Abstract
Neuropathic pain is often “spontaneous” or “stimulus-independent.” Such pain may result from spontaneous discharge in primary afferent nociceptors in injured peripheral nerves. However, whether axotomized primary afferent nociceptors give rise to pain is unclear. The rostral anterior cingulate cortex (rACC) mediates the negative affective component of inflammatory pain. Whether the rACC integrates the aversive component of chronic spontaneous pain arising from nerve injury is not known. Here, we used the principle of negative reinforcement to show that axotomy produces an aversive state reflecting spontaneous pain driven from injured nerves. Additionally, we investigated whether the rACC contributes to the aversiveness of nerve injury induced spontaneous pain. Partial or complete hindpaw denervation was produced by sciatic or sciatic/saphenous axotomy, respectively. Conditioned place preference resulting from presumed pain relief was observed following spinal clonidinein animals with sciatic axotomy but not in sham-operated controls. Similarly, lidocaine administration into the rostral ventromedial medulla (RVM) produced place preference selectively in animals with sciatic/saphenous axotomy. In rats with spinal nerve ligation (SNL) injury lesion of the rACC blocked the reward elicited by RVM lidocaine but did not alter acute stimulus evoked hypersensitivity. Lesion of the rACC did not block cocaine-induced reward indicating that rACC blockade did not impair memory encoding or retrieval but did impair spontaneous aversiveness. These data indicate that spontaneous pain arising from injured nerve fibers produces a tonic aversive state that is mediated by the rACC. Identification of the circuits mediating aversiveness of chronic pain should facilitate the development of improved therapies.
Keywords: Spontaneous pain, axotomy, nerve injury, anterior cingulate cortex, negative reinforcement
Introduction
Many patients with pain due to nerve injury exhibit enhanced sensitivity to normally innocuous stimuli of touch or cold (allodynia) and most current preclinical models use enhanced responses to evoked stimuli as the primary measure of neuropathic pain [5; 42; 50; 28]. However, most patients with neuropathic pain report that it has either a continuous or paroxysmal component that is not related to any applied somatic stimulus. Because the measurement of non-evoked pain in animals has been difficult, its underlying mechanisms are poorly understood and this represents a major barrier to the development of effective treatments.
Nerve injury induced spontaneous pain has recently been shown to be demonstrable through negative reinforcement [28]. Importantly, agents and manipulations that are clinically effective in alleviating neuropathic pain in patients, such as clonidine, produced conditioned place preference in nerve injured, but not sham-operated, rats. Moreover, administration of lidocaine into the RVM induced conditioned place preference in nerve-injured rats indicating that blocking descending facilitation alleviates nerve-injury induced spontaneous pain [28]. Importantly, these treatments elicited conditioned place preference when administered at sites (e.g., spinally or within the rostral ventromedial medulla) that are not a part of the reward pathways [28]. Critically, the studies confirm that partial injury to peripheral nerves in rats elicits spontaneous pain and support place conditioning as a valid approach to study the clinically important problem of neuropathic pain and to assess the efficacy of treatments for it.
One prominent mechanistic hypothesis of neuropathic pain is that it results from ectopic discharge produced following injury to peripheral nerves [14; 15]. However, whether the pain state is a consequence of increased activity in injured fibers, or in adjacent uninjured but abnormal, fibers remains unresolved with conflicting data in the literature [45; 59; 32]. A related question is whether complete axotomy induces pain or simply produces numbness and whether the autotomy behaviors following axotomy are a measure of pain [44]. These questions remain unresolved as denervation prevents the assessment of evoked behavioral responses as well as classification of the primary afferent fibers that are ectopically active. Here, we have used the principle of negative reinforcement to determine whether animals with axotomy, producing partial or complete hindpaw denervation, exhibit tonic pain.
The use of negative reinforcement to reveal spontaneous pain relies on the aversive motivational properties of the affective component of pain. Both human and preclinical studies implicate the anterior cingulate cortex (ACC) in processing the affective component of pain [41; 7; 24; 25]. In the current work we investigated whether the tonic aversive state resulting from injuries to peripheral nerves is mediated by the ACC.
Materials and Methods
Male Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN), 250–350 g at the time of testing, were maintained in a climate-controlled room on a 12 hr light/dark cycle (lights on at 7:00 A.M.), and food and water were available ad libitum. All experiments were performed in accordance with the policies and recommendations of the International Association for the Study of Pain and the National Institutes of Health guidelines for the handling and use of laboratory animals and received approval from the Institutional Animal Care and Use Committee of the University of Arizona. All efforts were made to minimize animal suffering and reduce the number of animals used.
Surgical Procedures
Intrathecal catheterization
Rats underwent surgery for implantation of an intrathecal catheter under halothane anesthesia (polyethylene-10 tubing; 7.5 cm) as described previously [58] for drug administration at the level of the lumbar cord. Rats then received gentamicin and were allowed to recover 7 days before any behavioral testing and undergoing axotomy or sham axotomy surgeries. Pilot studies demonstrated that shorter recovery times resulted in reduced exploration of conditioned place preference (CPP) chambers and resultant preconditioning chamber bias, likely due to incomplete recovery from the surgery. The behavior of the rats was monitored carefully for any visual indication of motor disorders or change in weight or general health. Any rats showing any signs of motor disorders or severe change in weight were immediately euthanized (<10% of the rats).
