Abstract
Treatment of the protected and unprotected nucleosides with 1,3-dibromo-5,5- dimethylhydantoin in aprotic solvents such as CH2Cl2, CH3CN, or DMF effected smooth bromination of uridine and cytidine derivatives at C-5 of pyrimidine rings as well as adenosine and guanosine derivatives at C-8 of purine rings. Addition of Lewis acids such as trimethylsilyl trifluoromethanesulfonate enhanced efficiency of bromination.
Keywords: Bromination; 1,3-Dibromo-5,5-dimethylhydantoin; Lewis acids; Purines; Pyrimidines; Nucleosides
Halogen-substituted nucleosides and especially uracil derivatives substituted at C-5 and adenine derivatives substituted at C-8 with bromine have been shown to possess interesting synthetic and biological properties.1–2 The halogenated C-5 pyrimidine and C-8 purine nucleosides are often used in reactions involving direct displacement with nucleophiles1–2 and in transition metal catalyzed cross-coupling reactions3 resulting in the syntheses of a variety of unnatural nucleosides of biological interest and fluorescent probes.4 A number of 5-substituted uracil derivatives, especially arabinofuranosyl- and 2′-deoxyuridines, have been investigated extensively for the clinical treatment of viral diseases.5 For instance, the high-yield coupling of 5-iodouracil derivatives with terminal alkynes afforded 5-alkynyluracil nucleosides with antiviral activity6–7 and such products can be transformed into furanopyrimidine-2-one derivatives which possess potent and selective inhibition of Varicella-Zoster virus.6,8 Radiolabeled 5-bromo- and 5- iodouracil nucleosides are used in cellular biochemistry.9
Halogenated pyrimidine2 and purine1 nucleosides have been prepared by direct reaction with halogens and other halogenating agents but some of these methods required vigorous conditions. The 5-bromination of uracil derivatives has been effected with Br2/Ac2O/AcOH,2 Br2/H2O,10 N- bromosuccinimide (NBS) in DMF11 or ionic liquids,12 combination of 3-chloroperoxybenzoic acid/HBr in aprotic solvents,13 ceric ammonium nitrate (CAN)/LiBr in protic or aprotic solvents,14 or KBr/Oxone.15 Bromination of cytidine at C-5 has been accomplished with Br2/CCl4/hν 16 or NBS in DMF11 or ionic liquids.12 The 8-bromination of adenine or guanine nucleosides has been typically achieved with Br2/AcOH/AcONa17 or NBS/DMF.11
The 1,3-dibromo-5,5-dimethylhydantoin (DBDMH or DBH) is a useful reagent for various organic transformations18–20 including aromatic bromination.21–25 Enhanced reactivity of DBH towards aromatic bromination in the presence of acids has been noted.22–24 Furthermore, Lewis acid-catalyzed benzylic bromination with DBH26 and efficient oxidation of thiols to disulfides with DBH27–28 have been reported. The combination of DBH/TsOH was also used for α-bromination of aliphatic ketones.29 Herein, we report an efficient bromination of pyrimidine (at C-5 position) and purine (at C-8 position) nucleosides with 1,3-dibromo-5,5-dimethylhydantoin in aprotic solvents and the effect of Lewis acids.
