Abstract
Our understanding of the key players involved in the differential regulation of T-cell responses during inflammation, infection and auto-immunity is fundamental for designing efficient therapeutic strategies against immune diseases. With respect to this, the inhibitory role of the lipid mediator prostaglandin E2 (PGE2) in T-cell immunity has been documented since the 1970s. Studies that ensued investigating the underlying mechanisms substantiated the suppressive function of micromolar concentrations of PGE2 in T-cell activation, proliferation, differentiation and migration. However, the past decade has seen a revolution in this perspective, since nanomolar concentrations of PGE2 have been shown to potentiate Th1 and Th17 responses and aid in T-cell proliferation. The understanding of concentration-specific effects of PGE2 in other cell types, the development of mice deficient in each subtype of the PGE2 receptors (EP receptors) and the delineation of signalling pathways mediated by the EP receptors have enhanced our understanding of PGE2 as an immune-stimulator. PGE2 regulates a multitude of functions in T-cell activation and differentiation and these effects vary depending on the micro-environment of the cell, maturation and activation state of the cell, type of EP receptor involved, local concentration of PGE2 and whether it is a homeostatic or inflammatory scenario. In this review, we compartmentalize the various aspects of this complex relationship of PGE2 with T lymphocytes. Given the importance of this molecule in T-cell activation, we also address the possibility of using EP receptor antagonism as a potential therapeutic approach for some immune disorders.
Keywords: T cells, PGE2, EP receptors, immunosuppression, EP receptor antagonism, pro-inflammatory role
Biosynthesis and function of prostanoids
Lipid mediators have long been considered as regulators of homeostasis and inflammation. These molecules are usually produced by a conserved biosynthetic pathway controlled by specific enzymes that exert their sequential action on lipid precursors that are released from the plasma membrane. One of the most important families of lipid mediators is the prostanoid family, comprises prostaglandins (PGs) and thromboxanes (Txs).1 The precursor molecule for prostanoids is Arachadonic acid. Arachadonic acid is released from the plasma membrane phospholipids by the action of phospholipase A2, and is further processed by cyclooxygenase (COX) enzymes COX-1 and COX-2.2 COX-1 is constitutive and has a role in the maintenance of homeostasis and normal physiology. COX-1 is expressed in most tissues and is responsible for the production of ‘housekeeping' PGs that control normal physiological processes. On the other hand, COX-2 is inducible and can be activated by a variety of pro-inflammatory stimuli, especially during infection and inflammation.3, 4 Both COX-1 and COX-2 activation results in the generation of PGG2, which is then reduced to the intermediate PGH2 via a separate peroxidase site. Various specific isomerases and oxidoreductases convert PGH2 to the different types of PGs, such as PGE2, PGI2, PGD2 and PGF2α and additionally TXA2.5
Most PGs act as potent pro-inflammatory mediators, thereby making it a desirable therapeutic goal for the treatment of cancer, rheumatoid arthritis, intestinal inflammation, Alzheimer's disease and chronic musculoskeletal pain.6 However, some PGs may exert anti-inflammatory actions.7
PGE2: synthesis, function and importance
The isomerization of PGH2 to PGE2 is catalyzed by three different PGE synthases, namely cytosolic PGE synthase and two membrane-bound PGE synthases, mPGES-1 and mPGES-2. Cytosolic PGE synthase and mPGES-2 are constitutive, whereas mPGES-1 is mainly induced. It is postulated that cytosolic PGE synthase uses PGH2 produced by COX-1, whereas mPGES-1 uses COX-2-derived PGH2. mPGES-2 can use both sources of PGH2.8, 9 mPGES-1 is upregulated in response to various pro-inflammatory and mitogenic stimuli with a concomitantly increased expression of COX-2. Cytokines such as interleukin (IL)-1β and tumor necrosis factor-α and Toll-like receptor 4 signalling activated by lipopolysaccahride are defined as some of the inducers of m-PGES-1.10, 11 Results obtained from m-PGES-1 knockout mice suggest that this enzyme has key roles in normal physiology and pathological conditions such as inflammation, pain, fever, arthritis, stroke, atherosclerosis and cancer,9 hence making it an innovative therapeutic target.
PGE2 is the most abundant prostanoid found in the human body. It has many important functions in physiology and is ubiquitously produced in pathophysiological conditions.3, 12, 13 This molecule stereo-specifically exerts potent tissue- and cell type-selective actions.14, 15 The biological functions of PGE2 range from effects on the reproductive, gastro-intestinal, immune, cardiovascular and nervous systems. PGE2 has been implicated in multiple physiological processes mainly because of its ability to induce vasodilation or vasoconstriction.16 This is especially important in processes such as embryo implantation, modulation of hemodynamics in kidney, blood pressure control, childbirth and gastro-intestinal motility. Apart from these functions, PGE2 has been shown to be a key player in regulating body temperature and sleep–wake mechanisms and gastrointestinal secretion along with mucosal barrier functions.16, 17 In the field of tumor biology, COX-2 overexpression leads to increased levels of PGE2 and has been associated particularly with colorectal, pancreatic, lung and breast cancer.18 PGE2 has been implicated in tumor progression through stimulation of angiogenesis, cell invasion and metastasis, and promotes cell survival by inhibiting apoptosis via numerous signalling pathways.19, 20 Besides, PGE2 also has a role in tumor evasion of immunosurveillance and has been known to alter cytokine expression profiles of dendritic cells (DCs) in order to suppress antitumor cytotoxic T cells.21, 22 PGE2-secreting cancer cells have been shown to induce human Treg cell formation and increase their inhibitory activity against Th cells that are specific for tumors.23
It is in the area of inflammation that PGE2's actions are most diverse. Over the past decades, extensive research using COX-2, m-PGES1 and EP receptor knockout mice yielded novel and important findings proving that prostanoids exert both pro-inflammatory and anti-inflammatory effects, and that these actions are often produced through directed regulation of gene expression in relevant tissues.
PGE2 usually serves as an important pro-inflammatory mediator that is involved in the production of all cardinal signs of inflammation: edema, redness, swelling and pain.3, 24 This is produced as a result of the effect of PGE2 on increased microvascular permeability, increase in blood flow to the inflamed site, hyperalgesia and action on peripheral sensory neurons within the affected area.25 The effect of PGE2 on immune cell types is much more complex. Apart from favoring the production of inflammation, PGE2 has been proved to favor DC maturation, antigen uptake and homing to lymph nodes.26, 27 In addition, it has been also demonstrated that PGE2 can induce the expression of co-stimulatory molecules on DCs, thus augmenting T-cell activation.28 In macrophages, PGE2 acts as a positive aid in macrophage activation by interferon (IFN)-γ and tumor necrosis factor-α via its capacity to modulate intracellular cyclic adenosine monophosphate (cAMP) levels.29 It has also been shown to be an inducer of matrix metallo-proteinases MMP-2 and MMP-9 in macrophages.30
However, there have been a large number of reports that compile evidence supporting the notion that PGE2 acts also as an anti-inflammatory molecule that dampens the immune response (reviewed in Smyth et al.4). PGE2 has been demonstrated to suppress Th1 differentiation, B-cell functions, T-cell activation and allergic reactions.3, 31 Furthermore, PGE2 can exert anti-inflammatory actions on innate immune cells like neutrophils, monocytes and natural killer cells.3, 31 However, the past decade has seen a revolution in the outlook of PGE2 as a T-cell immunosuppressor, owing to different reports that substantiate a beneficial role of PGE2 in T-cell differentiation and immune functions, as discussed in the later sections of this review.
