasoning and sentence comprehension (Baldo & Dronkers, 2007; Baldo, Bunge, Wilson, & Dronkers, 2010). It has also been proposed that a non-linguistic spatial representation might underlie our understanding of thematic relations in language (Chatterjee, Maher, Rothi, & Heilman, 1995; Coslett, 1999). These speculations can tested by future studies evaluating whether damage to the junction of temporal and parietal areas leads to broader spatial and/or relational deficits as measured by non-linguistic tasks and the comprehension of other relational linguistic structures.
VLPFC
Earlier studies reported evidence from Broca’s aphasia in favor of a critical role for Broca’s area in the syntactic aspects of comprehension (Caramazza & Zurif, 1976). Such studies seldom had the anatomical precision required to make brain-function correlations. Several linking assumptions in inferring this brain-function relation have been called into question. First, not all Broca’s aphasics have damage to Broca’s area (Dronkers, 2000). Second, not all Broca’s aphasics have impaired syntactic comprehension (Berndt, Mitchum, & Haendiges, 1996). Last but not least, patients with Broca’s area damage do not invariably show syntactic comprehension deficits. One recent study tested comprehension of passives in 38 agrammatic aphasics with verified damage to Broca’s area (Caramazza, Capasso, Capitani, & Miceli, 2005). More patients performed better than chance than would be expected if Broca’s area were critical for the comprehension of non-canonical sentences. Collectively, these findings call into question the proposed link from Broca’s aphasia to Broca’s area damage to poor syntactic comprehension. A look at the Broca’s aphasics in our sample offers a similar cautionary note. Some of the patients with extensive Broca’s area (BA 44) damage performed well above chance in our comprehension task. Temporoparietal (BA 39) damage better separated the poor comprehenders from the good ones.
Neuroimaging studies are equivocal about the correct interpretation of VLPFC activation during comprehension tasks. While some authors propose that VLPFC, particularly Brocas’s area, supports critical syntactic operations (Ben-Shachar et al., 2003), others have attributed a less central role to Broca’s area during sentence comprehension. For example, Caplan, Stanczak and Waters (2008) suggest that the initial assignment of thematic roles based on sentence structure is carried out by left temporal areas and that left frontal areas may be involved only in later checking of those roles under certain conditions. Fiebach, et al. (2005: pp 89) state: “We suggest that BA 44 is recruited mainly in cases when syntactic information has to be maintained temporarily in working memory. Parsing processes that are more computational in nature and temporally more circumscribed might be carried out partly in other brain regions”.
Our interpretation of the negative VLPFC results from the current study is consistent with such interpretations. We suggest that Broca’s area does not play a task-independent, core syntactic parsing role in comprehension. Instead it may support the cognitive control and/or working memory resources that are often but not always associated with sentence comprehension. This hypothesis is consistent with the fact that in the current study, the only suggestion of a preferential VLPFC effect was found when we contrasted 2-proposition with 1-proposition sentences –a manipulation that has been hypothesized to tap working memory and other task-related resources (Waters et al., 1998).
There is widespread evidence that VLPFC is involved in the selection of a task-relevant representation from amongst mutually incompatible alternatives (Petrides, 2005; Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997). In sentence comprehension, VLPFC involvement may be particularly important, crucial even, in cases where conflicting cues (e.g., semantics and syntax) must be resolved in order to converge on the correct interpretation. Future research can determine whether patients with damage in Broca’s area will be particularly impaired in such tasks. For the present, results from our VLSM and regional analyses suggest that VLPFC does not play a major role in successful performance in a widely used reversible sentence comprehension test.
Closing Remarks
Our analyses show a robust association between impaired reversible sentence comprehension and damage in left temporoparietal areas. In lesion studies, areas may show up as important either because they play a direct causal role in the behavior of interest or because they are functionally affected by disrupted connectivity from elsewhere. Our VLSM and regional analyses are congruent in suggesting that a functional left temporoparietal cortex is necessary for normal reversible sentence comprehension. Nevertheless, both are “static” techniques that allow us to correlate behavioral impairments with brain damage. A fuller picture of how sentence comprehension unfolds in the brain requires the integration of several methods, including electrophysiological techniques that have higher temporal resolution and functional connectivity studies that shed light on the transfer of information between different areas.