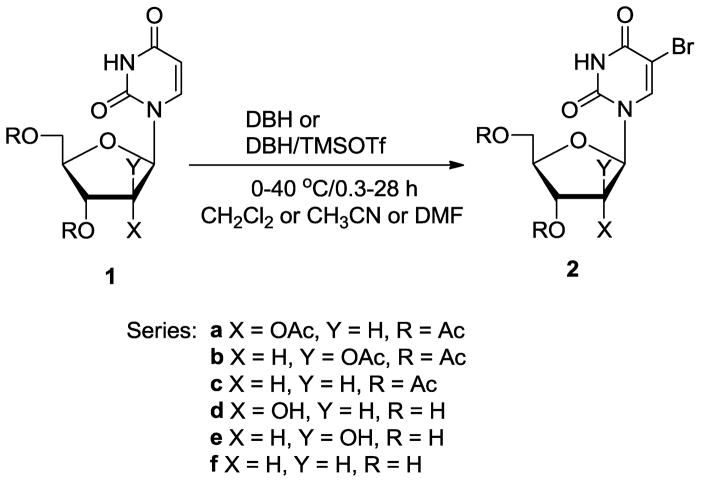
Treatment of 2′,3prime;,5prime;-tri-O-acetyluridine 1a with DBH (1.1 equiv.) in CH2Cl2 at ambient temperature for 28 h gave protected 5-bromouridine 2a in 95% yield (Scheme 1; Table 1, entry 1). Although the DBH reagent can deliver two bromonium equivalents, reaction of 1a with 0.55 eqiuv. of DBH was completed in only 60% yield even after prolonged reaction time (48 h). We found, however, that addition of 0.55 equiv. of a Lewis acid such as trimethylsilyl trifluoromethanesulfonate (TMSOTf) significantly enhanced the efficiency of bromination yielding 2a in 94% yield after only 6 h. (entry 2). Bromination of 1a at elevated temperature (40 °C) afforded 2a quantitatively in only 2 h (entry 3), while bromination at lower temperature was incomplete even after 8 h and required 1.1 equiv. of DBH for complete conversion (entry 4). Increasing the amount of TMSOTf to 1.1 equiv. had no effect on the rate of reaction (entry 5). Bromination with DBH or the DBH/TMSOTf combination was also effective in polar aprotic solvents such as CH3CN and DMF (entries 6–9) providing 2a in shorter reaction times. However, it is noteworthy that bromination in CH2Cl2 provides pure 2a after aqueous workup only and does not require prior evaporation of the solvent from the crude reaction mixture.30 Moreover, other organic acids such as p-toluenesulfonic acid (TsOH) also efficiently catalyzed the bromination (entry 10). Bromination was much less efficient in protic solvents (e.g., MeOH).
Scheme 1.
Bromination of uracil-derived nucleosides 1 with 1,3-dibromo-5,5-dimethylhydantoin (DBH). See Table 1 and 2 for specific reaction parameters.
Table 1.
Effect of various reaction parameters on 5-bromination of 2′,3prime;,5prime;-tri-O-acetyluridine 1a with DBHa
| Entry | Solvent | Temp. (°C) | DBH (equiv.) | TMSOTf (equiv.) | Time (h) | Yieldb,c 2a (%) |
|---|---|---|---|---|---|---|
| 1 | CH2Cl2 | 25 | 1.1 | - | 28 | 95 |
| 2 | CH2Cl2 | 25 | 0.55 | 0.55d | 6 | 94 |
| 3 | CH2Cl2 | 40 | 0.55 | 0.55 | 2 | 98 |
| 4 | CH2Cl2 | 0 | 1.1e | 0.55 | 3 | 98 |
| 5 | CH2Cl2 | 25 | 0.55 | 1.10 | 6 | 91 |
| 6 | CH3CN | 25 | 0.55 | - | 11 | 86f |
| 7 | CH3CN | 25 | 0.55 | 0.55 | 2.5 | 90 |
| 8 | DMF | 25 | 0.55 | - | 0.6 | 95 |
| 9 | DMF | 25 | 0.55 | 0.55 | 0.3 | 98 |
| 10 | CH2Cl2 | 25 | 0.75 | 0.75g | 8 | 94 |
Bromination was performed on 0.1 mmol scale of 1a.
Isolated yield after aqueous work-up.
Purity of the product 2a was determined by TLC and 1H NMR and was higher than 97% unless otherwise noted.
Reaction without TMSOTf showed 60% conversion to 2a (TLC) after 48 h and complete conversion after 68 h with purity over 90% (1H NMR).
Reaction with 0.55 eq. of DBH was complete in 65% after 8 h.
With purity over 90%.
TsOH was used instead of TMSOTf.