RVM cannulation
Animals were anesthetized with injection of ketamine–xylazine (100 mg/kg, i.p.) and placed in a stereotaxic apparatus. Bilateral cannulation of the RVM was performed as previously described [3]. Two 26 gauge guide cannulas separated by 1.2 mm (Plastics One, Roanoke, VA) were directed toward the lateral portions of the RVM (anteroposterior, −11.0 mm from bregma; lateral, ±0.6 mm; dorsoventral, −7.5 mm from the dura mater [35]). The guide cannulas were cemented in place and secured to the skull by small stainless steel machine screws. Rats then received gentamicin and were allowed to recover 7 days before any behavioral testing and undergoing surgeries for SNL, axotomy, and their respective sham operated controls. No signs of weight loss or distress were observed following cannulation surgeries.
Axotomy
Sciatic nerve axotomy resulting in partial denervation was performed as previously described [51; 1] 7 days following implantation of intrathecal catheters. Rats were anaesthetized with isoflurane (Dose: 2–3 l/min, 4%/vol until anesthetized, then 2.5%/vol) throughout the surgical procedure. A 1 cm incision was made and the sciatic nerve was exposed and sectioned proximal to its bifurcation into the tibial and peroneal divisions. A 5–10 mm segment of nerve was removed to prevent regeneration. Separate groups of rats received axotomy of both the sciatic and saphenous nerves for total denervation of the hindpaw as previously described [51; 1]. For saphenous nerve axotomy, a 1 cm incision was made along the inner thigh and a 5–10 mm segment of the nerve was removed to prevent regeneration. Sham controls underwent surgery in which the sciatic nerve or the sciatic and saphenous nerves were exposed, but not severed. All rats received gentamicin following surgery.
Spinal Nerve Ligation
The surgical procedure for L5/L6 spinal nerve ligation was performed according to that described previously [27]. Sham-operated control rats were prepared in an identical manner except that the L5/L6 spinal nerves were not ligated. The behavior of the rats was monitored carefully for any visual indication of motor disorders or change in weight or general health.
rACC Lesions
Rats underwent rACC lesions immediately followed by RVM cannulation to diminish the number of surgeries performed. Ibotenic acid lesions of the rostral ACC were performed as previously described[25]. Ibotenic acid (0.5 mg/ml) was dissolved in 0.1 M PBS and adjusted to pH 7.2–7.4. Animals were anesthetized with injection of ketamine–xylazine (100 mg/kg, i.p.) and placed in a stereotaxic apparatus. An injection cannula (30- gauge stainless steel tubing) filled with the ibotenic acid or 0.1 M PBS was connected to a microinfusion pump (Stoelting, Wood Dale, IL) via PE 10 tubing. The cannula was directed towards the rostral ACC (AP +2.6 mm from bregma, DV −2.5 mm, ML ±0.6 mm [35]). Six minutes after lowering the cannula to rostral ACC, 0.6 µlibotenic acid or PBS was slowly microinjected over a period of 6 min. Following microinjection, the cannula remained in the rostral ACC for 7 min to allow diffusion of the drug. This procedure was then repeated in the opposite hemisphere. While still anesthestized, bilateral cannulation of the RVM was performed as previously described [3]. Two 26 gauge guide cannulas separated by 1.2 mm (Plastics One, Roanoke, VA) were directed toward the lateral portions of the RVM (anteroposterior, −11.0 mm from bregma; lateral, ±0.6 mm; dorsoventral, −7.5 mm from the dura mater [35]). The guide cannulas were cemented in place and secured to the skull by small stainless steel machine screws. Rats then received gentamicin and were allowed to recover 7 days before SNL or SNL sham surgeries. No overt signs of distress (e.g. prolonged lethargy, weight loss) were observed in any animals following these surgeries. Animals recovered for 7 days before any behavioral tests were performed.
Drug administration
Spinal drug administration was performed by injection of 5 µl saline or 10 µg clonidine in 5 µl saline followed by a 9 µl saline flush as previously demonstrated to elicit CPP in rats with nerve injury [28].Drug administration into the RVM was performed by slowly expelling 0.5 µl of saline or lidocaine (4% w/v) through a 33 gauge injection cannula inserted through the guide cannula and protruding an additional 1mm into fresh brain tissue to prevent backflow of drug into the guide cannula as previously demonstrated to elicit CPP in rats with nerve injury [28]. Cocaine (1mg/kg) was administered into awake rats by intravenous injection into the tail vein. All injections were delivered in a separate room, and rats were placed into the CPP chambers within 2 min of injection.
Tactile hypersensitivity
Rats were placed in suspended wire-mesh cages and allowed to acclimate for 30 min. The withdrawal threshold of the hindpaw was measured in response to probing of the plantar surface with a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) in logarithmically spaced increments ranging from 0.41 to 15 gm (4–150 N). Withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (“up and down” method) and analyzed using a Dixon nonparametric test[16; 9].
Thermal hypersensitivity
Rats were allowed to acclimate for 30 min in Plexiglas enclosure on a glass plate maintained at room temperature on a plantar test analgesia meter Hargreaves Apparatus (UgoBasile, Italy). An infrared radiant heat source was directed onto the plantar surface of the hindpaw. A motion detector halted both infrared source and timer when the paw was withdrawn. Baseline latencies were established at 17–25 sec to allow a sufficient window for the detection of possible hyperalgesia. A maximal cutoff of 33 sec was used to prevent tissue damage.