PGE2 receptors: the EP receptors (1–4)
PGE2 binds to four specific G-protein-coupled receptors termed EP receptors (EP1–4). EP receptors are distinguished by the signal transduction pathway that is activated upon ligand binding.32 Some of the signalling pathways that are generated by PGE2 are under the control of the secondary messenger cAMP. cAMP is derived from adenosine triphosphate by 1 of at least 10 currently identified isoforms of the adenylyl cyclase (AC) enzymes (AC 1–9 and soluble AC), which differ in cell-specific expression, regulation and effects, providing an intracellular system suited for finely targeted signalling.33 The phosphatidylinositol and its phosphorylated products have been shown to be the precursors for messengers generated by phospholipases, although they have been directly implicated in signalling.34, 35 Another level of control of signalling by PGs is attributed to Ca2+, which is a highly versatile intracellular signal that modifies various cellular processes through spatial and temporal dynamic remodelling of a variety of signalling constituents.36 Activation of EP receptors leads to changes in the production of cAMP and/or phosphoinositol turnover and intracellular Ca2+ mobilization.32
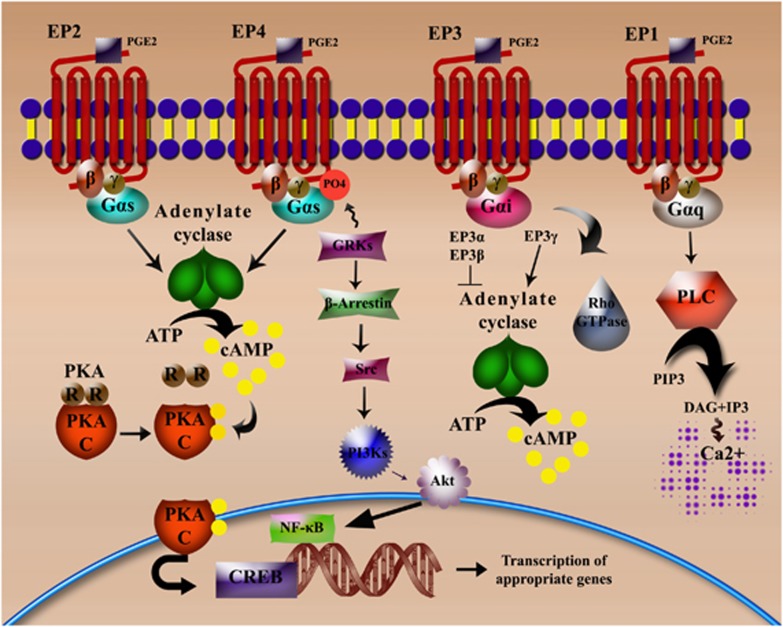
EP1 was first described as involved in constriction of smooth muscle.37 The C-terminal domain of the EP1 receptor binds to Gα-q heterotrimeric guanine nucleotide-binding protein. Activation of EP1 by ligand binding results in increased phosphatidylinositol hydrolysis and elevation of the intracellular Ca2+ through activation of phospholipase-C (Figure 1).
Figure 1.
EP receptors: types and signalling. The four different EP receptors are high-affinity G-protein coupled receptors characterized by the activation of different signalling pathways. EP2 and EP4 are linked to Gαs proteins and function by inducing the adenylate cyclase (AC) system and concomitant increases in the secondary messenger cAMP. cAMP acts by activating PKA, resulting in the dissociation of the regulatory and catalytic subunits of the kinase. The catalytic subunits initiate the corresponding transactivation of the transcription factor CREB. EP4 is also capable of activating the phosphatidylinositol 3 kinase (PI3K) signalling pathway by phosphorylation induced by G-protein-coupled receptor kinases. This ultimately results in the triggering of NF-κB-mediated transcription programs. EP3 isoforms differ in their ability to modulate signal transduction. EP3α and EP3β are capable of blocking induction of AC while EP3γ potentiates AC and cAMP production. EP1, on the other hand, couples to Gαq protein and signals through the phospholipase C (PLC)/inositol-1,4,5-trisphosphate (IP3) pathway resulting in the formation of the second messengers diacylglycerol (DAG) and IP3, with the latter rapidly liberating Ca2+ ions from intracellular stores.
In contrast, EP2 was originally believed to have a role in smooth muscle relaxation.38 Both EP2 and EP4 are coupled to Gs-proteins, leading to increased production of cAMP and activation of protein kinase A (PKA)32, 39 (Figure 1). Although both receptors share the same signalling pathway, they differ in the length of their C-terminal sequence and hence have differing sensitivities to phosphorylation and desensitization.40 The distinguishing feature of EP4 is, however, the ability to activate phosphatidylinositol 3 kinase signalling pathways following phosphorylation by G-protein coupled receptor kinases41 or by virtue of the ability to bind Gi proteins42, 43 (Figure 1). Both EP2 and EP4 are capable of stimulating the T-cell factor/lymphoid enhancer factor and inhibiting glycogen synthase kinase-3 through the PKA and phosphatidylinositol 3 kinase-dependent signalling pathways, respectively.44
The major signalling pathway activated by EP3 receptor-ligand binding goes through the pertussis toxin-sensitive Gi protein, resulting in inhibition of AC and decrease in cAMP levels.45 However, EP3 receptors have different C-terminal splice variants that exhibit varied specificities for downstream G-proteins. In this context, EP3α and EP3β couple to Gi and inhibit AC, whereas EP3γ couples to Gs in addition to Gi, and evokes cAMP production.46, 47 Moreover, EP3 has been demonstrated to activate the small GTPase Rho in various cell types48 (Figure 1).
A difference in the structure of the C-terminal domain of EP receptors determines the differential nature of agonist-induced desensitization and internalization. Till date, knowledge of EP1 receptor trafficking has been limited. But with respect to EP3, the existence of different variants generated by alternative splicing of the C-terminal tail reflects on the variations observed in signal transduction and intracellular trafficking. EP3α undergoes rapid agonist-induced desensitization and sequestration followed by long-term downregulation, whereas no such changes were observed in EP3β trafficking.49 The long C-terminal of the EP4 receptor contributes to its susceptibility to rapid agonist-induced internalization and desensitization.50, 51 However, the EP2 receptor undergoes neither rapid agonist-induced internalization nor desensitization owing to a shorter C-terminal sequence.40, 51
With respect to their tissue distribution and cellular localization, it has been demonstrated that EP2-4 are widely distributed in almost all mouse tissues, whereas EP1 mRNA expression is restricted to distinct organs such as the kidney, lung and stomach. EP2 is the least abundant of all the receptors. As each EP receptor is committed to a defined signalling pathway and its associated function, they follow a restricted expression pattern within each organ system. Interestingly, this precise cellular localization of EP receptors is found in mice, humans and rabbits.52, 53 A detailed summary of described physiological functions of each subtype of the four EP receptors are enlisted in supplementary Table 1.
