Abstract
Numerous atypical mycobacteria, including Mycobacterium abscessus (Mabc), cause nontuberculous mycobacterial infections, which present a serious public health threat. Inflammasome activation is involved in host defense and the pathogenesis of autoimmune diseases. However, inflammasome activation has not been widely characterized in human macrophages infected with atypical mycobacteria. Here, we demonstrate that Mabc robustly activates the nucleotide binding and oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome via dectin-1/Syk-dependent signaling and the cytoplasmic scaffold protein p62/SQSTM1 (p62) in human macrophages. Both dectin-1 and Toll-like receptor 2 (TLR2) were required for Mabc-induced mRNA expression of pro-interleukin (IL)-1β, cathelicidin human cationic antimicrobial protein-18/LL-37 and β-defensin 4 (DEFB4). Dectin-1-dependent Syk signaling, but not that of MyD88, led to the activation of caspase-1 and secretion of IL-1β through the activation of an NLRP3/apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) inflammasome. Additionally, potassium efflux was required for Mabc-induced NLRP3/ASC inflammasome activation. Furthermore, Mabc-induced p62 expression was critically involved in NLRP3 inflammasome activation in human macrophages. Finally, NLRP3/ASC was critical for the inflammasome in antimicrobial responses to Mabc infection. Taken together, these data demonstrate the induction mechanism of the NLRP3/ASC inflammasome and its role in innate immunity to Mabc infection.
Keywords: Dectin-1, inflammasome, innate immunity, Mycobacterium abscessus, NLRP3
The nontuberculous mycobacteria (NTM), also called atypical mycobacteria, are ubiquitous in soil and water and exhibit varied pathogenicity. NTM infections are now increasingly recognized as a cause of chronic lung disease, lymphadenitis, skin disease and disseminated infections.1, 2 Rapidly growing mycobacteria such as Mycobacterium abscessus (Mabc), M. fortuitum and M. chelonae are the principal pathogens involved in NTM infections.1, 2 Mabc (formerly M. chelonae subspecies abscessus) is one of the most common NTM species that causes invasive mycobacterial disease and disseminated infections in patients with cystic fibrosis.3, 4 Antimicrobial peptides, including human cathelicidins and defensins, constitute a major component of innate host defense systems.5, 6, 7 Human cationic antimicrobial protein (hCAP-18)/LL-37 is the only member of the cathelicidin family to have been identified in humans.5, 6 The small (3–5 kDa) human cationic defensins are a delineated family of effector molecules that contribute to host defense, inflammation and cytotoxicity.7 Mabc infection is often notoriously difficult to eradicate;8 however, the mechanisms of innate immune defense in host cells against Mabc infection remain unknown.
Nucleotide binding and oligomerization domain-like receptors (NLRs) are pattern-recognition receptors that recognize bacterial products in the cytosol.9 Recent studies have revealed that these receptors can activate caspase-1 via the inflammasome, a multiprotein complex.9, 10 Upon cellular infection or stress, inflammasome activation has been shown to be important in the maturation of proinflammatory cytokines such as interleukin (IL)-1β, thereby triggering innate immune responses and inflammation.9, 10, 11, 12 Accumulating studies have highlighted the roles of several pathways that activate the inflammasome.10, 12 Recent studies have revealed that the cytoplasmic protein p62/SQSTM1 (p62) functions as a nodal point in various signaling pathways, including those that control inflammation.13, 14, 15 In Shigella-infected cells, p62 is recruited to polyubiquinated membrane remnants that are associated with inflammasome components and caspase-1.13 However, the possible role of p62 in the activation of the inflammasome in innate immune cells has not been characterized. Additionally, the ability of atypical mycobacteria to activate the inflammasome and its regulation in monocytes/macrophages remain to be clarified.
Dectin-1, a C-type lectin-like pattern-recognition receptor present on monocytes/macrophages and dendritic cells, can recognize β-glucans.16 Dectin-1 contains an immunoreceptor tyrosine-based activation motif-like motif in its cytoplasmic tail, and when phosphorylated upon ligand binding, it can activate the spleen tyrosine kinase (Syk)-dependent pathway.17, 18 Recent studies have demonstrated the role of dectin-1 in the activation of the inflammasome in fungal infections, including those with Candida albicans19, 20 and Aspergillus fumigatus.21 Moreover, a recent study has shown that the dectin-1/Syk signaling pathway is involved in β-glucan-mediated nucleotide binding and oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3 also called NALP3 and cryopyrin) inflammasome activation.19, 21, 22 However, the role of dectin-1 in inflammasome activation in mycobacterial infection, particularly in human cells, has not been fully characterized.