Conditioned place preference
The single trial conditioned place preference protocol was performed as previously described [28]. Starting 7 days post nerve-injury/sham surgery, all rats underwent a 2 day habituation period in which they were exposed to the environment with full access to all chambers for 30 min. Behavior was recorded for 15 min on day 3 and analyzed to verify no pre-conditioning chamber preference. The following day, rats received the appropriate control (i.e. vehicle) paired with a randomly chosen chamber in the morning, and the appropriate drug treatment paired with the other chamber 4 hr later in the afternoon. Chamber pairings were counterbalanced. On test day, 20–24 hours following the afternoon pairing, rats were placed in the CPP box with access to all chambers and their behavior recorded for 15 min for analysis for chamber preference.
Tissue verification
Following behavioral testing, 0.5 µl Indian ink was injected bilaterally into the RVM for verification of cannula placement as previously described [3; 56]. Rats were perfused transcardially with 0.9% 100–150 ml saline followed by 10% formalin solution, brains were rapidly removed and post-fixed in 10% formalin. Following cryopreservation by immersion into 30% sucrose solution overnight at 4°C, RVM sections were cut serially into 30 µm thick coronal sections on a freezing microtome (Microm, HM 525; International GmbH, Germany). Data from animals with incorrectly placed cannulae were not included within the data analysis and were used as “off-site” controls. For rACC lesion verification, 30 mm thick coronal sections were stained with cresyl violet. Lesions were indicated by neuronal cell loss and glial cell proliferation that was especially apparent around the lesion borders. Inclusion criteria had a minimum percent bilateral damage of 50% and at least 30% damage in the least damaged hemisphere. As previously reported, histological processing produced unavoidable tearing in the lesioned area in some sections across all experimental groups [25]. Only animals with verified cannula placement within the RVM were included in the data analysis.
Statistical Analysis
For analysis of evoked pain behaviors, significant changes from pre-surgery baseline control values were detected by ANOVA, followed by Bonferronipost test. These evaluations were all performed using GraphPad Prism 5.03 for Windows (GraphPad Software, San Diego CA, http://www.graphpad.com). For CPP experiments, data was analyzed before conditioning (baseline) and after conditioning using two-factor ANOVA (chambers vs. treatment) followed by Bonferroni test. Difference from baseline scores were calculated for each rat using the formula: test time in chamber - preconditioning time spent in chamber. Difference scores from baseline for the drug paired chamber between nerve injured and sham operated rats were analyzed using paired t-tests. For all analyses, significance was set at P<0.05.
Results
Sciatic axotomy (partial hindpaw denervation) induces spontaneous pain
Axotomy of the sciatic nerve results in denervation of the plantar and lateral portions of the hindpaw [51], as well as ectopic discharge from the injured fibers [20] and development of autotomy starting approximately 2 weeks following injury [52; 53]. Therefore, all rats underwent CPP, with conditioning day occurring 8 days following axotomy, a time-point prior to observable autotomy behaviors. Axotomized and sham axotomy rats showed equivalent pre-conditioning time in the saline and clonidine paired chambers indicating no pre-conditioning bias for either group, so data were pooled for graphical representation (Fig 1a). The number of chamber crossings were slightly decreased in the axotomy group but this difference did not reach statistical significance (13 ± 2 for sham vs 9 ± 2 for axotomized rats, p>0.1). Spinal administration of clonidine (10 µg) resulted in a robust increase in time spent in the clonidine paired chamber in the axotomized rats (Fig 1a, *p<0.05 vs pre-conditioning). Difference from baseline score confirmed that axotomized, but not sham control rats, increased time spent in the clonidine paired chamber compared to baseline (Fig 1b, *p<0.05 vs sham).
Figure 1.
Spinal clonidine blocks sciatic axotomy-induced spontaneous pain. A) Axotomized and sham rats showed equivalent time in the pairing chambers prior to conditioning day. As no differences were observed between axotomized and sham operated rats, pre-conditioning values were pooled for graphical representation. Axotomized, but not sham operated rats showed clear preference for the chamber paired with spinal clonidine (10 mg).* indicates significant difference from pre-conditioning time, p<0.05. B) Difference scores, calculated as test time – preconditioning time spent in the clonidine paired chamber confirms that axotomized, but not sham operated rats increased time spent in the clonidine paired chamber.* indicates significant difference from shams, p<0.05. Graphs depict means ± SEM, n=5.
Sciatic and saphenous (complete hindpaw denervation) induces spontaneous pain
Axotomy of the sciatic and sapheneous nerves results in denervation of the entire hindpaw [51], as well as ectopic discharge from injured fibers [20] and autotomy behaviors that are observed earlier than sciatic nerve axotomy alone [52]. All rats were tested with conditioning day at 8 days following axotomy, a time point that preceded observable autotomy. Full denervation of the hindpaw in the rats included in the study was verified by lack of response to administration of radiant heat to the hindpaw, with all axotomized rats reaching cut-off latencies of 32-s on this test (data not shown). Axotomized and sham axotomy rats showed equivalent pre-conditioning time in the saline and lidocaine paired chambers indicating no pre-conditioning bias for either group, so data were pooled for graphical representation (Fig 2a). There were no differences in the total number of chamber crossings (16 ± 1 for sham vs 14 ± 2 for axotomized rats, p>0.05) indicating that axotomy did not impair the ability of these animals to explore the chambers. RVM administration of lidocaine induced a significant preference for the lidocaine paired chamber (Fig 2a, *p<0.05 vs pre-conditioning). Difference from baseline score confirmed that the axotomized, but not sham control rats increased time spent in the RVM lidocaine paired chamber (Fig 2b, *p<0.05 vs sham).
