Abstract
Objectives/Hypothesis
To evaluate outcomes of salvage surgery with free flap reconstruction for recurrent squamous cell carcinoma of the oropharynx and oral cavity with increased use of chemoradiotherapy.
Study Design
Retrospective patient review.
Methods
All patients undergoing salvage surgery with free flap reconstruction for oropharynx (n = 36) and oral cavity (n = 36) squamous cell carcinomas between January 2001 and January 2008 were obtained. Mean follow-up was 14 months. Previous chemoradiotherapy was used in 40% and radiotherapy alone in 60%.
Results
Complications were more frequent in oropharynx than oral cavity tumors (36% and 14%, respectively; P = .05) requiring more secondary procedures (15 for oropharynx vs. six for oral cavity). Few patients returned to a normal diet (8%), and a majority retained an enterogastric feeding tube (56%). Median survival overall following salvage surgery was 44.8 months for oral cavity and 53.8 months for oropharynx head and neck squamous cell carcinoma. Overall estimated 1-, 2-, and 5-year observed survivals were 98%, 77.2%, and 43.7%, respectively. Twelve patients had a disease-free interval of <6 months, 92% of whom died of disease. Of 17 patients with disease at the primary site and involved regional lymph nodes, 94% died of disease.
Conclusions
Salvage surgery with free flap reconstruction for recurrent oral and oropharyngeal tumors after chemoradiotherapy has acceptable morbidity and similar cure rates as salvage following radiotherapy without chemotherapy. Concurrent nodal recurrence and short disease-free interval are associated with reduced cure rates. A significant proportion will require enterogastric feeding and few will tolerate a normal diet.
Keywords: Squamous cell carcinoma, oral cavity, oropharynx, surgery, salvage, survival outcome, treatment outcome
INTRODUCTION
A recent review of 54,801 primary oropharyngeal squamous cell carcinomas (SCC) recorded in the National Cancer Database showed that concurrent chemoradiotherapy has increasingly replaced primary surgery or radiotherapy alone as the first line of treatment, especially for stage III and IV disease.1 In the oral cavity, surgical management remains the first line of treatment with postoperative (chemo)radiotherapy being used for those at higher risk of recurrence.2 Hyperfractionation and concurrent chemotherapy are increasingly being used to improve organ preservation rates at the expense of worse functional outcomes.3,4 Furthermore, chemotherapy has been shown to improve overall mortality by reducing both distant and local failures.5
After locoregional chemoradiotherapy failure, salvage surgery remains the primary curative option but is associated with high morbidity compared to salvage after radiotherapy without chemotherapy.6 Functional results and survival after salvage surgery in the age of chemoradiation has not been well described. It has previously been shown that the use of free flaps reduces morbidity in salvage laryngectomy by introducing well-vascularized, nonirradiated tissue.7
The purpose of this study is to review the current morbidity, functional, and survival outcomes of salvage surgery with free flap reconstruction for recurrent oropharyngeal and oral cavity cancer given the increased use of primary and adjuvant chemoradiotherapy.
MATERIALS AND METHODS
Institutional review board approval was obtained. All patients undergoing salvage surgery following previous treatment for oral (n = 36) or oropharyngeal SCC (n = 36) that included >45 Gy of radiotherapy from January 2001 to January 2008 were identified from the prospectively maintained Division of Otolaryngology reconstructive database.
Minimum work-up included direct laryngoscopy and biopsy, esophagoscopy, computed tomography (CT) neck with contrast, chest x-ray, and liver function tests. Patients without contraindications then received salvage surgery if complete macroscopic resection was deemed possible. Free flap reconstruction is commonly used following salvage surgeries especially if there is a through-and-through defect or mandibular resection. Flaps used for oropharynx included radial forearm (27), anterolateral thigh (four), rectus (three), fibula (one), and ulnar (one). Flaps used for oral cavity included radial forearm (17), fibula (13), rectus (nine), and pectoralis major (one) with four patients receiving two flaps. The external carotid system (facial artery representing the majority, 86%) was the most common recipient arterial vessel. An end-to-end anastomosis performed to the facial vein was most common (76%), followed by an end-to-side anastomosis with the internal jugular vein (16%).