Our previous studies have shown that Mabc is actively internalized and induces proinflammatory cytokine secretion in macrophages through dectin-1-dependent signaling and cooperation between Toll-like receptor (TLR) 2 and dectin-1.23 In the present study, we investigated how Mabc activates the inflammasome, a process that is required for efficient innate immune activation in response to Mabc. We show that Mabc activates the NLRP3/ASC inflammasome via dectin-1/Syk-dependent signaling. Both dectin-1 and TLR2 were required for the induction of pro-IL-1β, cathelicidin hCAP-18/LL-37 and β-defensin 4 (DEFB4) mRNA expression. Notably, dectin-1-dependent Ca2+ influx and Syk signaling contributed to the activation of the NLRP3/ASC inflammasome. Additionally, p62 expression was critical for Mabc-induced proinflammatory responses and inflammasome activation in human macrophages. Finally, NLRP3/ASC inflammasome activation contributed to antimicrobial responses against Mabc infection in human macrophages.
RESULTS
Mabc robustly induces caspase-1 activation and IL-1β maturation in human macrophages
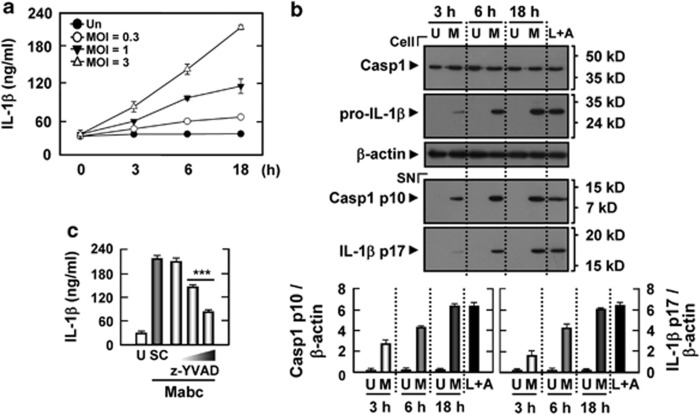
We first examined whether Mabc activates caspase-1 and IL-1β secretion in human primary monocyte-derived macrophages (MDMs) at various time points after infection and at various moi (multiplicities of infection). When MDMs were treated with Mabc, IL-1β levels were increased significantly in culture supernatants beginning 3 h after infection (at moi of 1 and 3), and peaked at 18 h (Figure 1a). Because there was no significant Mabc-dependent IL-1β secretion between moi of 3 and 10, cells were infected at a moi of 3 in subsequent experiments (data not shown). Given that caspase-1 (an IL-1β-converting enzyme) cleaves the IL-1β precursor to mature IL-1β,24 we investigated caspase-1 activation and IL-1β processing using western blot analysis. After 3 h of Mabc infection, MDMs displayed clear activation of caspase-1, as evidenced by an increased amount of the cleaved p10 subunit (Figure 1b, bottom). Consistent with caspase-1 activation, the processed active form of IL-1β was evident in culture supernatants after 6 h of Mabc infection (Figure 1b, bottom). Additionally, IL-1β secretion after Mabc infection was considerably reduced when human MDMs were pretreated with a caspase-1-specific inhibitor, z-YVAD-fmk (Figure 1c).25 Together, these data suggest that Mabc robustly activates the inflammasome and induces IL-1β secretion in human macrophages.
Figure 1.
Mabc robustly induces inflammasome activation in human MDMs. (a, b) Human MDMs were infected with Mabc for the indicated times (0–18 h) at a moi of 0.3, 1 or 3 (a) and 3 (b). The cells were then subjected to IL-1β ELISA (a) and western blot analysis (b) for caspase-1 (Casp1) and IL-1β (cell, caspase-1 p45 and 31 kDa pro-IL-1β SN, cleaved caspase-1 p10 and 17 kDa mature IL-1β). As a positive control, cells were stimulated with ATP (1 mM) for 6 h after 4 h of incubation with LPS (100 ng ml–1). (b, bottom) Densitometry values for caspase-1 p10 and 17 kDa mature IL-1β normalized to β-actin level. (c) Human MDMs were infected with Mabc for 18 h in the absence or presence of z-YVAD-fmk (10, 20 and 50 μM). SN were then collected and subjected to IL-1β ELISA analysis. Data are from a representative of at least three independent experiments (for a, b, bottom and c; mean values±s.d. of triplicate samples). ***P<0.001 versus control cultures. A, ATP; L, LPS; M, Mabc; SC, solvent control (0.1% DMSO); SN, supernatants; U, uninfected.
Dectin-1 and TLR2 have essential roles in the expression of pro-IL-1β, cathelicidin hCAP-18/LL-37 and DEFB4 in macrophages infected with Mabc
Previously, we showed that dectin-1 is a receptor for Mabc recognition and cooperates with TLR2 to induce inflammatory cytokines in response to Mabc infection.23 In the current study, we examined the time course of the mRNA expression of LL-37 and DEFB4 in human macrophages after Mabc infection. Mabc infection rapidly led to expression of LL-37 and DEFB4 mRNA in human primary MDMs (Supplementary Figure S1a). Levels of LL-37 and DEFB4 mRNA peaked within 6 h of Mabc infection and then decreased (Supplementary Figure S1a).