Figure 2.
RVM lidocaine blocks sciatic and saphenous axotomy induced spontaneous pain. A) Axotomized and sham rats showed equivalent time in the pairing chambers prior to conditioning day. As no differences were observed between axotomized and sham operated rats, pre-conditioning values were pooled for graphical representation. Axotomized, but not sham operated rats spent more time in the lidocaine paired chamber. * indicates significant difference from pre-conditioning time, p<0.05. B) Difference scores confirm that axotomized, but not sham operated rats increased time spent in the RVM lidocaine paired chamber. * indicates significant difference from shams, p<0.05. Graphs depict means ± SEM, n=6.
Histological verification of rostral ACC lesion
Consistent with previous reports [25], bilateral infusions of ibotenic acid (0.5 µg/ml) into the rostral ACC (rACC) produced neuronal cell loss and proliferation of small glial cells with clearly definable borders between lesioned and healthy areas (Fig 3). Lesion areas were mapped as demonstrated in Fig 3 showing the smallest (dark gray) and largest (light gray) lesions of the rACC. All animals included in analyses met lesion inclusion criterion as described in Materials and Methods.
Figure 3.
Representations of the largest and smallest rACC lesions are depicted on map images representing slices at Bregma +2.20, +2.70, and +3.20 mm. Photomicrographs of representative coronal sections through the rostral ACC at Bregma +2.70 mm. Sections from a rostral ACC sham lesion (left) and a rostral ACC lesion (right) at the same antero-posterior level. Lesion areas are evidenced by neuronal cell loss and by proliferation of smaller glial cells, especially apparent around the borders of the lesions. Sections were stained with cresyl violet.
Rostral ACC lesions do not block evoked pain
The paw withdrawal thresholds to probing with von Frey filaments and the withdrawal latencies from noxious radiant heat were determined 7 days following SNL or sham SNL surgeries in animals with rACC lesion or sham rACC lesion (Fig 4 a,b respectively). Following SNL, both rACC lesioned and rACC sham animals demonstrated reduced withdrawal thresholds to von Frey filaments (Fig 4a, *p<0.05 vsrACC sham/sham) and reduced withdrawal latencies to noxious thermal stimulation (Fig 4b, *p<0.05 vsrACC sham/sham). Direct comparison of post-SNL values in rats with rACC sham and rACC lesion shows no difference (p>0.05) indicating that lesions of the rACC failed to alter SNL-induced tactile allodynia or thermal hyperalgesia. No changes in sensory thresholds were observed in animals with sham SNL and either sham rACC or lesion or the rACC.
Figure 4.
Lesions of the rACC did not block SNL-induced evoked pain. SNL surgeries produced equivalent levels of tactile allodynia (A) and thermal hyperalgesia (B) in rats with rACC sham and rACC lesion when tested 7 days following surgery. *indicates significant difference from pre-SNL values, p<0.05. Rats that received SNL sham surgeries did not show tactile allodynia or thermal hyperalgesia. Graphs depict means ± SEM, n=9–13.
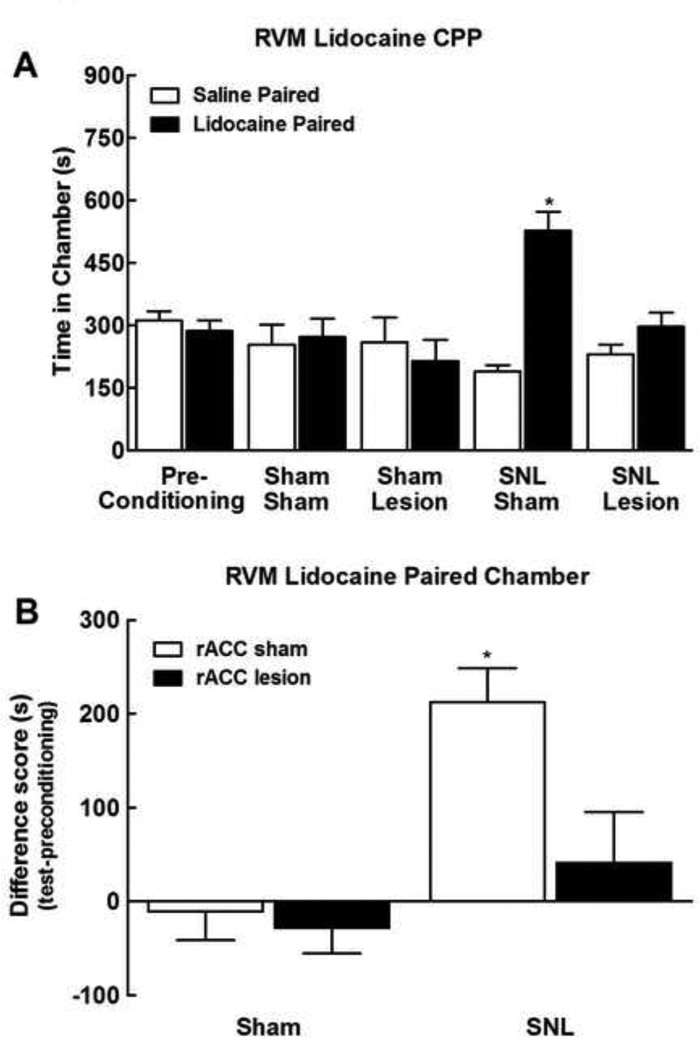
Rostral ACC lesions block CPP from pain relief
The week following testing of SNL-induced thermal and tactile allodynia, rats underwent the single-trial CPP procedure in which RVM administration of lidocaine was paired with a distinct chamber as detailed in the Materials and Methods. Pre-conditioning times spent in the saline or lidocaine paired chambers were equivalent across all treatment groups (p>0.05). As no group differences were observed, data were pooled across groups for graphical representation (Fig 5a). Rats with SNL that had received sham rACC surgeries (SNL/Sham) showed clear preference for the lidocaine paired chamber (Fig 5a, *p<0.05 vs pre-conditioning). In contrast, SNL treated rats with rACC lesions (SNL/Lesion) showed no preference for the lidocaine paired chamber. Sham SNL rats had equivalent post conditioning times spent in the saline and lidocaine paired chambers irrespective of whether they received sham rACC lesion (Sham/Sham) or rACC lesion (Sham/Lesion). Difference from baseline scores verified that rACC lesions blocked RVM lidocaine induced CPP in the SNL rats, as only SNL rats with sham rACC lesions demonstrated increased time spent in the lidocaine paired chamber (Fig 5b, *p<0.05 vs sham/rACC sham).