Effect of PGE2 on T-cell activation and differentiation
Although most of the PGE2 secreted in the body comes from professional APCs and stromal cells, in vitro findings have shown that PTGS2 (gene for COX2) is transcriptionally upregulated in human T cells during T cell receptor (TCR)/CD3 triggering and that it behaves as an early inducible gene in the T-cell activation process.54
With respect to EP receptor expression, while mRNA for all types of EP receptors were detected in murine T cells, expression of EP1 and EP3 has not been fully documented.55 Recent studies have confirmed that EP2 and EP4 are the main receptor subtypes to mediate the actions of PGE2 in human and murine CD4+ T cells.56
Immunosuppressive role of PGE2 on T-cell function
PGE2-induced activation of AC and production of cAMP and its role in producing an inhibitory effect on T-cell activation was documented in the early 1970s.57, 58 Starting from the early 1980s, it has been strongly believed that PGE2 has a largely immunosuppressive role to have in T-cell activation and proliferation. Many attempts were made to describe the working mechanism of this process. The immunomodulatory role of PGE2 in T-cell activation was documented >30 years ago, when it was postulated that PGE2 concentration, as well as the state of differentiation of the target cell, and length of PGE2–target cell interaction were important factors controlling the process (reviewed in Goodwin and Ceuppens59).
Initial findings reported a role of PGE2 in mediating induction of nonspecific T lymphocyte suppressor activity,60 and a drastic inhibition of T-cell proliferation, hence modifying T-cell blastogenic responses in mice lymphoid organs61, 62 and suppressing proliferation of lymphoma in mice.63 Later studies suggested that PGE2 primarily exerts its inhibitory effect on lymphocyte proliferation through an inhibition of IL-2 production.64, 65 This was followed by reports that stated that inhibition of lymphocyte response was brought about by PGE2-producing macrophages,66 which were found to inhibit IL-1-dependent T-lymphocyte differentiation.67 Subsequent research substantiated the suppressive function of PGE2 in T-cell responses.
However, it was not until the late 1980s that research began to delineate the underlying inhibitory pathways of PGE2 in T cells, mainly through the production of cAMP. It was found that cAMP exerts its anti-proliferative effects through interference with IL-2-mediated gene-expression.68, 69 cAMP was also shown to downregulate transferrin receptor expression in an IL-2-dependent manner70 and abrogate TCR-mediated cytosolic increases in Ca2+,71 later confirmed by studies in sepsis.72 cAMP was also found to negatively regulate the phosphoinositide cycle-related transduction pathway including inhibition of phosphatidylinositol hydrolysis and diacylglycerol and inositol phosphate (IP) production.73, 74 Increases in cAMP were also found to inhibit expression of IL-2 receptors.75, 76 Increasing intracellular concentrations of cAMP may result in a reduction of K+ movements and in negative modulation of signal transduction via G-proteins, impairing T-cell activation further.77
The suggestion that PGE2 might alter polarization of T helper cells to Th1 and Th2 subtypes was demonstrated first in a study by Betz and Fox,78 where they showed that PGE2 inhibits IL-2 and IFN-γ production (Th1) but not IL-4 and IL-5 production (Th2). This was further re-confirmed by the demonstration that PGE2 upregulates IL-5 production in T cells.79 It was later demonstrated that while PGE2 primed Th cells to produce higher amounts of IL-4, IL-10 and IL-13,80 it was found to inhibit IL-12 production and IL-12 receptor responsiveness,81 consolidating its role in the Th1/Th2 balance.
On the other hand, there are various reports that suggest that PGE2 enhances induction and differentiation of FOXP3+CD4+CD25+ adaptive regulatory T cells that thereby suppress effector T-cell stimulation pathways.82, 83, 84 In addition, PGE2 has been shown to induce T-cell anergy85 to maintain the survival of CD45RO+ T cells86 and to inhibit γδ T-cell cytotoxicity triggered by the TCR through cAMP-mediated PKA type I-dependent signalling.87
With respect to transcription factors and nuclear proteins, it was found that cAMP signalling interfered with the activation pathway for NF-κB,88 and counteracted calcineurin-dependent pathways.89 Yet, decreased IL-2 production in the presence of PGE2 was shown to be due to targeting of AP-1 and NF-AT transcription factors in human T cells.90 Therefore, qualitative differences in the concentration of cAMP and PKA activity can be considered as important elements in modulating T-cell proliferative responses.91
Several molecular mechanisms have been proposed for the inhibition of T-cell activation by PGE2. PGE2 signalling has been proved to attenuate p59(fyn) protein tyrosine kinase activity92, 93 and interfere with the protein-kinase C pathway.94, 95 The enzyme Csk has been shown to negatively regulate Lck, a kinase responsible for TCR signalling following antigen recognition.96, 97, 98, 99 PGE2-mediated cAMP was also shown to regulate raft-associated Csk in a spatial and enzymatic manner.100 It is well known that TCR ligation results in the activation of mitogen-activated protein kinase cascades involving different members such as ERK and p38 mitogen-activated protein kinases. These kinases are important for regulating transcription factors that control growth, survival and differentiation of T cells.101, 102 Hematopoietic protein tyrosine phosphatase phosphorylation by PKA in T cells and its negative regulation of extracellular signal-regulated protein kinase and mitogen-activated protein kinase pathways has also been reported.103 The inhibition of the kinase Lck was also proposed as a mechanism of suppression of T-cell activation triggered by PGE2.104 Stimulation of prolactin expression (a negative regulator of T-cell proliferation) was also shown to be mediated through Ca2+ and cAMP signalling through EP3 and EP4 receptors by PGE2 in T cells.105
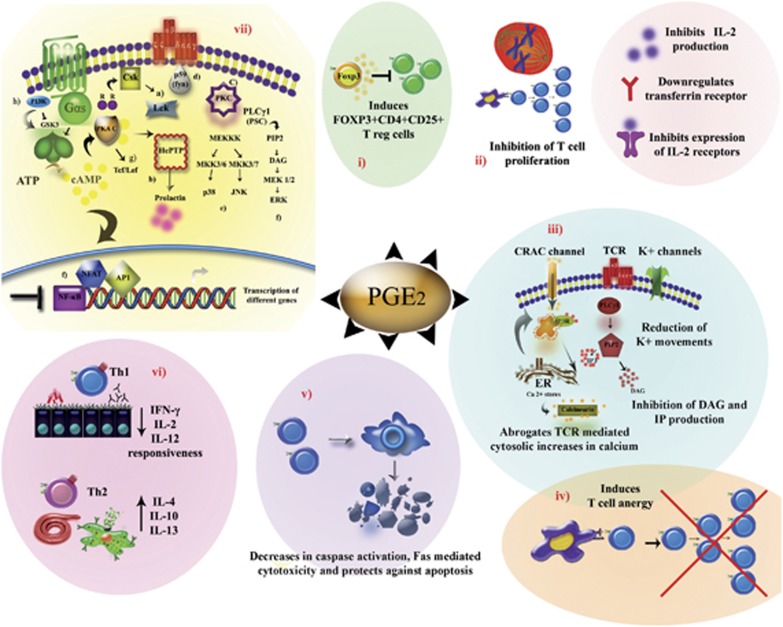
The immunosuppressive role of PGE2 in T-cell responses has been summarized in Figure 2.