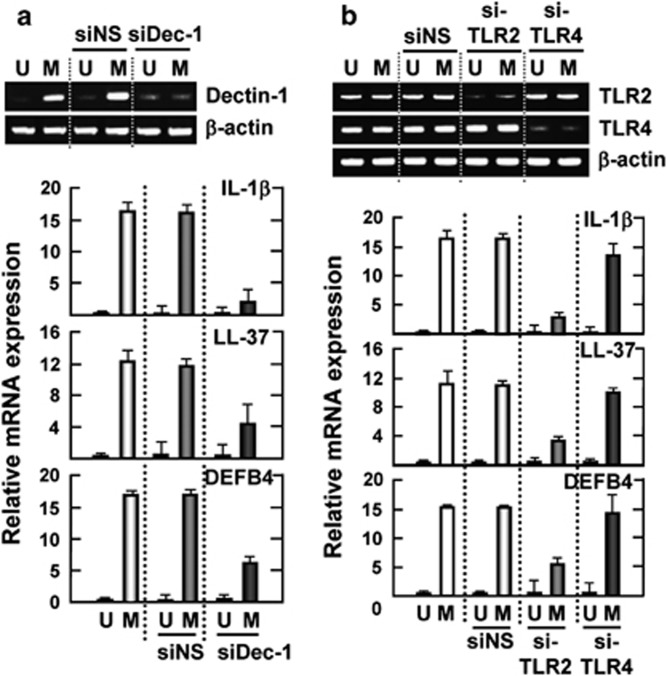
To investigate whether dectin-1 is involved in Mabc-induced pro-IL-1β mRNA expression, we performed dectin-1 small-interfering RNA (siRNA) transfection to THP-1 cells before infection. As shown in Figure 2a, knockdown of dectin-1 significantly attenuated Mabc-induced mRNA expression of pro-IL-1β, LL-37 and DEFB4 in human monocytic THP-1 cells. Additionally, Mabc-induced expression of pro-IL-1β, LL-37 and DEFB4 mRNA was significantly inhibited by pretreatment with a neutralizing dectin-1 antibody (Ab; Supplementary Figure S1b). Expression of these mRNAs was not modulated by an isotype-matched control (IC) monoclonal Ab (mAb; Supplementary Figure S1b).
Figure 2.
Dectin-1 and TLR2 are required for Mabc-induced expression of IL-1β, LL-37 and DEFB4. THP-1 cells were transfected with nonspecific and scrambled siRNA (siNS) or specific siRNAs against hDectin1 (siDec-1; a), hTlr2 (siTLR2) or hTlr4 (siTLR4; b). The cells were then infected with Mabc (moi=3) for 6 h and subjected to semi-quantitative RT–PCR analysis for IL-1β, LL-37 and DEFB4. Data show densitometric analyses (mean values±s.d. of the mRNA levels) from three independent experiments with similar results. (Top) RT–PCR analysis to assess transfection efficiency. M, Mabc; U, uninfected.
We further investigated the role of TLR2 in Mabc-induced expression of pro-IL-1β, LL-37 and DEFB4 in human macrophages. As shown in Figure 2b, specific hTLR2 gene silencing in human THP-1 cells resulted in a profound inhibition of Mabc-induced pro-IL-1β, LL-37 and DEFB4 mRNA expression, whereas neither hTLR4 gene silencing nor control vectors demonstrated this effect. These data strongly suggest that dectin-1 and TLR2 have essential roles in the Mabc-induced mRNA expression of pro-IL-1β, LL-37 and DEFB4 in human macrophages.