Figure 5.
Lesions of the rACC blocked CPP induced by RVM administration of lidocaine. A) SNL treated rats showed increased time spent in the RVM lidocaine paired chamber (SNL/Sham) whereas rats that had rACC lesions did not show preference to the RVM lidocaine paired chamber (SNL/Lesion). *indicates difference from pre-conditioning time, p<0.05. B) Difference scores, calculated as test time – preconditioning time spent in the RVM lidocaine paired chamber confirms that SNL rat with sham rACC lesions increased time spent in the RVM lidocaine paired chamber, whereas rACC lesions failed to increase time spent in the RVM lidocaine paired chamber. *indicates difference from SNL sham with rACC sham, p<0.05. Graphs depict means ± SEM, n=6.
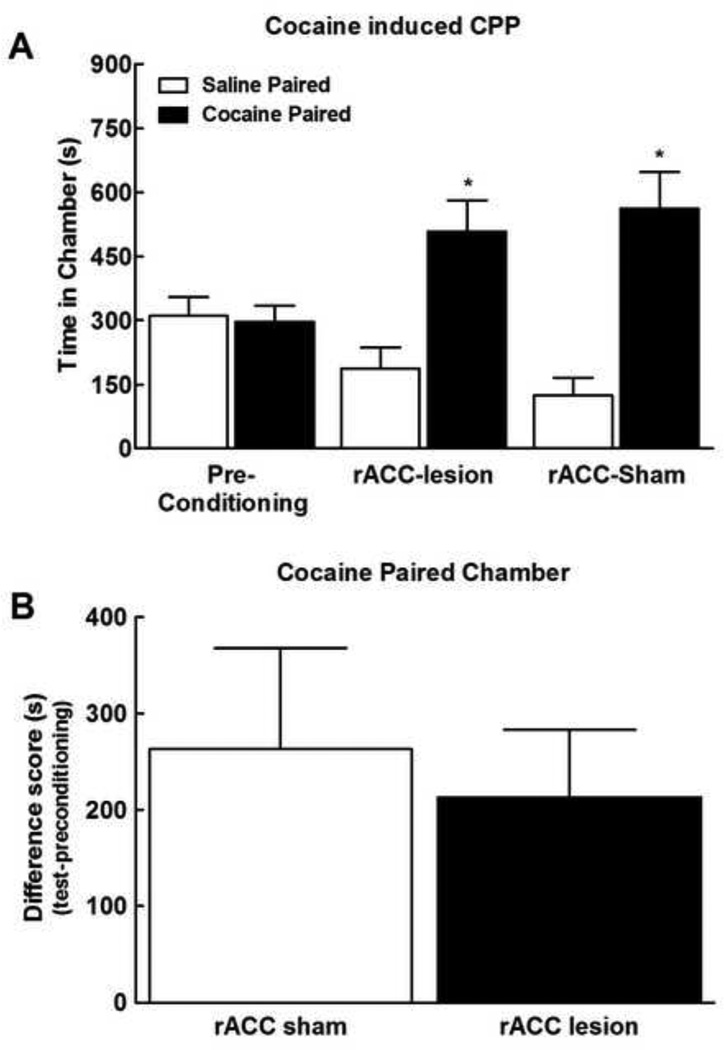
Rostral ACC lesions do not block cocaine induced reward
To verify that the rACC lesions did not block the ability of the rats to acquire rewarding stimuli independent of noxious input, we determined whether rACC lesions block cocaine-induced reward. Systemic administration of 1 mg/kg cocaine (i.v.) produces CPP in animals with sham rACC lesions. Similar levels of CPP were demonstrated in rACC lesioned rats, with time-spent in the cocaine paired chamber elevated in both sham and rACC lesioned rats (Fig 6a, *p<0.05 vs preconditioning). Difference from baseline scores verified that both the sham rACC and the lesioned rACC rats demonstrated equivalent increased time spent in the lidocaine paired chamber (Fig 6b, p>0.05).