Figure 2.
Negative regulation of T-cell responses by PGE2. PGE2 mediates its anti-inflammatory effects on T cells through different mechanisms: (i) PGE2 has been shown to induce differentiation of FOXP3+CD4+CD25+ adaptive regulatory T cells that were found to inhibit effector T-cell responses, (ii) PGE2 has also been demonstrated to suppress T-cell proliferation through different mechanisms, (iii) PGE2 is involved in the inhibition of secondary messenger generation including the abrogation of Ca2+, K+, diacylglycerol (DAG) and IP production, (iv) T-cell anergy has been known to be promoted by high concentrations of PGE2, (v) PGE2 favors cell survival by blocking activation-induced apoptosis, cellular cytotoxicity and caspase activation, (vi) PGE2 at micromolar concentrations was found to be inhibitory for Th1 differentiation and beneficial for Th2 differentiation, (vii) modulation of TCR-mediated signal transduction pathways by PGE2. (a) regulation of Csk, (b) hematopoietic protein tyrosine phosphatase (HePTP) phosphorylation by cAMP-dependent protein kinase and promotion of prolactin expression, (c) interference of PKC signalling, (d) attenuation of p59(fyn) protein tyrosine kinase activity, (e, f) negative regulation of extracellular signal-regulated protein kinase (ERK) and mitogen-activated protein kinase (MAPK) pathways (g) PKA-mediated signalling potentiates T-cell factor (Tcf)/lymphoid enhancer factor (Lef) signalling pathways, (h) while PI3K inhibits glycogen synthase kinase-3 (GSK3) signal-mediation.
Pro-inflammatory role of PGE2 in T-cell function
An indirect pro-inflammatory role for PGE2 in human T lymphocytes was shown to be mediated by the induction of IL-8 (CXCL8) gene transcription following activation of C/EBP homologous protein.106 IL-8 (CXCL8) thus produced by T cells was then shown to mediate neutrophil recruitment and sustain inflammation.106 However, a different perspective on the suppressive nature of PGE2 came into view when it was shown that nanomolar concentrations of PGE2 potentiated Th1 and Th17 differentiation through phosphatidylinositol 3 kinase and PKA signalling, respectively, in a process mediated by EP2 and EP4 receptors.107 Interestingly, administration of an EP4 antagonist suppressed Th1 and Th17 expansion within draining lymph nodes in two disease models of inflammation: contact hypersensitivity and experimental autoimmune encephalomyelitis.107 The role of PGE2 in Th17 expansion was also reported by Boniface et al.,56 who showed that PGE2 in combination with IL-1β and IL-23 promoted differentiation of Th17 cells by upregulating the IL-1βR and IL-23R expression through the EP2/EP4–cAMP pathway. In this elegant report, investigators propose that PGE2 promotes the development and maturation of Th17 cells through activation of the EP2 receptor, while inhibiting IL-10 and IFN-γ synthesis through the EP4 receptor in human and mouse T cells, substantiating a role for PGE2 in regulation of Th17 responses.56 PGE2 was also found to synergize with IL-23 and increase the number of Th17 cells derived from human CD4+CD45RO+ (memory) T cells but not from CD4+CD45RO− (naive) T cells.108 The favoring of IL-17 production and down-modulation of IFN-γ production by memory CD4+ T cells through PGE2-mediated EP2/EP4 signalling, when present in micromolar concentrations, was also demonstrated in another study.109 Esaki et al.110 indicated an essential role of PGE2-EP2/EP4 signalling in T-cell proliferation as well as IFN-γ and IL-17 cytokine production within the draining lymph nodes of mice during the course of experimental autoimmune encephalomyelitis. The unique ability of PGE2 to differentially modulate Th1 and Th17 differentiation at different concentrations, could bring a new dimension to the PGE2-mediated determination of the type of effector response and hence the outcome of the inflammatory reaction.
On the other hand, indirect control of T-cell differentiation through regulation of cytokine patterns produced by DCs has also been reported. Exogenous PGE2 was found to enhance lipopolysaccahride-induced IL-23 production by DCs, which could therefore promote Th17 differentiation.111, 112 In addition, DCs cultured in the presence of PGE2 enhanced the differentiation of naive T cells toward the Th1 type.113 This was further emphasized in another report where the addition of PGE2 and tumor necrosis factor-α for the maturation of human monocyte-derived DCs enhanced CD4+ and CD8+ T-cell proliferative responses, and favored Th1-type responses.114 Interestingly, PGE2 was found to enhance T-cell proliferation by inducing the co-stimulatory molecules OX40L, CD70 and 4-1BBL on DCs.28 This study also shows that PGE2-matured DCs upregulate the expression of OX-40L, OX-40 and CD70 on the surface of T cells, enlisting a possible role in T-cell–T-cell interactions and sustained antigen-specific immune responses.28
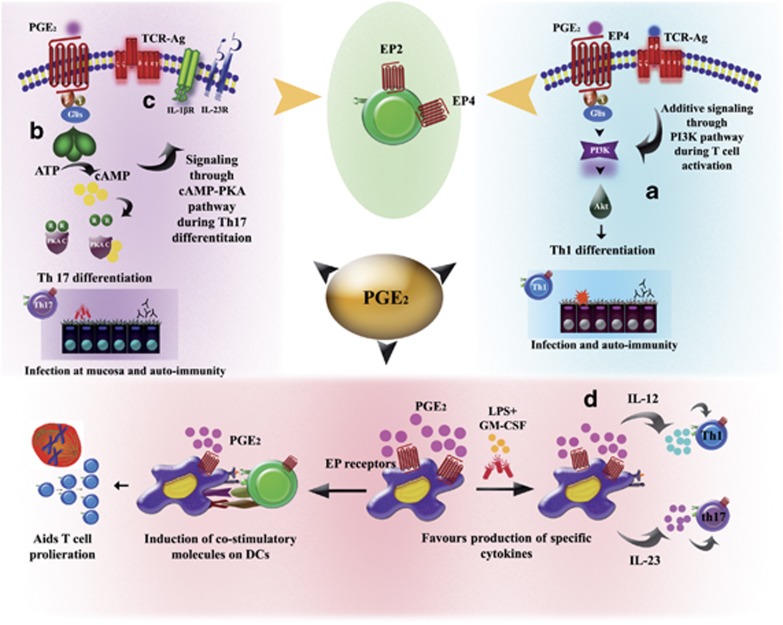
A comprehensive summary of the pro-inflammatory role of PGE2 in T-cell response is shown in Figure 3.
Figure 3.