Dectin-1-dependent Syk signaling, but not MyD88, leads to activation of caspase-1 and secretion of IL-1β in macrophages infected with Mabc
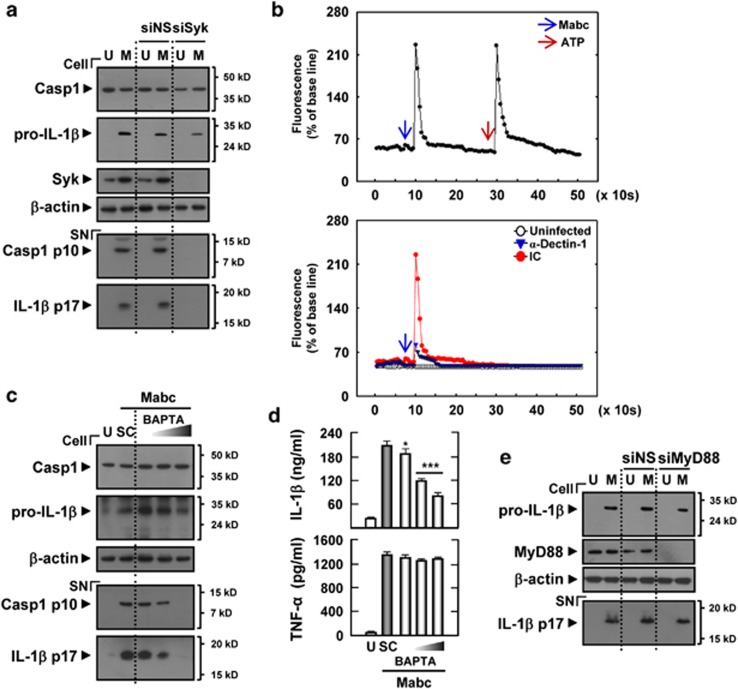
Dectin-1 activates innate signaling pathways through the adaptor molecule Syk kinase.18 Additionally, dectin-1 induces Ca2+ flux in dendritic cells through phospholipase C-γ2 activation.26 We further examined the role of Syk and Ca2+ signaling in Mabc-induced inflammasome activation. Either silencing of Syk by specific siRNA transfection or pharmacological inhibition of Syk significantly inhibited IL-1β maturation as well as caspase-1 cleavage in response to Mabc infection in THP-1 cells (Figure 3a) and MDMs (Supplementary Figure S2a), indicating that Syk activity modulates caspase-1 activation and IL-1β secretion in macrophages infected with Mabc. As a control, Mabc-induced tumor necrosis factor (TNF)-α production was not inhibited in MDMs pretreated with picetannol (Supplementary Figure S2b).
Figure 3.
Syk, but not MyD88, has a role in Mabc-induced caspase-1 activation and IL-1β secretion in human MDMs. (a, e) THP-1 cells were transfected with control nonspecific siRNA (siNS) or specific siRNAs against hSyk (siSyk, a) or hMyD88 (siMyD88, e). (c, d) Human MDMs were pretreated with BAPTA (5, 10 or 25 μM) for 45 min. Cells were then infected with Mabc (moi=3) for 18 h. (a, c, e) Western blots of cell lysates (cell) and supernatants (SN) from THP-1 cells (a, e) and MDMs (c) were probed with anti-caspase-1 (Casp1) Ab and anti-IL-1β Ab. (b) (Top) THP-1 cells were infected with Mabc (moi=3). (Bottom) THP-1 cells were pretreated with or without anti-dectin-1 Ab (α-Dectin-1) or the same concentration of an IC Ab before infection with Mabc (moi=3). The cells were then loaded with Fluo-4/AM and imaged by LSM510 confocal microscope objective lens at 5 s intervals. Changes are shown as mean fluorescence intensity from 15 cells per microscopic field over time. (d) IL-1β and TNF-α ELISA analysis. Data are from a representative of at least three independent experiments (d; mean values±s.d. of triplicate samples). *P<0.05; ***P<0.001 versus control cultures. M, Mabc; SC, solvent control (0.1% DMSO); U, uninfected.
We next examined whether Mabc-dependent dectin-1 activation could induce intracellular Ca2+ influx in human monocytic cells. As shown in Figure 3b, Mabc treatment of THP-1 cells led to a significant induction of intracellular Ca2+ influx, which was considerably dampened by preincubation of these cells with a blocking anti-dectin-1 Ab. These data imply that Mabc-dependent dectin-1 activation could trigger Ca2+ signaling in these cells. We additionally found that pretreatment with the intracellular Ca2+-specific chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N,N-tetraacetic acid tetra-(acetoxymethyl) ester (BAPTA-AM) significantly blocked Mabc-induced caspase-1 activation and cleavage and secretion of IL-1β in human MDMs (Figures 3c and d). However, Mabc-dependent TNF-α production was not substantially modulated in MDMs pretreated with BAPTA-AM (Figure 3d, bottom). Silencing of MyD88 by specific siRNA transfection did not affect formation of mature IL-1β in THP-1 cells after Mabc infection (Figure 3e). These data imply that dectin-1-dependent Ca2+ and Syk signaling, but not TLR2-dependent MyD88 signaling, contribute to the production of mature IL-1β in macrophages infected with Mabc.