Figure 6.
Systemic (i.v.) administration of cocaine (1 mg/kg) produced significant place preference as indicated by increased time spent in the cocaine paired chamber. *indicates difference from pre-conditioning time, p<0.05. Difference from baseline scores confirmed that rACC sham and rACC lesion treated rats demonstrated equivalent increased time spent in the cocaine paired chamber. Graphs depict means ± SEM, N=11; 7 ACC lesion and 4 ACC sham.
Discussion
Pain elicits an aversive state that can serve as a motivating force to shape behavior. Our previous work demonstrated that relief of the tonic component of nerve injury-induced pain elicits reward manifested as a preference for a context associated with pain relief (i.e., conditioned place preference) [28]. Here, we demonstrate that (a) rACC lesion blocks aversiveness of chronic pain in a widely used model of experimental neuropathic pain; (b) aversiveness and evoked thresholds can be dissociated within the rACC; (c) axotomy elicits spontaneous pain; and (d) such pain results from injured nerves. Thus, peripheral nerve injury elicits a tonic aversive state that likely results from increased activity of injured nerve fibers and that requires neuronal activity in the rACC.
Peripheral nerve injury is characterized by increased excitability and ectopic discharge in primary afferent nociceptors, thought to contribute to pain by direct activation of pain pathways and/or by establishing and maintaining a state of central sensitization [51; 13]. However, the contribution of specific classes of injured or uninjured primary afferent fibers in these processes remains unclear. Partial nerve injury models reveal increased activity in injured, myelinated A fibers, but not in C-nociceptors [55]. However, such injuries are accompanied by increased discharge of uninjured C-fibers from neighboring ganglia (for review see [43]). Studies employing dorsal rhizotomy have led to conflicting data with support for both injured and uninjured fibers in mediating pain [45; 59; 32] and evidence exists that dorsal rhizotomy itself can elicit pain [33]. However, clinical observations have consistently supported the idea that injured fibers are important in driving pain as stimulation of a neuroma produces pain and infiltration with local anesthetics relieves both ongoing pain and evoked hyperalgesia [21; 36].
To date, pain resulting from axotomy of peripheral nerves has not been conclusively demonstrated as 1) ectopically discharging axons in a neuroma are not identified as nociceptors; 2) evoked behavioral hypersensitivity following axotomy is difficult or impossible to measure; 3) autotomy or self-mutilation that occurs after axotomy may reflect the presence of pain but might also suggest lack of sensation, or numbness [44; 12]; and 4) there were no validated models of spontaneous pain. The present studies evaluated reward from pain relief in animals with either partial or complete hindpaw denervation allowing for direct assessment of the role of injured fibers in promoting spontaneous neuropathic pain. Our data indicate selective place preference to either spinal clonidine or RVM lidocaine in animals with sciatic or sciatic/saphenous axotomy suggesting that spontaneous pain arises from injured nerve fibers, consistent with findings in humans. Notably, place preference occurs with a single pairing to pain relief, a finding consistent with severe and sustained aversiveness resulting from peripheral nerve injury. Our observations in animals with complete denervation of the hindpaw also provide an important control eliminating concerns for pain resulting from tactile stimulation potentially arising fromambulation within the testing apparatus. These data cannot address, however, whether additional contributions to spontaneous pain, or evoked hypersensitivity, may result from uninjured, but abnormal adjacent fibers.
Use of negative reinforcement to demonstrate ongoing pain [28] relies on the presumption that such pain elicits a tonic aversive motivational state. The rACC has been implicated in processing of the aversive consequences of noxious stimulation that motivates avoidance learning [11; 25; 26]. Human studies have implicated the ACC in the processing of acute painful stimuli. Altered perception of pain-related unpleasantness in the absence of changes in stimulus intensity has been demonstrated to correlate with activity in the ACC, but not the somatosensory cortex [41]. Although imaging studies have investigated changes in brain activity in response to acute noxious stimulation across a variety of modalities [6; 10; 7; 48; 8; 2; 46], studies of changes in the brain associated with clinical pain, such as neuropathic pain, have occurred relatively recently [34; 47]. Neuropathic pain patients show increased activity in the insula and ACC without significant changes in the thalamus and somatosensory cortices (S1 and S2) [34]. In contrast, brain activity changes to brush-evoked allodynia indicate that dynamic allodynia is predominately associated with changes in lateral thalamus, and S1, S2 regions rather than the ACC and insula [39; 37; 18; 54].