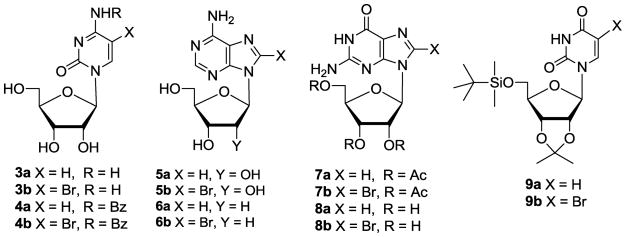
The optimized procedure for the 5-bromination of the uracil ring with DBH has general applicability. For example 1-(2,3,5-tri-O-acetyl-β,D-arabinofuranosyl)uracil 1b and 3′,5′-di-O-acetyl-2′-deoxyuridine 1c were efficiently transformed into 2b30 and 2c using this approach (Scheme 1; Table 2, entries 2–6). Furthermore, bromination of the unprotected uridine 1d using DBH in DMF was completed in only 20 min. producing 5-bromouridine 2d in 75% crystallized yield (entry 7).31 DBH also effected efficient bromination of 1-(β,D-arabinofuranosyl)uracil 1e and the acid sensitive 2′-deoxyuridine 1f (entries 8 and 9). The 5-bromination of cytidine 3a and 4-N-benzoylcytidine 4a with DBH in DMF proceeded smoothly as well providing 3b and 4b31 (Figure 1; Table 3, entries 1 and 2).
Table 2.
5-Bromination of the uracil-derived nucleosides 1a-fa
| Entry | Substrate | Product | Solvent | Temp. (°C) | DBH (equiv.) | TMSOTf (equiv.) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 2a | CH2Cl2 | 25 | 0.55 | 0.55 | 6 | 94c |
| 2 | 1b | 2b | CH2Cl2 | 25 | 0.55 | 0.55 | 10 | 91c |
| 3 | 1b | 2b | CH3CN | 25 | 0.55 | 0.55 | 2 | 98c |
| 4 | 1c | 2c | CH2Cl2 | 25 | 1.10 | - | 18 | 72d |
| 5 | 1c | 2c | CH2Cl2 | 25 | 0.55 | 0.55 | 2.5 | 90c |
| 6 | 1c | 2c | CH2Cl2 | 40 | 0.55 | 0.55 | 0.5 | 93c |
| 7 | 1d | 2d | DMF | 25 | 0.55 | - | 0.33 | 75e |
| 8 | 1e | 2e | DMF | 25 | 0.55 | - | 1 | 65d |
| 9 | 1f | 2f | DMF | 25 | 0.55 | - | 0.75 | 80d |
Bromination was performed on 0.25–2.0 mmol scale.
Isolated yield.
After aqueous work-up with purity higher than 97% (1H NMR).
After column chromatography.
After crystallization.
Figure 1.
Selected nucleoside precursors (series a) and their brominated products (series b).
Table 3.
Bromination of selected purine and pyrimidine nucleosides at ambient temperature (see Figure 1 for structures)
| Entry | Substrate | Product | Solvent | DBH (equiv.) | TMSOTf (equiv.) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| 1 | 3a | 3b | DMF | 0.55 | - | 0.5 | 72c |
| 2 | 4a | 4b | DMF | 0.55 | - | 0.5 | 74c,d |
| 3 | 5a | 5b | DMF | 1.75 | - | 5 | 48c,e |
| 4 | 6a | 6b | DMF | 1.50 | - | 3.5 | 68c,e |
| 5 | 7a | 7b | DMF | 0.55 | - | 2.5 | 83c |
| 6 | 7a | 7b | CH3CN | 0.55 | - | 4 | 98f |
| 7 | 8a | 8b | DMF | 0.75g | - | 2.5 | 51h |
| 8 | 8a | 8b | DMF | 0.60 | 0.55 | 0.5 | 48h |
| 9 | 9a | 9b | DMF | 0.55 | - | 0.5 | 98f |
Bromination was performed on 0.5–1 mmol scale.
Isolated yield.
After column chromatography.
Direct crystallization of the crude reaction mixture from MeOH gave 4b in 46% yield.