NLRP3 and ASC are involved in the maturation and secretion of IL-1β by human macrophages after Mabc treatment
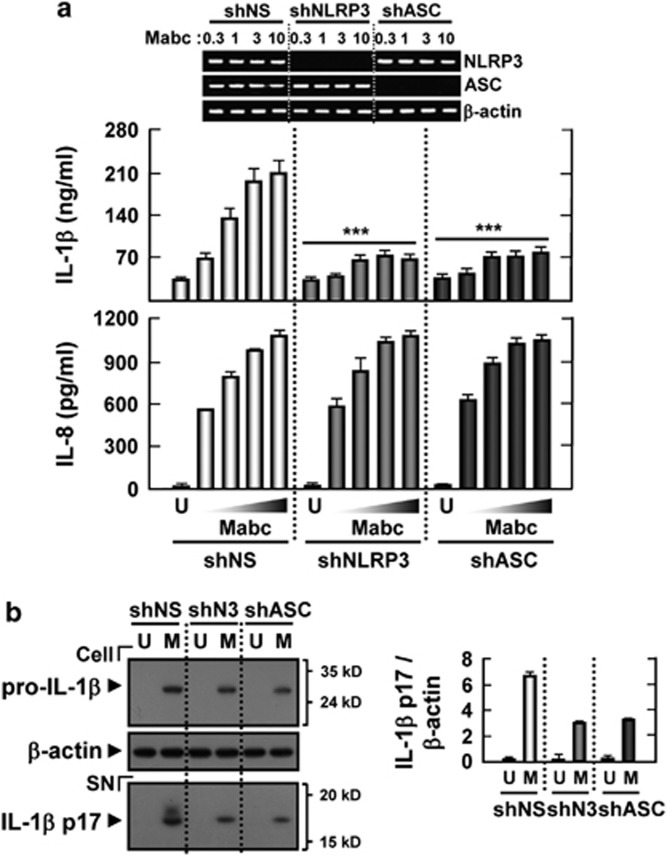
Recent studies have shown that NLRP3/ASC is required for caspase-1 activation and mature IL-1β secretion by M. tuberculosis protein early-secreted antigenic target (ESAT)-6 in human macrophages.27 Thus, we determined the effects of NLRP3 and ASC on Mabc-induced IL-1β secretion by human primary MDMs. As shown in Figure 4a, knockdown of NLRP3 or ASC via lentiviral short hairpin RNA (shRNA)-mediated RNA interference resulted in a decrease of >90% in the mRNA levels of NLRP3 or ASC. Based on enzyme-linked immunosorbent assay (ELISA), analysis of IL-1β and IL-8 secretion, knockdown of NLRP3 and ASC caused a significant reduction in IL-1β, but not IL-8, production by human MDMs (Figure 4a). Additionally, lentiviral shRNA specific to the NLRP3 (shNLRP3) or ASC (shASC) genes significantly inhibited mature IL-1β secretion in human primary MDMs after Mabc infection, compared with secretion in MDMs transduced with nonspecific control shRNA lentiviral particles (shNS; Figure 4b). These results suggest that Mabc-induced secretion and maturation of IL-1β is dependent on NLRP3 and ASC expression in human macrophages.
Figure 4.
Mabc-induced IL-1β release is mediated through NLRP3/ASC inflammasome activation. Human MDMs were transduced with nonspecific control shRNA lentiviral particles (shNS) or lentiviral shRNA specific to NLRP3 (shNLRP3) or ASC (shASC) following infection with Mabc (moi=1, 3 or 10 (a); 3 (b)) for 18 h. The supernatants (SN) were harvested and subjected to ELISA analysis (a) and to western blot analysis for mature IL-1β detection (b; densitometric analysis shown on the right). Data are from a representative of at least three independent experiments (a, b, mean values±s.d. of triplicate samples). (a, top) RT–PCR analysis to assess transduction efficiency. ***P<0.001 versus control cultures. M, Mabc; SN, supernatant; U, uninfected.
Mabc-induced IL-1β secretion does not require LL-37, but require potassium efflux
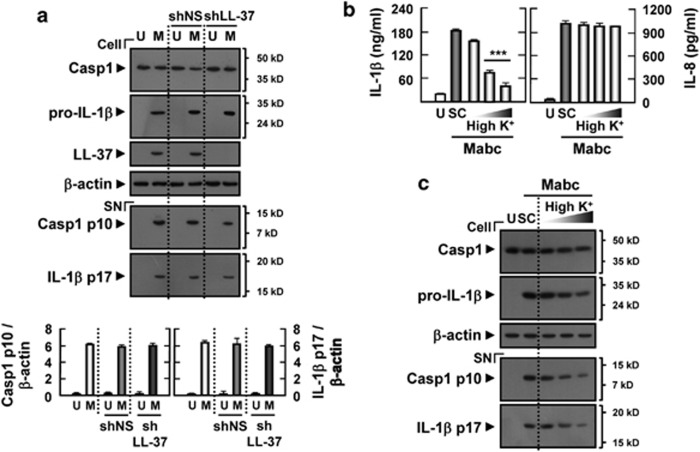
It has been shown previously that human cathelicidin-derived peptide LL-37 induces IL-1β release in lipopolysaccharide (LPS)-primed monocytes through activation of the P2X7 receptor.28 We examined whether Mabc-induced LL-37 is required for the caspase-1 activation and IL-1β maturation in MDMs. To examine this, MDMs transduced with shRNA specific for LL-37 (shLL-37) or shNS were treated with Mabc. The silencing of LL-37 using shLL-37 did not affect IL-1β processing or caspase-1 activation in human macrophages (Figure 5a), indicating that LL-37 is not responsible for Mabc-induced activation of IL-1β processing and release in macrophages.