Preclinical studies have shown that lesion of the rACC prevented conditioned place aversion to a chamber paired with formalin, whereas formalin-induced acute pain-related behaviors, which require the localization of the stimulus and its identification as painful (e.g. lifting, licking, and flinching the stimulated paw) were unaffected [25]. Fuchs and colleagues demonstrated that rACC lesions block avoidance learning in nerve injured rats, while not altering mechanical hyperalgesia [17; 31]. These studies support the role of the rACC in mediating the aversive component of chronic pain. Importantly, the above studies focus on avoidance of the aversive motivational state of evoked pain, rather than on the rewarding properties of relief of spontaneous pain. To date, no studies have directly assessed the role of the ACC in spontaneous pain resulting from nerve injury or in the mechanism of negative reinforcement. Our studies demonstrate that the rACC is required for the reward associated with relief from nerve-injury induced spontaneous pain. Thus, RVM lidocaine, previously demonstrated to block nerve injury-induced evoked hypersensitivity and to produce place preference selectively in animals with nerve injury [28], no longer produced reward. One interpretation of these data is that rACC lesions removes the tonic aversive state resulting from the peripheral nerve injury, thus removing the motivational drive to achieve pain relief. Importantly evoked hypersensitivity remained following rACC lesion, consistent with previous observations [31].This finding is critical because it indicates that evoked pain resulting from spontaneous movement of the affected limb is insufficient to fully account for the aversive state of the animal. This is consistent with the clinical observation that many neuropathic pain patients have pain in the absence of movement or stimulation. Notably, Xu and colleagues showed that bilateral microinjection of CNQX, a non-NMDA antagonist, into the ACC of mice with ligation of the peroneal nerve reduced allodynia in both the contralateral and ipsilateral sides, without effect on control animals [57]. The reasons for this finding are not completely clear but may result from differences in methodology and specific site of injection within the ACC. Nevertheless, our studies are consistent with human data demonstrating that 1) brain activity changes associated with dynamic allodynia are observed in the lateral thalamus, and S1, S2 regions rather than the ACC and insula [22; 39; 37; 18; 54]; 2) the unpleasantness of pain can be altered without disrupting the localization or intensity of the stimulus [10; 41] and 3) patients with cingulotomies report diminished pain-related unpleasantness without altering the human subject’s ability to discriminate intensity or localization of the noxious stimulus [19; 23].
An alternate explanation of the present results is that rACC lesions disrupt neural processing relating to learning and memory. The rACC is known to mediate the emotional-affective component of pain whereas the caudal ACC (cACC) mediates cognitive tasks including attention [4]. Importantly, the role of the rACC was determined to be specific to the affective component of the formalin-induced pain as systemic injection of a kappa agonist, known to produce dysphoria in humans [40] produced conditioned place avoidance in both rACC lesioned and rACC sham lesioned rats [25]. The present studies are dependent on reward induced by pain relief, rather than aversion induced by pain. Therefore, it remains possible that these lesions block the animal’s ability to associate the context to the reward induced by pain relief rather than reflecting a reduction in the primary aversive quality of the tonic pain per se. To address this, consistent with previous reports, we showed that rACC lesions did not reduce the animal’s ability to acquire place preference to the positive reinforcer cocaine [49]. These data support the conclusion that rACC lesions do not have a general disruptive effect on reward learning, but instead reflect a deficit specifically relating to the acquisition or expression of pain-relief induced negative reinforcement. This is in line with studies indicating that ACC neurons can acquire responses to environmental cues predicting a painful stimulus [29; 30]. Moreover, this is also consistent with studies implicating the rACC in opioid analgesia and expectation of pain relief (i.e. the placebo effect) [38]. Such studies implicate the rACC in both the acute response to noxious stimulation and to the learning that underlies recognition of pain-predictive cues [25] and cues predictive of pain relief [38].
In summary, we have demonstrated that nerve injury produces spontaneous pain mediated by injured afferent fibers. Additionally, rACC lesion blocked the aversiveness of pain resulting from peripheral nerve injury in spite of maintained evoked hypersensitivity. Efforts focusing on mechanisms underlying nerve-injury induced spontaneous pain and the circuits that elicit the aversiveness of this condition may yield insights into molecular targets with translational relevance for treatment of neuropathic pain. Additionally, as a subset of patients with neuropathic pain also suffer from tactile and cold allodynia, further exploration of differences in mechanisms underlying spontaneous pain and allodynia are critical as treatment for each of these components of neuropathic pain likely differ.
Acknowledgements
This work was supported by NS 066958.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
References
- 1.Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol. 2001;85(2):644–658. doi: 10.1152/jn.2001.85.2.644. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22(12):5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol. 1996;76(1):571–581. doi: 10.1152/jn.1996.76.1.571. [DOI] [PubMed] [Google Scholar]
- 7.Casey KL. Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci U S A. 1999;96(14):7668–7674. doi: 10.1073/pnas.96.14.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey KL, Morrow TJ, Lorenz J, Minoshima S. Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography. J Neurophysiol. 2001;85(2):951–959. doi: 10.1152/jn.2001.85.2.951. [DOI] [PubMed] [Google Scholar]
- 9.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 10.Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384(6606):258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 11.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 12.Devor M. Sensory basis of autotomy in rats. Pain. 1991;45(2):109–110. doi: 10.1016/0304-3959(91)90174-V. [DOI] [PubMed] [Google Scholar]
- 13.Devor M. Neuropathic pain: what do we do with all these theories? Acta Anaesthesiol Scand. 2001;45(9):1121–1127. doi: 10.1034/j.1399-6576.2001.450912.x. [DOI] [PubMed] [Google Scholar]