Reaction showed formation of the product in approximately 80% yield (TLC).
Isolated yield after aqueous work-up.
Reaction with 0.55 equiv. of DBH was completed in 24 h.
After crystallization from water. Bromination was quantitative as judged by TLC.
The DBH and DBH/TMSOTf combination also effected bromination of purine nucleosides at the 8 position, although reactions usually required higher equivalency of DBH and longer reaction time. Thus, adenosine 5a and 2′-deoxyadenosine 6a afforded 8-bromo products 5b and 6b, albeit in lower isolated yield when compared to the 5-bromination of pyrimidine nucleosides (Table 3, entries 3 and 4). The 2′,3′,5′-tri-O-acetylguanosine 7a and guanosine 8a were converted to 7b and 8b (entries 5–8). Both reactions appear to be quantitative (TLC). However, protected product 7b was isolated in 98% yield after aqueous workup, while 8-bromoguanosine 8b was obtained in approximately 50% yield after crystalization of the crude reaction mixture from H2O. Treatment of inosine with DBH or DBH/TMSOTf failed to afford 8-bromo product.32
The bromination with DBH is also compatible with common protecting groups used in nucleoside chemistry. Thus, treatment of 5′-O-(tert-butyldimethylsilyl)-2′,3′-O-isopropylideneuridine 9a with 0.55 equiv. of DBH in DMF afforded the corresponding 5-bromo product 9b in quantitative yield (entry 9).
In summary, we have developed an efficient procedure for the bromination of all RNA nucleobases with 1,3-dibromo-5,5-dimethylhydantoin in polar aprotic solvents at ambient temperature with or without the presence of Lewis acids. The method offers a general and convenient procedure for the synthesis of C-5 pyrimidine and C-8 purine brominated nucleosides and 2′-deoxynucleosides. The protocol is also compatible with common protecting groups used in nucleoside chemistry.
Acknowledgments
This investigation was supported by an award from NIGMS/NCI (SC1CA138176). We thank Ms. Patricia Theard for her assistance during the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and footnotes
- 1.Srivastava PC, Robins RK, Meyer RB. In: Chemistry of Nucleosides and Nucleotides. Townsend LB, editor. Vol. 1. Plenum Press; New York: 1988. pp. 113–281. [Google Scholar]
- 2.Ueda T. In: Chemistry of Nucleosides and Nucleotides. Townsend LB, editor. Vol. 1. Plenum Press; New York: 1988. pp. 1–112. [Google Scholar]
- 3.Agrofoglio LA, Gillaizeau I, Saito Y. Chem Rev. 2003;103:1875. doi: 10.1021/cr010374q. [DOI] [PubMed] [Google Scholar]
- 4.Clima L, Bannwarth W. Helv Chim Acta. 2008;91:165. [Google Scholar]
- 5.Machida H, Sakata S. In: Nucleosides and Nucleotides as Antitumor and Antiviral Agents. Chu CK, Baker DC, editors. Plenum Press; New York: 1993. pp. 245–264. [Google Scholar]
- 6.Robins MJ, Barr PJ. J Org Chem. 1983;48:1854. [Google Scholar]
- 7.De Clercq E, Descamps J, Balzarini J, Giziewicz J, Barr PJ, Robins MJ. J Med Chem. 1983;26:661. doi: 10.1021/jm00359a008. [DOI] [PubMed] [Google Scholar]
- 8.McGuigan C, Yarnold CJ, Jones G, Velázquez S, Barucki H, Brancale A, Andrei G, Snoeck R, De Clercq E, Balzarini J. J Med Chem. 1999;42:4479. doi: 10.1021/jm990346o. [DOI] [PubMed] [Google Scholar]
- 9.Mercer JR, Xu LH, Knaus EE, Wiebe LI. J Med Chem. 1989;32:1289. doi: 10.1021/jm00126a024. [DOI] [PubMed] [Google Scholar]