Positive regulation of T-cell responses by PGE2. PGE2 has diverse pro-inflammatory effects on T cells. (a) Nanomolar physiological concentrations of PGE2 induce phosphatidylinositol 3 kinase (PI3K)/Akt signalling pathways through the EP4 receptor that serve to promote Th1 differentiation patterns. (b) PGE2 has also been shown to potentiate Th17 differentiation through EP2-cAMP-PKA signalling pathways, primarily through (c) induction of IL-1β and IL-23 receptor (d) PGE2 has been demonstrated to induce co-stimulatory molecules on the surface of DCs, thereby promoting T-cell proliferation. It has also been shown to promote secretion of specific cytokines by DCs, for example, IL-12, which further directs Th1 differentiation and IL-23, which enhances Th17 polarization.
PGE2-based T-cell-targeted therapies for inflammatory disorders
Modulating T-cell effector functions is a promising therapeutic approach for various diseases, owing to the multi-faceted roles of T cells in immuno-pathogenesis of auto-immunity, allergy and human immunodeficiency virus and parasitic infections. Given the importance of PGE2 signalling in the modulation of T-cell responses, several reports have focused on the development of PGE2-targeted therapies for immune disorders.
The non-steroidal anti-inflammatory drugs are a varied group of pharmacologic compounds used for the treatment of processes of inflammation, since the introduction of acetylsalicylic acid in 1899. The first-generation non-steroidal anti-inflammatory drugs exert anti-inflammatory, analgesic and antipyretic effects through the blockade of PG synthesis via nonspecific inhibition of COX-1 and COX-2. However, their employment as drugs over prolonged periods of time is not favored, since they cause pronounced side effects such as gastrointestinal and renal toxicity.25, 115, 116, 117 This has resulted in the shift of focus of therapeutic interventions from COX enzymes to PGE2 synthases such as m-PGES-1.118
The past decade has experienced a major change in the outlook of treatment regimens that aim to inhibit the actions of PGE2. Extensive work on the tissue, organ and cell-specific functions of PGE2 has given place to the generation of EP receptor antagonists and agonists, which have already been applied in diverse experimental animal models. Interestingly, the antagonism of EP receptors has been proved to be efficient in ameliorating Th1 and Th17 responses, thereby proving to be a potential treatment option for arthritis, autoimmune encephalitis and contact hypersensitivity.108, 119 EP receptor antagonists have been employed for the inhibition of inflammatory pain hypersensitivity, paw edema and cancer.120, 121, 122, 123, 124 The targeted modulation of T-cell function by blocking or potentiating specific EP receptor signalling pathways could thus be a revolutionary approach for the treatment of a variety of immune dysfunction-related diseases.
However, there have been various limitations to the use of receptor antagonists for therapy. One of them is the mild effectiveness of these compounds as compared with non-steroidal anti-inflammatory drugs: the reason being the inhibition of only one/two specific receptors as opposed to the robust inhibition of all downstream PGs by COX inhibitors. The antagonism/agonism of only one specific receptor would not be efficient enough to potentially curtail/cure a disease state. To complicate issues further, a lot of emphasis has been laid on the different additive, compensatory or opposing roles of EP receptors in a given disease setup or inflammatory condition. Therefore, it is not advisable to design treatments based solely on the blocking or triggering of individual prostanoid receptors. Extensive study of calculated combinations of specific agonists and antagonists will be required in order to design efficient therapies to treat inflammatory disorders.
Conclusion and remarks
The ‘classical' perspective of the role of PGE2 as only an immunosuppressor of T-cell function has changed over the past decade. This has been due to the description of concentration-dependent and somewhat opposed effects in different scenarios of homeostasis and inflammation and the interplay of signalling events generated by the EP2 and EP4 receptors during the process of T-cell responses. The pro-inflammatory actions of PGE2 in T cells and its promotion of the Th1 and Th17 differentiation have been well defined over the past few years. Determination of factors that cause the oscillation of PGE2 from a T-cell immunosuppressor to a T-cell immunoactivator, such as (1) local concentration of PGE2 during diverse phases of inflammation, (2) differential use of EP receptors and signalling pathway involved in T-cell subsets and (3) targeted effects of application of EP receptor antagonists in different disease scenarios, would be fundamental for the design of tailor-made therapies in infection, inflammatory disorders and autoimmunity.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50 (Suppl:S423–S428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B, Goldyne M, Granstrom E, Hamberg M, Hammarstrom S, Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Yedgar S, Krimsky M, Cohen Y, Flower RJ. Treatment of inflammatory diseases by selective eicosanoid inhibition: a double-edged sword. Trends Pharmacol Sci. 2007;28:459–464. doi: 10.1016/j.tips.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Goodwin JS. Are prostaglandins proinflammatory, antiinflammatory, both or neither. J Rheumatol Suppl. 1991;28:26–29. [PubMed] [Google Scholar]
- Radmark O, Samuelsson B. Microsomal prostaglandin E synthase-1 and 5-lipoxygenase: potential drug targets in cancer. J Intern Med. 2010;268:5–14. doi: 10.1111/j.1365-2796.2010.02246.x. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59:207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci USA. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampey AV, Monrad S, Crofford LJ. Microsomal prostaglandin E synthase-1: the inducible synthase for prostaglandin E2. Arthritis Res Ther. 2005;7:114–117. doi: 10.1186/ar1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Ushikubi F, Sugimoto Y, Ichikawa A, Narumiya S. Roles of prostanoids revealed from studies using mice lacking specific prostanoid receptors. Jpn J Pharmacol. 2000;83:279–285. doi: 10.1254/jjp.83.279. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Fortier MA, Krishnaswamy K, Danyod G, Boucher-Kovalik S, Chapdalaine P. A postgenomic integrated view of prostaglandins in reproduction: implications for other body systems. J Physiol Pharmacol. 2008;59 (Suppl 1:65–89. [PubMed] [Google Scholar]
- Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006;149:611–623. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007;26:525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- Iniguez MA, Rodriguez A, Volpert OV, Fresno M, Redondo JM. Cyclooxygenase-2: a therapeutic target in angiogenesis. Trends Mol Med. 2003;9:73–78. doi: 10.1016/s1471-4914(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Ahmadi M, Emery DC, Morgan DJ. Prevention of both direct and cross-priming of antitumor CD8+ T-cell responses following overproduction of prostaglandin E2 by tumor cells in vivo. Cancer Res. 2008;68:7520–7529. doi: 10.1158/0008-5472.CAN-08-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc'h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Legler DF, Krause P, Scandella E, Singer E, Groettrup M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J Immunol. 2006;176:966–973. doi: 10.4049/jimmunol.176.2.966. [DOI] [PubMed] [Google Scholar]
- van Helden SF, Krooshoop DJ, Broers KC, Raymakers RA, Figdor CG, van Leeuwen FN. A critical role for prostaglandin E2 in podosome dissolution and induction of high-speed migration during dendritic cell maturation. J Immunol. 2006;177:1567–1574. doi: 10.4049/jimmunol.177.3.1567. [DOI] [PubMed] [Google Scholar]
- Krause P, Bruckner M, Uermosi C, Singer E, Groettrup M, Legler DF. Prostaglandin E(2) enhances T-cell proliferation by inducing the costimulatory molecules OX40L, CD70, and 4-1BBL on dendritic cells. Blood. 2009;113:2451–2460. doi: 10.1182/blood-2008-05-157123. [DOI] [PubMed] [Google Scholar]
- Mauel J, Ransijn A, Corradin SB, Buchmuller-Rouiller Y. Effect of PGE2 and of agents that raise cAMP levels on macrophage activation induced by IFN-gamma and TNF-alpha. J Leukoc Biol. 1995;58:217–224. doi: 10.1002/jlb.58.2.217. [DOI] [PubMed] [Google Scholar]
- Cipollone F, Prontera C, Pini B, Marini M, Fazia M, De Cesare D, et al. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation. 2001;104:921–927. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]
- Kunikata T, Yamane H, Segi E, Matsuoka T, Sugimoto Y, Tanaka S, et al. Suppression of allergic inflammation by the prostaglandin E receptor subtype EP3. Nat Immunol. 2005;6:524–531. doi: 10.1038/ni1188. [DOI] [PubMed] [Google Scholar]
- Alfranca A, Iniguez MA, Fresno M, Redondo JM. Prostanoid signal transduction and gene expression in the endothelium: role in cardiovascular diseases. Cardiovasc Res. 2006;70:446–456. doi: 10.1016/j.cardiores.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Risoe PK, Wang Y, Stuestol JF, Aasen AO, Wang JE, Dahle MK. Lipopolysaccharide attenuates mRNA levels of several adenylyl cyclase isoforms in vivo. Biochim Biophys Acta. 2007;1772:32–39. doi: 10.1016/j.bbadis.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Hokin LE. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Gardiner PJ. Characterization of prostanoid relaxant/inhibitory receptors (psi) using a highly selective agonist, TR4979. Br J Pharmacol. 1986;87:45–56. doi: 10.1111/j.1476-5381.1986.tb10155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nishigaki N, Negishi M, Ichikawa A. Two Gs-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol Pharmacol. 1996;50:1031–1037. [PubMed] [Google Scholar]
- Sakata D, Yao C, Narumiya S. Prostaglandin E2, an immunoactivator. J Pharmacol Sci. 2010;112:1–5. doi: 10.1254/jphs.09r03cp. [DOI] [PubMed] [Google Scholar]
- Fujino H, Regan JW. EP(4) prostanoid receptor coupling to a pertussis toxin-sensitive inhibitory G protein. Mol Pharmacol. 2006;69:5–10. doi: 10.1124/mol.105.017749. [DOI] [PubMed] [Google Scholar]
- Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278:12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- Sonnenburg WK, Zhu JH, Smith WL. A prostaglandin E receptor coupled to a pertussis toxin-sensitive guanine nucleotide regulatory protein in rabbit cortical collecting tubule cells. J Biol Chem. 1990;265:8479–8483. [PubMed] [Google Scholar]
- Irie A, Sugimoto Y, Namba T, Harazono A, Honda A, Watabe A, et al. Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. Eur J Biochem. 1993;217:313–318. doi: 10.1111/j.1432-1033.1993.tb18248.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Negishi M, Hayashi Y, Namba T, Honda A, Watabe A, et al. Two isoforms of the EP3 receptor with different carboxyl-terminal domains. Identical ligand binding properties and different coupling properties with Gi proteins. J Biol Chem. 1993;268:2712–2718. [PubMed] [Google Scholar]
- Katoh H, Negishi M, Ichikawa A. Prostaglandin E receptor EP3 subtype induces neurite retraction via small GTPase Rho. J Biol Chem. 1996;271:29780–29784. doi: 10.1074/jbc.271.47.29780. [DOI] [PubMed] [Google Scholar]
- Negishi M, Sugimoto Y, Irie A, Narumiya S, Ichikawa A. Two isoforms of prostaglandin E receptor EP3 subtype. Different COOH-terminal domains determine sensitivity to agonist-induced desensitization. J Biol Chem. 1993;268:9517–9521. [PubMed] [Google Scholar]
- Bastepe M, Ashby B. Identification of a region of the C-terminal domain involved in short-term desensitization of the prostaglandin EP4 receptor. Br J Pharmacol. 1999;126:365–371. doi: 10.1038/sj.bjp.0702291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S, April H, Nwaneshiudu C, Ashby B. Comparison of agonist-induced internalization of the human EP2 and EP4 prostaglandin receptors: role of the carboxyl terminus in EP4 receptor sequestration. Mol Pharmacol. 2000;58:1279–1286. doi: 10.1124/mol.58.6.1279. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Namba T, Shigemoto R, Negishi M, Ichikawa A, Narumiya S. Distinct cellular localization of mRNAs for three subtypes of prostaglandin E receptor in kidney. Am J Physiol. 1994;266:F823–F828. doi: 10.1152/ajprenal.1994.266.5.F823. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Davis L, Jacobson HR, Breyer RM. Differential localization of prostaglandin E receptor subtypes in human kidney. Am J Physiol. 1996;270:F912–F918. doi: 10.1152/ajprenal.1996.270.5.F912. [DOI] [PubMed] [Google Scholar]
- Iniguez MA, Punzon C, Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol. 1999;163:111–119. [PubMed] [Google Scholar]
- Nataraj C, Thomas DW, Tilley SL, Nguyen MT, Mannon R, Koller BH, et al. Receptors for prostaglandin E(2) that regulate cellular immune responses in the mouse. J Clin Invest. 2001;108:1229–1235. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Steiner AL, Newberry WM, Jr, Parker CW. Cyclic adenosine 3′,5′-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971;50:432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Steiner AL, Parker CW. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971;50:442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Fischer A, Durandy A, Griscelli C. Role of prostaglandin E2 in the induction of nonspecific T lymphocyte suppressor activity. J Immunol. 1981;126:1452–1455. [PubMed] [Google Scholar]