Figure 5.
Potassium efflux, but not LL-37, is required for Mabc-induced IL-1β secretion in human MDMs. (a) Human MDMs were transduced with shNS or shLL-37 for 3 days before infection with Mabc (moi=3) for 18 h and then subjected to western blot analysis. (b, c) Human MDMs were pretreated with high K+ buffer (10, 50 or 150 mM) for 45 min before Mabc infection. (b) Supernatants were harvested at 18 h after Mabc infection and subjected to ELISA for IL-1β and IL-8. (a, c) Western blots of cell lysates (cell) and supernatants (SN) were probed with anti-caspase-1 (Casp1) Ab and anti-IL-1β Ab. Data are from a representative of at least three independent experiments (b; mean values±s.d. of triplicate samples). The densitometry values for cleaved caspase-1 p10 and 17 kDa IL-1β were normalized to β-actin levels (a, bottom). ***P<0.001 versus control cultures. M, Mabc; SC, solvent control (0.1% DMSO); U, uninfected.
Potassium efflux is implicated in the activation mechanism for the inflammasome, because NLRP3 inflammasome activation can be triggered by low intracellular potassium, but prevented by culturing cells in high potassium-containing media.29 To determine whether Mabc-induced IL-1β secretion was inhibited by high extracellular potassium levels, Mabc-infected MDMs were exposed to high potassium-containing media, and then IL-1β release was determined. As shown in Figure 5b, Mabc-stimulated MDMs in the presence of elevated potassium demonstrated significantly decreased IL-1β levels, showing that elevated extracellular potassium suppressed the ability of Mabc to induce secretion of IL-1β. However, Mabc-induced secretion of IL-8 was largely unaffected (Figure 5b). Furthermore, mature IL-1β secretion and caspase-1 cleavage in response to Mabc infection were significantly inhibited by treatment of MDMs with high concentrations of potassium (Figure 5c). These data suggest that potassium efflux, but not LL-37, is required for Mabc-induced NLRP3/ASC inflammasome activation in human MDMs.
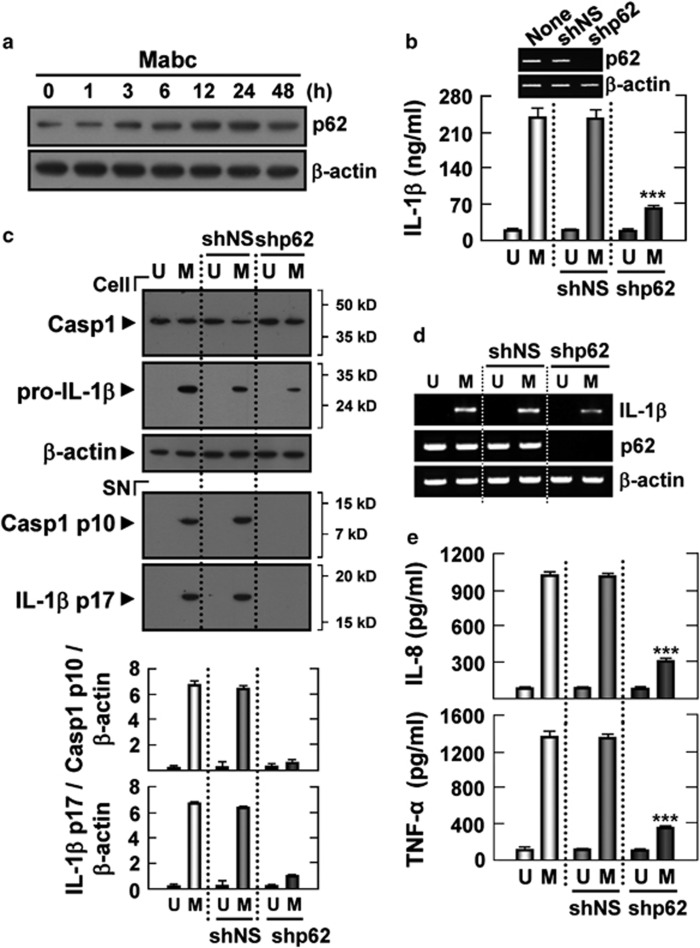
Mabc-induced p62 expression is involved in NLRP3 inflammasome activation in human macrophages
The autophagy adapter p62 can recruit and oligomerize important signaling molecules in cytosolic cellular speckles to control cell survival, apoptosis and inflammation.13, 14, 15, 30 We next examined whether p62 was induced in response to Mabc infection in human macrophages. Reverse transcription polymerase chain reaction (RT–PCR) and immunoblot analysis revealed that p62 transcripts and protein were constitutively expressed in unstimulated human macrophages. After Mabc treatment, p62 transcripts and protein were markedly increased after 3 h, peaked at 12–24 h and gradually decreased until 48 h (Figure 6a; Supplementary Figure S3a; human MDMs and THP-1 cells, respectively).