Demographic characteristics, initial and secondary tumor characteristics and treatment, surgical and pathologic records, treatment and disease related toxicity, enterogastrostomy, and tracheostomy requirement were taken from retrospective chart review. Retrospective staging was performed using the American Joint Commission on Cancer 6th Edition of the AJCC Cancer Staging Manual (2002). Outcome measures included hospital stay, complications, secondary procedures, postoperative diet, tracheostomy requirement, and survival. Complications were divided into minor (infection or fistula) and major (flap failure or major hemorrhage); reoperations were also divided into minor (neck washout) and major (microvascular take-back, carotid blowout, or second flap).
Statistical analysis was performed using SPSS software version 13.0 (SPSS Inc., Chicago IL).
Descriptive variables were summarized by mean (standard deviation) for continuous variables and n (%) for categorized variables. The relationship between patient, clinical, and treatment factors and disease-specific survival was calculated by using the Kaplan-Meier method from date of diagnosis to date of death taken from tumor registry records. Patients with unknown outcomes were excluded from analysis. For all analyses, a P value of <.05 was statistically significant.
RESULTS
Patient Characteristics
The majority of patients received radiotherapy for their first tumor (oral 75%, oropharynx 92%), and a significant number of patients received additional chemotherapy (oral 33%, oropharynx 47%) (Table I). All patients received wide-field radiotherapy (oral 67% and oropharynx 89% receiving high-dose [60–74 Gy], 33% and 6% low-dose radiation for oral and oropharynx patients, respectively, and unknown dose in 6% for oropharynx). Chemotherapy was administered during radiation therapy with concurrent cisplatin or carboplatin and paclitaxel in patients originally treated at our institution. Chemotherapy doses administered at other institutions were unknown. Six oropharyngeal and 10 oral recurrence patients received postoperative reirradiation after salvage surgery. The average time from first tumor to salvage treatment was 26 months (range, 1–132 months). Time after radiation therapy to salvage surgery is listed in Table I.
TABLE I.
Characteristics of Previously Irradiated Patients Undergoing Salvage Surgery for Oral and Oropharyngeal Tumors.
| Characteristic | Oral (n=36), No. (%) | Oropharynx (n=36), No. (%) |
|---|---|---|
| Age | ||
| Median | 61.6 | 59.3 |
| Range | 29–89 | 38–83 |
| Sex | ||
| Male | 17 (47) | 30 (83) |
| Female | 19 (53) | 6 (17) |
| Disease presentation | ||
| Recurrence | 35 (97) | 33 (92) |
| Second primary | 1 (3) | 3 (8) |
| Prior chemotherapy | ||
| Yes | 12 (33) | 17 (47) |
| No | 24 (67) | 21 (53) |
| Prior surgery | ||
| Yes | 24 (67) | 14 (39) |
| No | 12 (33) | 22 (61) |
| Recurrent T staging | ||
| 1 | 3 (8) | 2 (6) |
| 2 | 4 (11) | 9 (25) |
| 3 | 4 (11) | 13 (36) |
| 4 | 23 (64) | 12 (33) |
| Unknown | 2 (6) | 0 (0) |
| Recurrent N staging | ||
| 0 | 27 (75) | 25 (69) |
| 1 | 1 (3) | 1 (3) |
| 2 | 6 (17) | 9 (25) |
| 3 | 0 (0) | 1 (3) |
| Unknown | 2 (6) | 0 (0) |
| Year from prior radiotherapy | ||
| <0.5 | 7 (19) | 9 (25) |
| 0.5–2 | 16 (44) | 16 (44) |
| >2 | 13 (36) | 11 (31) |
The most common subsites of recurrent oropharyngeal tumors were base of tongue (n = 15, 42%) or tonsil (n = 13, 36%); and of oral tumors, oral tongue (n = 17, 47%), and floor of mouth (n = 12, 33%). At the time of recurrence after primary treatment, the majority of patients (78%) presented with stage III/IV recurrent disease. The oral tumors were more likely to have recurrence at the primary site only (78%) than oropharynx (64%), who more commonly had nodal disease as well (P= .19).