- Rojo JM, Portoles MP, Barasoain I, Portoles A. Exogenous additions of prostaglandins variably alter the blastogenic response of B and T lymphocytes from different mice lymphoid organs. Immunopharmacology. 1982;4:95–104. doi: 10.1016/0162-3109(82)90012-1. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Simon MM, Hahn H. Regulatory interactions between macrophages and T-cell subsets in Listeria monocytogenes-specific T-cell activation. Infect Immun. 1982;38:907–913. doi: 10.1128/iai.38.3.907-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemsa D, Leser HG, Deimann W, Resch K. Suppression of T lymphocyte proliferation during lymphoma growth in mice: role of PGE2-producing suppressor macrophages. Immunobiology. 1982;161:385–391. doi: 10.1016/s0171-2985(82)80096-x. [DOI] [PubMed] [Google Scholar]
- Maca RD. The effects of prostaglandins on the proliferation of cultured human T lymphocytes. Immunopharmacology. 1983;6:267–277. doi: 10.1016/0162-3109(83)90033-4. [DOI] [PubMed] [Google Scholar]
- Walker C, Kristensen F, Bettens F, deWeck AL. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983;130:1770–1773. [PubMed] [Google Scholar]
- Murray JL, Kollmorgen GM. Inhibition of lymphocyte response by prostaglandin-producing suppressor cells in patients with melanoma. J Clin Immunol. 1983;3:268–276. doi: 10.1007/BF00915351. [DOI] [PubMed] [Google Scholar]
- Cahill J, Hopper KE. Immunoregulation by macrophages. III. Prostaglandin E suppresses lymphocyte activation but not macrophage effector function during Salmonella enteritidis infection. Int J Immunopharmacol. 1984;6:9–17. doi: 10.1016/0192-0561(84)90029-8. [DOI] [PubMed] [Google Scholar]
- Farrar WL, Evans SW, Rapp UR, Cleveland JL. Effects of anti-proliferative cyclic AMP on interleukin 2-stimulated gene expression. J Immunol. 1987;139:2075–2080. [PubMed] [Google Scholar]
- Mary D, Aussel C, Ferrua B, Fehlmann M. Regulation of interleukin 2 synthesis by cAMP in human T cells. J Immunol. 1987;139:1179–1184. [PubMed] [Google Scholar]
- Chouaib S, Welte K, Mertelsmann R, Dupont B. Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985;135:1172–1179. [PubMed] [Google Scholar]
- Chouaib S, Robb RJ, Welte K, Dupont B. Analysis of prostaglandin E2 effect on T lymphocyte activation. Abrogation of prostaglandin E2 inhibitory effect by the tumor promotor 12.0 tetradecanoyl phorbol-13 acetate. J Clin Invest. 1987;80:333–340. doi: 10.1172/JCI113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry MA, Ahmad S, Sayeed MM. Role of Ca2+ in prostaglandin E2-induced T-lymphocyte proliferative suppression in sepsis. Infect Immun. 1995;63:3101–3105. doi: 10.1128/iai.63.8.3101-3105.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Jacobson B, Miller RA. Cyclic AMP concentrations modulate both calcium flux and hydrolysis of phosphatidylinositol phosphates in mouse T lymphocytes. J Immunol. 1988;140:936–940. [PubMed] [Google Scholar]
- Liang S, Ledbetter J, Goodwin JS. Phosphatidyl inositol hydrolysis after CD3 binding in human peripheral blood T cells: inhibition by prostaglandin E2. Int J Immunopharmacol. 1989;11:809–816. doi: 10.1016/0192-0561(89)90135-5. [DOI] [PubMed] [Google Scholar]
- Anastassiou ED, Paliogianni F, Balow JP, Yamada H, Boumpas DT. Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R alpha gene expression at multiple levels. J Immunol. 1992;148:2845–2852. [PubMed] [Google Scholar]
- Rincon M, Tugores A, Lopez-Rivas A, Silva A, Alonso M, De Landazuri MO, et al. Prostaglandin E2 and the increase of intracellular cAMP inhibit the expression of interleukin 2 receptors in human T cells. Eur J Immunol. 1988;18:1791–1796. doi: 10.1002/eji.1830181121. [DOI] [PubMed] [Google Scholar]
- Bastin B, Payet MD, Dupuis G. Effects of modulators of adenylyl cyclase on interleukin-2 production, cytosolic Ca2+ elevation, and K+ channel activity in Jurkat T cells. Cell Immunol. 1990;128:385–389. doi: 10.1016/0008-8749(90)90035-p. [DOI] [PubMed] [Google Scholar]
- Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–113. [PubMed] [Google Scholar]
- Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- Demeure CE, Yang LP, Desjardins C, Raynauld P, Delespesse G. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur J Immunol. 1997;27:3526–3531. doi: 10.1002/eji.1830271254. [DOI] [PubMed] [Google Scholar]
- Wu CY, Wang K, McDyer JF, Seder RA. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J Immunol. 1998;161:2723–2730. [PubMed] [Google Scholar]
- Bryn T, Yaqub S, Mahic M, Henjum K, Aandahl EM, Tasken K. LPS-activated monocytes suppress T-cell immune responses and induce FOXP3+ T cells through a COX-2-PGE2-dependent mechanism. Int Immunol. 2008;20:235–245. doi: 10.1093/intimm/dxm134. [DOI] [PubMed] [Google Scholar]
- Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–254. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- Mannie MD, Prevost KD, Marinakis CA. Prostaglandin E2 promotes the induction of anergy during T helper cell recognition of myelin basic protein. Cell Immunol. 1995;160:132–138. doi: 10.1016/0008-8749(95)80018-e. [DOI] [PubMed] [Google Scholar]
- Pace E, Bruno TF, Berenger B, Mody CH, Melis M, Ferraro M, et al. Elevated expression of prostaglandin receptor and increased release of prostaglandin E2 maintain the survival of CD45RO+ T cells in the inflamed human pleural space. Immunology. 2007;121:427–436. doi: 10.1111/j.1365-2567.2007.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet L, Jean C, Dietrich G, Fournie JJ, Poupot R. PGE2 inhibits natural killer and gamma delta T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem Pharmacol. 2010;80:838–845. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Yssel H, Harada Y, Arai K. Effects of prostaglandin E2 on Th0-type human T cell clones: modulation of functions of nuclear proteins involved in cytokine production. Int Immunol. 1994;6:523–532. doi: 10.1093/intimm/6.4.523. [DOI] [PubMed] [Google Scholar]
- Paliogianni F, Kincaid RL, Boumpas DT. Prostaglandin E2 and other cyclic AMP elevating agents inhibit interleukin 2 gene transcription by counteracting calcineurin-dependent pathways. J Exp Med. 1993;178:1813–1817. doi: 10.1084/jem.178.5.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliogianni F, Boumpas DT. Prostaglandin E2 inhibits the nuclear transcription of the human interleukin 2, but not the Il-4, gene in human T cells by targeting transcription factors AP-1 and NF-AT. Cell Immunol. 1996;171:95–101. doi: 10.1006/cimm.1996.0178. [DOI] [PubMed] [Google Scholar]
- Bauman GP, Bartik MM, Brooks WH, Roszman TL. Induction of cAMP-dependent protein kinase (PKA) activity in T cells after stimulation of the prostaglandin E2 or the beta-adrenergic receptors: relationship between PKA activity and inhibition of anti-CD3 monoclonal antibody-induced T cell proliferation. Cell Immunol. 1994;158:182–194. doi: 10.1006/cimm.1994.1266. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Ahmed Z, Sayeed MM. PGE(2)-mediated inhibition of T cell p59(fyn) is independent of cAMP. Am J Physiol. 1999;277:C302–C309. doi: 10.1152/ajpcell.1999.277.2.C302. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Uddin S, Sayeed MM. Prostaglandin E2 modulation of p59fyn tyrosine kinase in T lymphocytes during sepsis. J Immunol. 1998;160:929–935. [PubMed] [Google Scholar]