- 14.Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7(1 Suppl 1):S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196(1):115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 16.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 17.Donahue RR, LaGraize SC, Fuchs PN. Electrolytic lesion of the anterior cingulate cortex decreases inflammatory, but not neuropathic nociceptive behavior in rats. Brain Res. 2001;897(1–2):131–138. doi: 10.1016/s0006-8993(01)02103-5. [DOI] [PubMed] [Google Scholar]
- 18.Ducreux D, Attal N, Parker F, Bouhassira D. Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain. 2006;129(Pt 4):963–976. doi: 10.1093/brain/awl016. [DOI] [PubMed] [Google Scholar]
- 19.Foltz EL, White LE., Jr Pain "relief" by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 20.Govrin-Lippmann R, Devor M. Ongoing activity in severed nerves: source and variation with time. Brain Res. 1978;159(2):406–410. doi: 10.1016/0006-8993(78)90548-6. [DOI] [PubMed] [Google Scholar]
- 21.Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51(2):175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh J-C, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63(2):225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- 23.Hurt RW, Ballantine HT., Jr Stereotactic anterior cingulate lesions for persistent pain: a report on 68 cases. Clin Neurosurg. 1974;21:334–351. doi: 10.1093/neurosurgery/21.cn_suppl_1.334. [DOI] [PubMed] [Google Scholar]
- 24.Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2(5):403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- 25.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98(14):8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7(4):398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 28.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12(11):1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama T, Tanaka YZ, Mikami A. Nociceptive neurons in the macaque anterior cingulate activate during anticipation of pain. Neuroreport. 1998;9(11):2663–2667. doi: 10.1097/00001756-199808030-00044. [DOI] [PubMed] [Google Scholar]
- 30.Koyama T, Kato K, Mikami A. During pain-avoidance neurons activated in the macaque anterior cingulate and caudate. Neurosci Lett. 2000;283(1):17–20. doi: 10.1016/s0304-3940(00)00894-6. [DOI] [PubMed] [Google Scholar]
- 31.LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol. 2004;188(1):139–148. doi: 10.1016/j.expneurol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Dorsi MJ, Meyer RA, Belzberg AJ. Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers. Pain. 2000;85(3):493–502. doi: 10.1016/S0304-3959(00)00250-5. [DOI] [PubMed] [Google Scholar]
- 33.Lombard MC, Nashold BS, Jr, Albe-Fessard D, Salman N, Sakr C. Deafferentation hypersensitivity in the rat after dorsal rhizotomy: a possible animal model of chronic pain. Pain. 1979;6(2):163–174. doi: 10.1016/0304-3959(79)90123-4. [DOI] [PubMed] [Google Scholar]
- 34.Moisset X, Bouhassira D. Brain imaging of neuropathic pain. NeuroImage. 2007;37(Supplement 1):S80–S88. doi: 10.1016/j.neuroimage.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- 36.Petersen KL, Fields HL, Brennum J, Sandroni P, Rowbotham MC. Capsaicin evoked pain and allodynia in post-herpetic neuralgia. Pain. 2000;88(2):125–133. doi: 10.1016/S0304-3959(00)00311-0. [DOI] [PubMed] [Google Scholar]
- 37.Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83(3):459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 38.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and Opioid Analgesia-- Imaging a Shared Neuronal Network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 39.Peyron R, Garcia-Larrea L, Gregoire MC, Convers P, Lavenne F, Veyre L, Froment JC, Mauguiere F, Michel D, Laurent B. Allodynia after lateral-medullary (Wallenberg) infarct. A PET study. Brain. 1998;121(Pt 2):345–356. doi: 10.1093/brain/121.2.345. [DOI] [PubMed] [Google Scholar]
- 40.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233(4765):774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 41.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 42.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139(2):243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Ringkamp M, Meyer RA. Injured versus uninjured afferents: Who is to blame for neuropathic pain? Anesthesiology. 2005;103(2):221–223. doi: 10.1097/00000542-200508000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Rodin BE, Kruger L. Deafferentation in animals as a model for the study of pain: an alternative hypothesis. Brain Res. 1984;319(3):213–228. doi: 10.1016/0165-0173(84)90011-0. [DOI] [PubMed] [Google Scholar]
- 45.Sheen K, Chung JM. Signs of neuropathic pain depend on signals from injured nerve fibers in a rat model. Brain Res. 1993;610(1):62–68. doi: 10.1016/0006-8993(93)91217-g. [DOI] [PubMed] [Google Scholar]
- 46.Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol. 2005;15(4):478–487. doi: 10.1016/j.conb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain. 2009;10(11):1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79(2–3):105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 49.Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11(11):4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- 50.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135(1–2):7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Wall JT, Cusick CG. Cutaneous responsiveness in primary somatosensory (S-I) hindpaw cortex before and after partial hindpaw deafferentation in adult rats. J Neurosci. 1984;4(6):1499–1515. doi: 10.1523/JNEUROSCI.04-06-01499.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979;7(2):103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- 53.Wall PD, Scadding JW, Tomkiewicz MM. The production and prevention of experimental anesthesia dolorosa. Pain. 1979;6(2):175–182. doi: 10.1016/0304-3959(79)90124-6. [DOI] [PubMed] [Google Scholar]
- 54.Witting N, Kupers RC, Svensson P, Jensen TS. A PET activation study of brush-evoked allodynia in patients with nerve injury pain. Pain. 2006;120(1–2):145–154. doi: 10.1016/j.pain.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 55.Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early Onset of Spontaneous Activity in Uninjured C-Fiber Nociceptors after Injury to Neighboring Nerve Fibers. J Neurosci. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. (8; RC140:) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25(2):409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008;28(29):7445–7453. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiology and Behavior. 1976;17(2):1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 59.Yoon YW, Na HS, Chung JM. Contributions of injured and intact afferents to neuropathic pain in an experimental rat model. Pain. 1996;64(1):27–36. doi: 10.1016/0304-3959(95)00096-8. [DOI] [PubMed] [Google Scholar]