Surgical and Functional Outcomes
Average hospital stay was approximately 9 days (8.8 days for oropharynx and 8.9 days for oral cavity). There were 13 (36%) complications among oropharynx patients (eight minor and five major) compared to five (14%) among oral cavity patients (all minor), (P = .05). Oropharynx consequently required more second procedures than oral cavity: 15 (10 minor and five major) versus six (five minor and one major), respectively. Major complications included carotid blowout/major vessel bleed (n = 1, requiring ligation of an external carotid artery branch), microvascular complications requiring take-back (n = 3, all salvaged), fistula requiring local-regional flap coverage (n = 2). Radiation dose (low vs. high) did not affect complication rate (P = .34).
Postoperative diets were not significantly different between the oropharyngeal and oral cavity patients, with a majority of patients remaining gastrostomy tube dependent (52.9% vs. 58.3% for oropharynx and oral cavity, respectively) (Table II). There was no difference in gastrostomy tube dependence between patients primarily treated with radiation compared to chemoradiation, or in patients treated with low-dose compared to high-dose radiation. Bone reconstruction was not associated with diet or complication rates in either group.
TABLE II.
Best Postoperative Diet After Salvage Surgery for Recurrent Oral and Oropharyngeal Squamous Cell Carcinoma (N = 72).
| Diet | Oropharyx, No. (%) | Oral Cavity, No. (%) | Total, No. (%) |
|---|---|---|---|
| Regular | 4 (11) | 2 (6) | 6 (8) |
| Puree | 5 (14) | 4 (11) | 9 (13) |
| Liquid | 6 (17) | 3 (8) | 9 (13) |
| Gastrostomy tube dependent | 19 (52) | 21 (58) | 40 (56) |
| Unknown | 2 (6) | 6 (17) | 8 (11) |
Local Control and Survival Outcomes
Mean follow-up after salvage surgery was 14 months (range, 1–72 months). At last follow-up, 15 patients had no further locoregional recurrence. The average time to recurrence after salvage surgery was 10.5 months (range, 1–53 months). Mean time to recurrence after salvage surgery was longer for oropharynx (14.9 months) than oral cavity (6.6 months; P = .04). Compared to the recurrence after primary treatment in this patient cohort, after salvage surgery, patients recurred more quickly (40% within 6 months, 40% between 6–24 months, and 20% after 24 months).
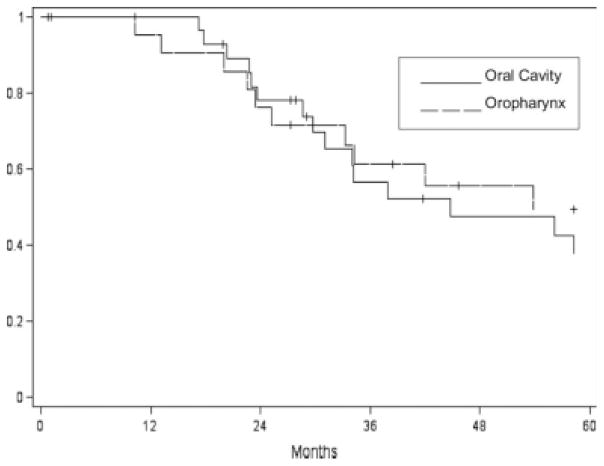
At last follow-up, 15 patients were alive following salvage surgery (eight oral and seven oropharyngeal tumors). Median survival overall following salvage surgery was 44.8 months for oral and 53.8 months for oropharynx SCC. Overall estimated 1-, 2-, and 5-year disease-specific survivals were 98%, 77.2%, and 43.7%, respectively (Fig. 1). However, overall only 28% were cured of disease.
Fig. 1.
Disease-specific survival following salvage surgery for recurrent oral (n = 36) and oropharyngeal (n = 36) cancers.