- Naito Y, Endo H, Arai K, Coffman RL, Arai N. Signal transduction in Th clones: target of differential modulation by PGE2 may reside downstream of the PKC-dependent pathway. Cytokine. 1996;8:346–356. doi: 10.1006/cyto.1996.0048. [DOI] [PubMed] [Google Scholar]
- Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Role of protein kinase C in cyclic AMP-mediated suppression of T-lymphocyte activation following burn injury. Biochim Biophys Acta. 1999;1455:45–53. doi: 10.1016/s0925-4439(99)00079-4. [DOI] [PubMed] [Google Scholar]
- Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Xu Z, Majeti R, Weiss A. Reciprocal regulation of lymphocyte activation by tyrosine kinases and phosphatases. J Clin Invest. 2002;109:9–14. doi: 10.1172/JCI14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- Werlen G, Palmer E. The T-cell receptor signalosome: a dynamic structure with expanding complexity. Curr Opin Immunol. 2002;14:299–305. doi: 10.1016/s0952-7915(02)00339-4. [DOI] [PubMed] [Google Scholar]
- Vang T, Abrahamsen H, Myklebust S, Horejsi V, Tasken K. Combined spatial and enzymatic regulation of Csk by cAMP and protein kinase a inhibits T cell receptor signaling. J Biol Chem. 2003;278:17597–17600. doi: 10.1074/jbc.C300077200. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Xu S, Hepler JE, Hutchison M, Frost J, Robbins DJ. Regulation of the MAP kinase cascade. Cell Mol Biol Res. 1994;40:253–256. [PubMed] [Google Scholar]
- Whitehurst CE, Geppert TD. MEK1 and the extracellular signal-regulated kinases are required for the stimulation of IL-2 gene transcription in T cells. J Immunol. 1996;156:1020–1029. [PubMed] [Google Scholar]
- Nika K, Hyunh H, Williams S, Paul S, Bottini N, Tasken K, et al. Haematopoietic protein tyrosine phosphatase (HePTP) phosphorylation by cAMP-dependent protein kinase in T-cells: dynamics and subcellular location. Biochem J. 2004;378:335–342. doi: 10.1042/BJ20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemnitz JM, Driesen J, Classen S, Riley JL, Debey S, Beyer M, et al. Prostaglandin E2 impairs CD4+ T cell activation by inhibition of lck: implications in Hodgkin's lymphoma. Cancer Res. 2006;66:1114–1122. doi: 10.1158/0008-5472.CAN-05-3252. [DOI] [PubMed] [Google Scholar]
- Gerlo S, Verdood P, Gellersen B, Hooghe-Peters EL, Kooijman R. Mechanism of prostaglandin (PG)E2-induced prolactin expression in human T cells: cooperation of two PGE2 receptor subtypes, E-prostanoid (EP) 3 and EP4, via calcium- and cyclic adenosine 5′-monophosphate-mediated signaling pathways. J Immunol. 2004;173:5952–5962. doi: 10.4049/jimmunol.173.10.5952. [DOI] [PubMed] [Google Scholar]
- Caristi S, Piraino G, Cucinotta M, Valenti A, Loddo S, Teti D. Prostaglandin E2 induces interleukin-8 gene transcription by activating C/EBP homologous protein in human T lymphocytes. J Biol Chem. 2005;280:14433–14442. doi: 10.1074/jbc.M410725200. [DOI] [PubMed] [Google Scholar]
- Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, et al. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T cells. Eur J Immunol. 2009;39:1301–1312. doi: 10.1002/eji.200838969. [DOI] [PubMed] [Google Scholar]
- Esaki Y, Li Y, Sakata D, Yao C, Segi-Nishida E, Matsuoka T, et al. Dual roles of PGE2-EP4 signaling in mouse experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:12233–12238. doi: 10.1073/pnas.0915112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 2004;18:1318–1320. doi: 10.1096/fj.03-1367fje. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Takei M, Hori S, Inoue Y, Harada Y, Tanosaki R, et al. The role of PGE(2) in the differentiation of dendritic cells: how do dendritic cells influence T-cell polarization and chemokine receptor expression. Stem Cells. 2002;20:448–459. doi: 10.1634/stemcells.20-5-448. [DOI] [PubMed] [Google Scholar]
- Rubio MT, Means TK, Chakraverty R, Shaffer J, Fudaba Y, Chittenden M, et al. Maturation of human monocyte-derived dendritic cells (MoDCs) in the presence of prostaglandin E2 optimizes CD4 and CD8 T cell-mediated responses to protein antigens: role of PGE2 in chemokine and cytokine expression by MoDCs. Int Immunol. 2005;17:1561–1572. doi: 10.1093/intimm/dxh335. [DOI] [PubMed] [Google Scholar]
- Chakraborti AK, Garg SK, Kumar R, Motiwala HF, Jadhavar PS. Progress in COX-2 inhibitors: a journey so far. Curr Med Chem. 2010;17:1563–1593. doi: 10.2174/092986710790979980. [DOI] [PubMed] [Google Scholar]
- Abdel-Tawab M, Zettl H, Schubert-Zsilavecz M. Nonsteroidal anti-inflammatory drugs: a critical review on current concepts applied to reduce gastrointestinal toxicity. Curr Med Chem. 2009;16:2042–2063. doi: 10.2174/092986709788682209. [DOI] [PubMed] [Google Scholar]
- Lai LH, Chan FK. Nonsteroid anti-inflammatory drug-induced gastroduodenal injury. Curr Opin Gastroenterol. 2009;25:544–548. doi: 10.1097/MOG.0b013e328331549f. [DOI] [PubMed] [Google Scholar]
- Koeberle A, Werz O. Inhibitors of the microsomal prostaglandin E(2) synthase-1 as alternative to non-steroidal anti-inflammatory drugs (NSAIDs)--a critical review. Curr Med Chem. 2009;16:4274–4296. doi: 10.2174/092986709789578178. [DOI] [PubMed] [Google Scholar]
- Chen Q, Muramoto K, Masaaki N, Ding Y, Yang H, Mackey M, et al. A novel antagonist of the prostaglandin E(2) EP(4) receptor inhibits Th1 differentiation and Th17 expansion and is orally active in arthritis models. Br J Pharmacol. 2010;160:292–310. doi: 10.1111/j.1476-5381.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudino RF, Kassuya CA, Ferreira J, Calixto JB. Pharmacological and molecular characterization of the mechanisms involved in prostaglandin E2-induced mouse paw edema. J Pharmacol Exp Ther. 2006;318:611–618. doi: 10.1124/jpet.106.102806. [DOI] [PubMed] [Google Scholar]
- Kundu N, Ma X, Holt D, Goloubeva O, Ostrand-Rosenberg S, Fulton AM. Antagonism of the prostaglandin E receptor EP4 inhibits metastasis and enhances NK function. Breast Cancer Res Treat. 2009;117:235–242. doi: 10.1007/s10549-008-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CR, Amaya F, Barrett L, Wang H, Takada J, Samad TA, et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Ther. 2006;319:1096–1103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- Ma X, Kundu N, Rifat S, Walser T, Fulton AM. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res. 2006;66:2923–2927. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- Piazuelo E, Jimenez P, Strunk M, Santander S, Garcia A, Esteva F, et al. Effects of selective PGE2 receptor antagonists in esophageal adenocarcinoma cells derived from Barrett's esophagus. Prostaglandins Other Lipid Mediat. 2006;81:150–161. doi: 10.1016/j.prostaglandins.2006.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.