Figure 6.
p62/SQSTM1 is involved in the production of proinflammatory cytokines and activation of the Mabc-induced inflammasome. (a) Kinetics of p62 expression. Human MDMs were infected with Mabc (moi=3) for the indicated times (0–48 h) and subjected to western blot analysis to determine the levels of p62. (b–e) Human MDMs were transduced with shNS or shp62 for 3 days before infection with Mabc. The cells were then infected with Mabc (moi=3) for 18 h and subjected to ELISA analysis (b, IL-1β e, IL-8 and TNF-α) and semi-quantitative RT–PCR analysis (d). (b, top) Gene-silencing efficiencies were determined by semi-quantitative RT–PCR. Western blots of cell lysates (cell) and supernatants (SN) were probed with anti-caspase-1 (Casp1) Ab and anti-IL-1β Ab (c). The densitometry values for cleaved caspase-1 p10 and 17 kDa IL-1β were normalized to the β-actin level (c, bottom). Data are from a representative of at least three independent experiments (b, e; mean values±s.d. of triplicate samples). ***P<0.001 versus control cultures. M, Mabc; U, uninfected.
To investigate whether p62 had a role in IL-1β secretion in macrophages, human THP-1 cells transfected with siRNA specific for p62 (sip62) or siNS were treated with Mabc. After 18 h, silencing of p62 significantly reduced Mabc-induced synthesis of IL-1β (Supplementary Figure S3b) and the release of the caspase-1 p10 fragment and mature IL-1β (Supplementary Figure S3c) in human THP-1 cells, indicating that p62 participates in the induction of Mabc-mediated caspase-1 activation and IL-1β secretion in macrophages. Consistent with the above data, Mabc-induced activation of caspase-1 and the secretion and maturation of IL-1β were significantly decreased in human primary MDMs transduced with shRNA specific to p62 (shp62), but not in MDMs transduced with shNS (Supplementary Figures 6b and c). Moreover, MDMs deficient in p62 showed decreased expression of pro-IL-1β mRNA (Figure 6d) and the production of IL-8 and TNF-α proteins (Figure 6e) after Mabc stimulation. Silencing of p62 using shp62 did not affect the cell viability of MDMs (Supplementary Figure S4). We have also examined whether p62 knockdown inhibits NLRP3 inflammasome activation by adenosine triphosphate (ATP) or nigericin. As shown in Supplementary Figure S5, ATP- or nigericin-induced inflammasome activation was not inhibited in MDMs transduced with shp62, indicating that p62 is specifically required for Mabc-induced caspase-1 activation and IL-1β secretion (Supplementary Figure S5). Together, these results suggest that p62 is critical for the Mabc-induced expression of pro-IL-1β mRNA (signal 1) and caspase-1 activation and mature IL-1β secretion (signal 2) in human macrophages.
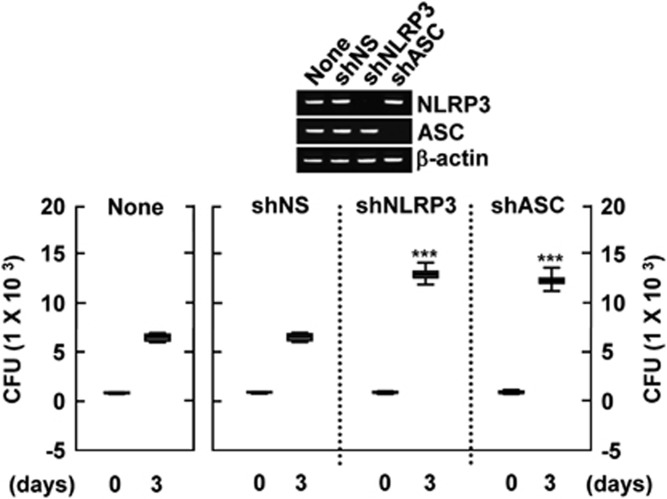
NLRP3 and ASC have critical roles in antimicrobial responses to Mabc in human macrophages
Recent studies have shown that inflammasome activation contributes to the antimicrobial defense against zmp1-deleted M. tuberculosis infection.31 Therefore, we decided to address whether NLRP3 or ASC affects the antimicrobial response to Mabc infection in human MDMs. Human primary MDMs transduced with shNLRP3, shASC or control shRNA, as well as untransduced MDMs, were infected with Mabc. After 4 h, all extracellular bacteria were removed by washing three times with warm phosphate-buffered saline. The cells were then cultured for the times indicated and intracellular bacteria were harvested and assayed for viability using colony forming unit (c.f.u.) assays. On day 3, intracellular bacterial viability was significantly increased in human MDMs transduced with shNLRP3 or shASC, compared with viability in untransduced and shNS-transduced cells (Figure 7). These data suggest that NLRP3 and ASC have important roles in human macrophage defenses against Mabc.