In focusing on the temporoparietal region, we do not mean to suggest that it is the sole area responsible for sentence comprehension under all circumstances. As mentioned above, some stimuli or tasks may tap working memory and cognitive control resources more than those used here. In such situations, VLPFC-supported executive functions may play a critical role. Our comprehension task also did not test fine-grained lexical processing. A small set of words -- 8 unique nouns, 4 unique verbs -- was repeated again and again in different sentences. A functioning anterior temporal lobe may be crucial when fine-grained lexico-semantic distinctions or the integration of meaning from multiple words is important for good performance (Warren et al., 2009). It is a virtual certainty that a complex task such as sentence comprehension involves a distributed network of regions subserving multiple functions (Caplan et al., 2007; Dronkers et al., 2004). Our results suggest that the temporoparietal region may be critical for a particular aspect of sentence comprehension, namely assigning thematic roles using sentence structure.
Acknowledgments
We would like to thank Olufunsho Faseyitan and Grant Walker for providing lesion information, Laurel Brehm, Maureen Gagliardi and Adelyn Brecher for help with the behavioral data, and Branch Coslett for valuable feedback. This work was supported by the National Institutes of Health (RO1 DC000191 to MFS and 5-T32-HD-007425 to the University of Pennsylvania).
Footnotes
Actives with prepositional phrases had the same syntax to thematic role mappings as Actives, but were more comparable in length to the relative clause sentence types. Throughout the manuscript, we use “actives” to refer only to bare actives (without prepositional phrases).
Standard short-term memory (STM) span measures may tap both semantic and phonological STM. Because temporoparietal areas have been specifically tied to phonological STM, we used a task that has been used to measure this component (Freedman & Martin, 2001).
Because VLSM compares two groups at each voxel (lesioned and non-lesioned), imposing such a threshold helps to ensure sufficient numbers in each group for obtaining a stable estimate of variance in the behavioral scores. The choice of threshold is arbitrary.
We obtained similar results when we included actives with prepositional phrases in the canonical sentence group (Mean = 85.7%. F(1,78)=97.1; p<.001). We did not include locatives in these analyses because there is no clear consensus on the thematic roles and their canonical mappings to syntax for such structures.
Separate VLSM analyses for canonical and non-canonical scores also support this conclusion. Suprathreshold peak voxels were found in BA 39 after co-varying out rhyme probe span (canonical: peak MNI -38, -53, 31; non-canonical: peak MNI -55, -54, 31) and non-word repetition (canonical: peak MNI -38, -53, 31; non-canonical: peak MNI -54, -55, 35 (t=5.19).
References
- Amici S, Brambati SM, Wilkins DP, Ogar J, Dronkers NL, Miller BL, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. Journal of Neuroscience. 2007;27(23):6282. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis. 2006;10(3):397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Bunge SA, Wilson SM, Dronkers NF. Is relational reasoning dependent on language? A voxel-based lesion symptom mapping study. Brain and Language. 2010 doi: 10.1016/j.bandl.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. Neural correlates of arithmetic and language comprehension: A common substrate? Neuropsychologia. 2007;45:229–235. doi: 10.1016/j.neuropsychologia.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Hendler T, Kahn I, Ben-Bashat D, Grodzinsky Y. The neural reality of syntactic transformations: Evidence from functional magnetic resonance imaging. Psychological Science. 2003;14(5):433–440. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Palti D, Grodzinsky Y. Neural correlates of syntactic movement: converging evidence from two fMRI experiments. NeuroImage. 2004;21:1320–1336. doi: 10.1016/j.neuroimage.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN. Comprehension of reversible sentences in “agrammatism”: A meta-analysis. Cognition. 1996;58(3):289–308. doi: 10.1016/0010-0277(95)00682-6. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. Journal of Cognitive Neuroscience. 1998;10(4):541–552. doi: 10.1162/089892998562843. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. PET studies of syntactic processing with auditory sentence presentation. NeuroImage. 1999;9:343–351. doi: 10.1006/nimg.1998.0412. [DOI] [PubMed] [Google Scholar]
- Caplan D, Stanczak L, Waters G. Syntactic and thematic constraint effects on blood oxygen level dependent signal correlates of comprehension of relative clauses. Journal of Cognitive Neuroscience. 2008;20(4):643–656. doi: 10.1162/jocn.2008.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Vijayan S, Kuperberg G, West C, Waters G, Greve D, et al. Vascular responses to syntactic processing: Event-related fMRI study of relative clauses. Human Brain Mapping. 2001;15:26–38. doi: 10.1002/hbm.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Waters G, Kennedy D, Alpert N, Makris N, DeDe G, et al. A study of syntactic processing in aphasia II: Neurological aspects. Brain and Language. 2007;101(2):151–177. doi: 10.1016/j.bandl.2006.06.226. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Capasso R, Capitani E, Miceli G. Patterns of comprehension performance in agrammatic Broca’s aphasia: A test of the Trace Deletion Hypothesis. Brain and Language. 2005;94(1):43–53. doi: 10.1016/j.bandl.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB. Dissociation of algorithmic and heuristic processes in language comprehension: Evidence from aphasia. Brain and Language. 1976;3:572–582. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Chang F, Dell GS, Bock K. Becoming syntactic. Psychological Review. 2006;113(2):234–272. doi: 10.1037/0033-295X.113.2.234. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Maher LM, Rothi LJG, Heilman KM. Syntactic thematic role assignment: The use of a temporal-spatial strategy. Brain and Language. 1995;49:125–139. doi: 10.1006/brln.1995.1024. [DOI] [PubMed] [Google Scholar]