- 10.Beltz RE, Visser DW. J Am Chem Soc. 1955;77:736. [Google Scholar]
- 11.Srivastava PC, Nagpal KL. Experientia. 1970;26:220. doi: 10.1007/BF01895597. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, Yap J, Muroyama A, Malhotra SV. Synthesis. 2009:3957. [Google Scholar]
- 13.Ryu EK, MacCoss MJ. J Org Chem. 1981;46:2819. [Google Scholar]
- 14.Asakura J, Robins MJ. J Org Chem. 1990;55:4928. [Google Scholar]
- 15.Ross SA, Burrows CJ. Tetrahedron Lett. 1997;38:2805. [Google Scholar]
- 16.Fukuhara TK, Visser DW. J Am Chem Soc. 1955;77:2393. [Google Scholar]
- 17.Holmes RE, Robins RK. J Am Chem Soc. 1964;86:1242. doi: 10.1021/ja01086a028. [DOI] [PubMed] [Google Scholar]
- 18.Markish I, Arrad O. Ind Eng Chem Res. 1995;34:2125. [Google Scholar]
- 19.Virgil SC. Encyclopedia of Reagents for Organic Synthesis. 2001 doi: 10.1002/047084289X.rd038. [DOI] [Google Scholar]
- 20.Alam A. Synlett. 2005;2005:2403. [Google Scholar]
- 21.Auerbach J, Weissman SA, Blacklock TJ, Angeles MR, Hoogsteen K. Tetrahedron Lett. 1993;34:931. [Google Scholar]
- 22.Eguchi H, Kawaguchi H, Yoshinaga S, Nishida A, Nishiguchi T, Fujisaki S. Bull Chem Soc Jpn. 1994;67:1918. [Google Scholar]
- 23.Herault X, Bovonsombat P, McNelis E. Org Prep Proced Int. 1995;27:652. [Google Scholar]
- 24.Chassaing C, Haudrechy A, Langlois Y. Tetrahedron Lett. 1997;38:4415. [Google Scholar]
- 25.Alam A, Takaguchi Y, Ito H, Yushida T, Tsuboi S. Tetrahedron. 2005;61:1909. [Google Scholar]
- 26.Shibatomi K, Zhang Y, Yamamoto H. Chem Asian J. 2008;3:1581. doi: 10.1002/asia.200800087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alam A, Takaguchi Y, Stuboi S. Synth Commun. 2005;35:1329. [Google Scholar]
- 28.Khazaei A, Zolfigol MA, Rostami A. Synthesis. 2004:2959. [Google Scholar]
- 29.Chen ZZ, Guan XX, Zheng ZB, Zou XZ. J East China Normal Univ; Natural Science. 2010;7:125. [Google Scholar]
- 30.Typical procedure for the bromination of protected nucleosides.DBH (161 mg, 0.56 mmol) and TMSOTf (0.1 mL, 125 mg, 0.56 mmol) were added to a stirred solution of 1a (380 mg, 1.03 mmol) in CH2Cl2 (15 mL). The resulting brownish-orange mixture was stirred at room temperature for 6 h or until TLC showed absence of starting material and formation of less polar product. The reaction mixture was diluted with CHCl3 (35 mL) and was washed with saturated NaHCO3/H2O (2 × 100 mL) and brine (100 mL). The organic layer was dried (MgSO4) and concentrated in vacuo to yield 2a (433 mg, 94%) as a colorless foam with purity over 98% (1H NMR) with data as reported.14 Compound 2b had: 1H NMR (400 MHz, CDCl3) δ 1.98 (s, 3, Ac), 2.07 (s, 3, Ac), 2.09 (s, 3, Ac), 4.12–4.16 (m, 1, H4′), 4.32 (dd, J = 3.9, 12.1 Hz, 1, H5″), 4.38 (dd, J = 5.8, 12.1 Hz, 1, H5′), 5.04 (“q”, J = 1.9 Hz, 1, H3′), 5.35 (dd, J = 3.7, 4.1 Hz, 1, H2′), 6.22 (d, J = 4.1 Hz, 1, H1′), 7.77 (s, 1, H6), 9.33 (br. s, 1, NH); 13C NMR (100 MHz, CDCl3): δ 20.4, 20.6, 20.8 (3 × Ac), 62.5 (C5′), 74.4 (C2′), 76.1 (C3′), 80.7 (C4′), 84.4 (C1′), 96.3 (C5), 139.8 (C6), 149.2 (C2), 158.6 (C4), 168.6, 169.6, 170.5 (3 × Ac); MS (ESI) m/z 447 (100, [79Br], MH−), 449 (98, [81Br], MH−). The products 2c,14 7b,35 and 9b37 had physical and spectroscopic properties as reported.