Prognostic Factors
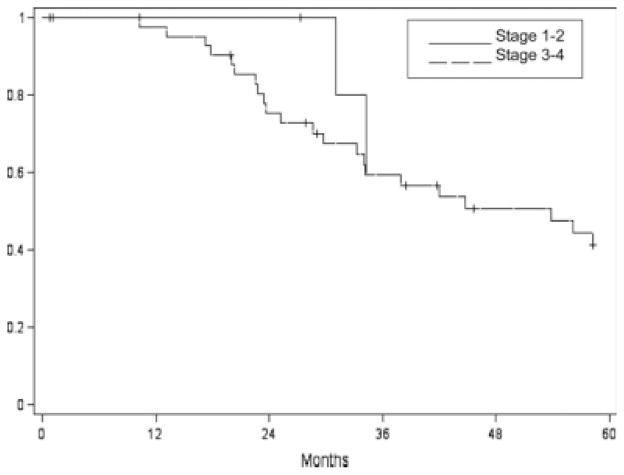
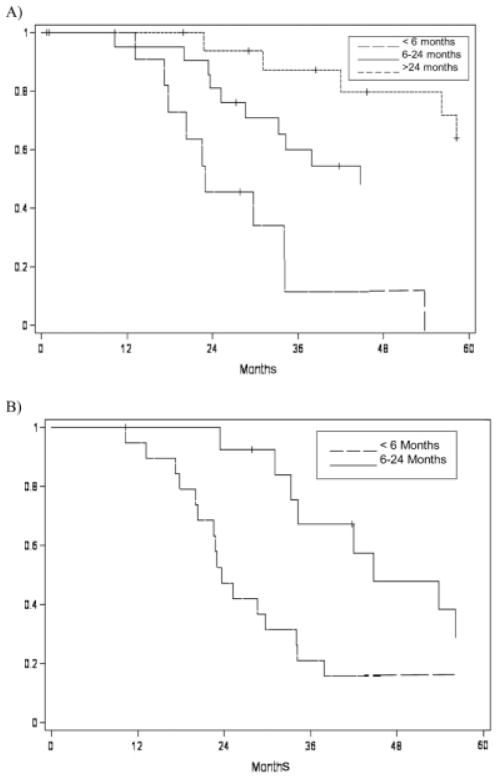
Survival following salvage surgery was not significantly correlated with restaging after recurrence (P = .66) (Fig. 2). Margin status following surgical salvage was not significantly correlated with survival (P = .81). Interestingly, survival was not significantly different between patients treated with primary radiation versus those treated with chemoradiation (P = .75) (Table III). Previous radiation dose did not correlate with survival either (P = .52). However, time to original recurrence (time between original treatment and first recurrence) was found to correlate with survival (P = .0076) (Table IV). All oropharyngeal and most (92%) oral cavity patients who had their original recurrence in the first 6 months, died of disease despite salvage surgery (Fig. 3A). Survival also correlated with time to recurrence after salvage surgery (P < .0001); many patients who had late (>24 months) further recurrences after salvage surgery survived after additional salvage surgery (Fig. 3B).
Fig. 2.
Disease-specific survival by stage for recurrent oral cavity and oropharyngeal tumors treated with salvage surgery (N = 72).
TABLE III.
Affect of Preoperative Chemotherapy on Survival Following Salvage Surgery for Recurrent Oral and Oropharyngeal Squamous Cell Carcinoma (N = 72).
| No. (%) | 5-Year Survival (Standard Error) | P Value | |
|---|---|---|---|
| Oral cavity | 36 (100) | 37.9 (10.2) | |
| Chemoradiotherapy | 12 (33) | 30.3 (11.6) | .82 |
| Radiotherapy | 24 (67) | 44.4 (13.8) | |
| Oropharynx | 36 (100) | 49.5 (11.5) | |
| Chemoradiotherapy | 17 (47) | 33.3 (18.0) | .52 |
| Radiotherapy | 19 (53) | 52.0 (15.7) |
TABLE IV.