- Coslett HB. Spatial influences on motor and language function. Neuropsychologia. 1999;37:695–706. doi: 10.1016/s0028-3932(98)00116-x. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon-Ralph MA, Warburton EA, Howard D, Wise RJS. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–1201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- Curtiss S, Yamada J. Unpublished test. University of California; Los Angeles, CA: 1988. The Curtiss-Yamada comprehensive language evaluation (CYCLE) [Google Scholar]
- Dronkers NF. The gratuitous relationship between Broca’s aphasia and Broca’s area. Behavioral and Brain Sciences. 2000;23(1):30–31. [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca’s area in sentence processing: Syntactic integration vs. syntactic working memory. Human Brain Mapping. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman ML, Martin RC. Dissociable components of short-term memory and their relation to long-term learning. Cognitive Neuropsychology. 2001;18(3):193–226. doi: 10.1080/02643290126002. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Santi A. The battle for Broca’s region. Trends in cognitive sciences. 2008;12(12):474–480. doi: 10.1016/j.tics.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22(2):324. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery test manual. Psychological Corp; 1982. [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF. Power in voxel-based lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19(7):1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D. A cortical network for semantics: (de)constructing the N400. Nature Reviews Neuroscience. 2008;9(12):920–933. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Luria AR. Traumatic aphasia. The Hague: Mouton & Co; 1970. [Google Scholar]
- Narain C, Scott SK, Wise RJS, Rosen S, Leff A, Iversen SD, et al. Defining a left-lateralized response specific to intelligible speech using fMRI. Cerebral Cortex. 2003;13:1362–1368. doi: 10.1093/cercor/bhg083. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cognitive, Affective, & Behavioral Neuroscience. 2005;5(3):263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- Ouden D-Bd, Fix S, Parrish TB, Thompson CK. Argument structure effects in action verb naming in static and dynamic conditions. Journal of Neurolinguistics. 2009;22:196–215. doi: 10.1016/j.jneuroling.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: Architectonic and functional organization. Philosophical transactions of the Royal Society B. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. 2009 doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of complex sounds. Current Opinion in Neurobiology. 1998;8(4):516–521. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5:813–9. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Saffran EM, Schwartz MF, Linebarger MC, Martin N, Bochetto P. Philadelphia comprehension battery. 1998 Unpublished. [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: A strategy for balancing patients’ privacy rights with researchers’ need for access. Archives of Physical Medicine and Rehabilitation. 2005;86(9):1807–1814. doi: 10.1016/j.apmr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132(12):3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87(2):245–251. [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S. Localization of syntactic comprehension by positron emission tomography. Brain and Language. 1996;52:452–473. doi: 10.1006/brln.1996.0024. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JE, Crinion JT, Ralph MAL, Wise RJS. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–3442. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Burchert F, Heinemann S, De Bleser R, Villringer A. Neural correlates of syntactic transformations. Human Brain Mapping. 2004;22(1):72–81. doi: 10.1002/hbm.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters GS, Rochon E, Caplan D. Task Demands and Sentence Comprehension in Patients with Dementia of the Alzheimer’s Type. Brain and Language. 1998;62:361–397. doi: 10.1006/brln.1997.1880. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der Aphasische Symptomencomplex. Breslau: Cohn and Weigert; 1874. [Google Scholar]
- Wise RJS. Language systems in normal and aphasic human subjects: functional imaging studies and inferences from animal studies. British Medical Bulletin. 2003;65:95–119. doi: 10.1093/bmb/65.1.95. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Watanabe J, Iwata K, Ikuta N, Haji T, Usui N, et al. Is Broca’s area involved in the processing of passive sentences? An event-related fMRI study. Neuropsychologia. 2007;45(5):989–996. doi: 10.1016/j.neuropsychologia.2006.09.003. [DOI] [PubMed] [Google Scholar]