- 31.Typical procedure for the bromination of unprotected nucleosides: DBH (323 mg, 1.13 mmol) was added to a stirred solution of 1d (500 mg, 2.05 mmol) in DMF (5 mL). The resulting pale-yellow solution was stirred at room temperature for 20 minutes or until TLC showed absence of starting material and formation of less polar product. Volatiles were evaporated and the residue was coevaporated with MeCN. The resulting pale solid was crystallized from hot acetone to give 2d (500 mg, 75%) as colorless crystals with data as reported.14 Compound 4b had: mp 193–195 °C; UV (MeOH) λmax 252, 335 nm (ε 8900, 13 900), λmin 228, 292 nm (ε 7300, 4200); 1H NMR (400 MHz, DMSO-d6) δ 3.63 (ddd, J = 2.1, 4.4, 12.2 Hz, 1, H5″), 3.74–3.82 (m, 1, H5′), 3.90–3.96 (m, 1, H4′), 4.04 (“q”, J = 5.9 Hz, 1, H3′), 4.07–4.13 (m, 1, H2′), 5.10 (d, J = 5.9 Hz, 1, 3′OH), 5.41(t, J =4.6 Hz, 1, 5′OH), 5.57 (d, J =3.9 Hz, 1, 2′OH), 5.7 (d, J = 3.6 Hz, 1, H1′), 7.53 (t, J = 7.6 Hz, 2H, Bz), 7.62 (t, J = 7.3 Hz, 1H, Bz), 8.10–8.24 (br. s, 2H, Bz), 8.79 (s, 1, H6), 12.81 (br. s, 1, NH); 13C NMR (100 MHz, DMSO-d6) δ 59.5 (C5′), 68.6 (C3′), 74.2 (C2′), 84.5 (C4′), 89.7 (C1′), 95.0 (C5), 128.4, 129.4, 132.8, 136.1 (Bz), 142.1 (C6), 147.2 (C4), 154.5 (C2), 177.8 (Bz); MS (ESI) m/z 426 (100, [79Br], MH+), 428 (98, [81Br], MH+). Anal. Calcd for C16H16BrN3O6 • 0.5 MeOH (442.24): C, 44.81; H, 4.10; N, 9.50. Found: C, 44.62; H, 3.71; N, 9.13. The products 2e,33 2f,14 3b,12 5b,34 6b,4 and 8b36 had physical and spectroscopic properties as reported.
- 32.Unsuccessful attempt of brominatian of inosine with NBS in DMF has been reported.11
- 33.Kumar R, Wiebe LI, Knaus EE. Can J Chem. 1994;72:2005. [Google Scholar]
- 34.Lin TS, Cheng JC, Ishiguro K, Sartorelli AC. J Med Chem. 1985;28:1481. doi: 10.1021/jm00148a018. [DOI] [PubMed] [Google Scholar]
- 35.Niles JC, Wishnok JS, Tannenbaum SR. Chem Res Toxicol. 2000;13:390. doi: 10.1021/tx0000318. [DOI] [PubMed] [Google Scholar]
- 36.Lin TS, Cheng JC, Ishiguro K, Sartorelli AC. J Med Chem. 1985;28:1194. doi: 10.1021/jm00147a012. [DOI] [PubMed] [Google Scholar]
- 37.Kotra L, Pai EF, Paige CJ, Bello AM. 2008/083465. WO. 2008:A1.