Prognostic Factors for Survival Following Salvage Surgery for Oral and Oropharyngeal Cancers (N = 72).
| Factor | Estimated 5-Year Survival (Standard Error) | P Value |
|---|---|---|
| Age | ||
| <60 | 52.6 (11.5) | .64 |
| >60 | 50.0 (13.8) | |
| Site recurrence | ||
| Oral | 37.9 (10.2) | .49 |
| Oropharynx | 49.5 (11.5) | |
| Recurrence | ||
| Primary only | 56.6 (9.6) | <.0001 |
| Primary and neck | 21.4 (10.8) | |
| Disease-free interval | ||
| <6 months | 11.4 (10.5) | <.0001 |
| 6–24 months | 54.4 (11.3) | |
| >24 months | 63.8 (13.1) | |
| Prior surgery | ||
| Yes | 56.8 (10.0) | .96 |
| No | 39.5 (11.1) | |
| Initial staging | ||
| I-II | 85.7 (13.2) | .14 |
| III-IV | 29.7 (10.1) | |
| Restaging | ||
| I-II | 60.0 (21.9) | .63 |
| III-IV | 41.2 (8.3) | |
| Surgical margins | ||
| Clear | 47.7 (9.6) | .83 |
| Close/involved | 40.0 (13.6) | |
Fig. 3.
Disease-specific survival for patients having salvage surgery for recurrent oral and oropharyngeal tumors by time to recurrence following (A) primary and (B) recurrent treatment.
Of the patients who presented with local disease only, 14 (39%) were disease free at 5 years. Simultaneous recurrence at the primary site and in regional lymph nodes had a dismal prognosis. Of the 17 patients (nine oropharynx and eight oral cavity) with positive neck disease at the time of recurrence, 94% died of disease.
DISCUSSION
Increasing use of concurrent chemotherapy has improved local control rates, but has been shown to alter recurrent tumor biology by increasing multifocality, contralateral spread, and perineural invasion.8 This study demonstrates that, in the era of chemoradiation, surgery remains a viable salvage option for locally recurrent SCCs of the oropharynx and oral cavity, with 28% cured of disease after salvage surgery. This study demonstrates the guarded prognosis of patients with recurrence in the neck and at the primary site after radiotherapy (6% at 5 years) and for those having a recurrence within 6 months of completing radiotherapy (8% at 5 years). For such patients, the significant functional impact of salvage surgery (56% require enterogastric feeding) may outweigh the benefits.
In a review of 3,887 head and neck SCC patients treated at a single institution since 1957, Layland et al, found that 40.5% of oral and 41.9% of oropharyngeal primaries recurred.9 Of these, 65.3% might be candidates for salvage surgery (42.5% recurred only at the primary site and 22.8% at the primary site and elsewhere). In a meta-analysis of 1,633 patients undergoing salvage surgery following radiotherapy between 1980 and 1998, Goodwin showed significant treatment related mortality (5.2%), frequent major complications (27%), and a modest 5-year survival of 43% for oral cavity and 26% for oropharynx.10 A more recent series of 38 recurrent SCCs, including eight oral and 16 oropharynx, undergoing salvage surgery following chemoradiation suggested salvage after chemoradiation may have even lower success, with 2-year estimated survival being 27%.11 Our study refutes this latter argument and shows that, despite significant morbidity and functional impact, a similar proportion are cured by salvage surgery following chemoradiotherapy as were cured following radiotherapy alone in historic series.
Tumors considered appropriate for salvage surgery had work-up to exclude unresectable status, especially carotid invasion or fixation to the skull base (direct laryngoscopy and biopsy, esophagoscopy, CT neck with contrast) or distant metastases (chest x-ray and liver function tests). Subsites considered for salvage were typical of oral (oral tongue 47%, floor of mouth 33%) and oropharyngeal (base of tongue 42%, tonsil 36%) distributions suggesting that no particular subsite precluded surgical salvage. The majority are detected at advanced stage (78% stage III/IV) due to tissue effects of edema, induration, and fibrosis, and the difficulty of obtaining a diagnostic fine needle aspiration material due to necrosis and inflammation. Consequently, our institutional practice has moved toward routine examination under anesthesia with biopsy 8 to 12 months after completion of chemoradiation, a protocol that others have advocated to improve early detection of local failures.12
Although minor complications are common after salvage surgery (36%), major complications are uncommon (11%) and can be managed without significant sequelae. Chemoradiotherapy has been shown to adversely effect mandibular healing compared with radiotherapy alone.13 However, our institution has shown that the use of free flaps may improve healing following salvage surgery by introducing well-vascularized, nonirradiated tissue.7 Free flaps are commonly used for surgical salvage to facilitate healing of through-and-through defects, protect the great vessels from salivary contamination, and reconstruct mandibular defects. Regional flaps, such as the pectoralis major, are now mainly used in addition to a free flap or for salvage after flap failure.
The majority (56%) of salvage cases retained enterogastric feeding at a mean 14 months follow-up, and few (8%) maintained a normal diet. Poor swallowing outcomes of open surgery have been central in the move toward primary chemoradiotherapy for advanced oropharyngeal tumors. Gillespie et al. compared swallowing outcomes in 10 advanced oropharyngeal SCCs treated with open surgery and 11 treated with chemoradiotherapy and found better airway protection and dysphagia-specific quality of life following chemoradiotherapy.14 However, in highly selected oropharyngeal cases, trans-oral resection with the CO2 laser has shown superior swallow outcomes to open surgery and comparable outcomes to (chemo)radiotherapy.15 As the majority (78%) of salvage cases presenting to our institution are advanced stage, few are amenable to transoral resection; robotic-assisted transoral resection is currently being evaluated in our institution and others in the hope of improving swallowing outcomes in salvage cases.
The estimated 5-year survival for surgical salvage following chemoradiotherapy in this series is consistent with previous series of salvage surgery after radiotherapy alone. In a review of 96 previously irradiated oral and oropharyngeal SCCs salvaged surgically, Agra et al. found a similar 5-year survival rate (25.3%).16 The role of adjuvant reirradiation after salvage surgery is controversial, as it has been shown to improve local control with no impact on survival and with significant toxicity.17 Reirradiation alone as an alternative to salvage surgery offers a low rate of cure.18 The only other option would be palliative chemotherapy for nonsurgical candidates, which has a median survival of 8.5 months.19
In a review of 246 salvage surgeries for all head and neck sites, others have found restaging to be the most important predictor of survival along with disease-free interval. Although restaging was not associated with survival outcomes in this series (P = .68), the data was skewed by a very high proportion (78%) presenting in advanced stage. Our series showed no patients recurring within 6 months of their initial treatment are cured by salvage surgery; of the 8% who were alive, none were disease free at last follow-up. Additionally, patients who simultaneously recur in the neck and the primary site have a grave prognosis (6% estimated 5-year survival). Layland et al. found that for recurrent oral tumors, N+ disease approximately halved 5-year survival from 22.5% to 11.8% (P = .02).
Based on these data, patients with the best chance of cure for recurrent oropharyngeal and oral cavity squamous cell carcinoma appear to be those with: 1) surgically resectible disease, 2) longer disease-free interval, 3) no neck disease, and 4) early stage primary tumors. It has been shown that patients who do not fit these criteria have a limited chance of survival. This information will guide clinicians and patients in determining whether the significant functional impact of salvage surgery is balanced by a realistic prospect of cure.
CONCLUSION
Salvage surgery for recurrent oral and oropharyngeal SCCs with free flap reconstruction after chemoradiotherapy can be performed with manageable complications and a similar prospect of cure as following radiation alone. Recurrence in regional lymph nodes and at the primary site and short disease-free interval (<6 months after primary treatment) are associated with reduced cure rates. A significant proportion of salvaged oral and oropharyngeal tumor patients will be gastrostomy tube dependent and few will tolerate a normal diet.
Footnotes
This work was accepted for oral presentation at Triological Society Annual Meeting, Orlando, Florida, U.S.A., February 4-7, 2010.
BIBLIOGRAPHY
- 1.Chen AY, Schrag N, Hao Y, et al. Changes in treatment of advanced oropharyngeal cancer, 1985–2001. Laryngoscope. 2007;117:16–21. doi: 10.1097/01.mlg.0000240182.61922.31. [DOI] [PubMed] [Google Scholar]
- 2.Robertson AG, Soutar DS, Webster M, et al. Early closure of a randomized trial: surgery and post-operative radiotherapy versus radiotherapy in the management of intra-oral tumours. Clin Oncol. 1998;10:155–160. doi: 10.1016/s0936-6555(98)80055-1. [DOI] [PubMed] [Google Scholar]
- 3.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 5.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-analysis of chemotherapy on head and neck cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 6.Ganly I, Patel S, Matsuo J, et al. Postoperative complications of salvage total laryngectomy. Cancer. 2005;103:2073–2081. doi: 10.1002/cncr.20974. [DOI] [PubMed] [Google Scholar]
- 7.Withrow KP, Rosenthal EL, Gourin CG, et al. Free tissue transfer to manage salvage laryngectomy defects after organ preservation failure. Laryngoscope. 2007;117:781–784. doi: 10.1097/MLG.0b013e3180332e39. [DOI] [PubMed] [Google Scholar]
- 8.Zbaren P, Nuyens M, Curschmann J, et al. Histologic characteristics and tumor spread of recurrent glottic carcinoma: analysis on whole organ sections and comparison with tumor spread of primary glottic carcinomas. Head Neck. 2007;29:26–32. doi: 10.1002/hed.20502. [DOI] [PubMed] [Google Scholar]
- 9.Layland MK, Sessions DG, Lenox J. The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx and hypopharynx: N0 versus N+ Laryngoscope. 2005;115:629–639. doi: 10.1097/01.mlg.0000161338.54515.b1. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin WJ. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110:1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 11.Richey LM, Shores CG, George J, et al. The effectiveness of salvage surgery after the failure of primary concomitant chemoradiation in head and neck cancer. Otolaryngol Head Neck Surg. 2007;136:98–103. doi: 10.1016/j.otohns.2006.06.1267. [DOI] [PubMed] [Google Scholar]
- 12.Yom SS, Machtay M, Biel MA, et al. Survival impact of planned restaging and early surgical salvage following definitive chemoradiation for locally advanced squamous cell carcinomas of the oropharynx and hypopharynx. Am J Clin Oncol. 2005;28:385–392. doi: 10.1097/01.coc.0000162422.92095.9e. [DOI] [PubMed] [Google Scholar]
- 13.Sharan R, Iyer S, Chatni SS, et al. Increased plate and osteosynthesis related complications associated with postoperative concurrent chemoradiotherapy in oral cancer. Head Neck. 2008;30:1422–1430. doi: 10.1002/hed.20886. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie MB, Brodsky MD, Day TA, Sharma AK, Lee F, Martin-Harris B. Laryngeal penetration and aspiration during swallowing after the treatment of advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2005;131:615–619. doi: 10.1001/archotol.131.7.615. [DOI] [PubMed] [Google Scholar]
- 15.Steiner W, Ambrosch P. In: Endoscopic Laser Surgery of the Upper Aerodigestive Tract With Special Emphasis on Cancer Surgery. Knape MV, translator. Stuttgart, Germany: Thieme; 2000. [Google Scholar]
- 16.Agra IM, Carvaiho AL, Ulbrich FS, et al. Prognostic factors in salvage surgery for recurrent oral and oropharyngeal cancer. Head Neck. 2006;28:107–113. doi: 10.1002/hed.20309. [DOI] [PubMed] [Google Scholar]
- 17.Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of post-operative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26:5518–5523. doi: 10.1200/JCO.2007.15.0102. [DOI] [PubMed] [Google Scholar]
- 18.Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008;30:281–288. doi: 10.1002/hed.20697. [DOI] [PubMed] [Google Scholar]
- 19.Gibson MK, Murphy B, Hussain MHA, DeConti RC, Ensley J, Forastiere AA. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the eastern cooperative oncology group. J Clin Oncol. 2005;23:3562–3567. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]