Figure 7.
Both NLRP3 and ASC are required for the antimicrobial response to Mabc infection in human macrophages. Human MDMs were either left untransduced or transduced with shNS, shNLRP3 or shASC (for 3 days) before addition of Mabc (moi=3). After infection, the cells were incubated for 3 days and then subjected to c.f.u. assays. Data are representative of nine independent experiments that yielded similar results. The transduction efficiency was determined by RT–PCR analysis (top). ***P<0.001. Data included in the subfigures represent the medians, interquartile ranges and 95% confidence intervals.
DISCUSSION
Mabc is a common and rapidly growing mycobacterium that causes human diseases, including chronic pulmonary disease and skin and soft tissue infections.3, 4, 32 The management and treatment of Mabc infections is often difficult, and the specific host factors involved in the antimicrobial immune response to Mabc, in contrast to tuberculosis, remain largely unknown.33 Previous studies have shown that dectin-1, a natural killer cell-receptor-like C-type lectin, is required for the recognition of Mabc, M. ulcerans, M. tuberculosis and other mycobacteria.23, 32, 34 The engagement of dectin-1 by mycobacteria initiates intracellular signaling that activates nuclear factor-κB and mitogen-activated protein kinases, leading to the production of proinflammatory cytokines in macrophages and keratinocytes.23, 32, 34 Recent studies have defined the roles of dectin-1 and Syk in the activation of the NLRP3 inflammasome, which participates in host antifungal defense against the dissemination of mucosal infection and mortality in vivo.19, 21 However, whether atypical Mabc activates the inflammasome and how it is regulated in human macrophages have not been determined. In the present report, we demonstrate that dectin-1/Syk signaling has a critical role in Mabc-induced inflammasome activation and IL-1β secretion in human macrophages.
We demonstrated that both dectin-1 and TLR2 act as inducers of pro-IL-1β mRNA. Our data partially correlate with previous studies showing that TLR2 and dectin-1 control pro-IL-1β gene transcription, whereas NLRP3 and caspase-1 regulate the processing and maturation of IL-1β in macrophages infected with C. albicans.19, 20 Many fungi and mycobacteria are recognized by both TLR2 and dectin-1, whose interactions have an important role in the induction of inflammatory responses.9, 23, 32 Dectin-1-dependent signaling pathways can promote the synthesis of cytokines through an intracellular immunoreceptor tyrosine-based activation motif in a Syk-dependent manner.18 In human macrophages, we have defined an essential role for Syk in caspase-1 activation and IL-1β secretion in response to Mabc infection. Moreover, we also revealed the importance of intracellular Ca2+ influx, as well as the NLRP3 inflammasome (NLRP3 and ASC), in controlling Mabc-mediated IL-1β secretion. Our data correlate with a recent study, demonstrating that the dectin-1/Syk signaling pathway has a vital role in fungus- or β-glucan-mediated NLRP3 inflammasome activation.19, 21, 22 Either genetic deletion or pharmacological inhibition of Syk selectively abrogated NLRP3 inflammasome activation by Candida infection, but not by inflammasome activators such as Salmonella typhimurium or the bacterial toxin nigericin.19 Additionally, IL-1β processing and release were found to be dependent on K+ efflux and Ca2+ influx, which are also required for lysosome exocytosis.35 Recent studies have reported that Ca2+-dependent activation of the calmodulin-dependent kinase II and proline-rich tyrosine kinase 2 pathway downstream of dectin-1 and Syk promotes signaling, leading to the generation of an oxidative burst and IL-10 secretion in human macrophages.36 Together, these data imply that dectin-1/Syk pathway activation is important for Mabc-induced NLRP3/ASC inflammasome activation through Ca2+-dependent signaling in macrophages.
Our data show that both dectin-1 and TLR2 are required for expression of mRNA encoding the antimicrobial peptides hCAP-18/LL-37 and DEFB4. Previous studies have demonstrated that TLR2/1 activation in monocytes results in the vitamin D-dependent production of hCAP-18/LL-37, which mediates antimicrobial activity against intracellular M. tuberculosis infection.37, 38 Additionally, our recent studies have shown that hCAP-18/LL-37 has an essential role in vitamin D-induced phagosome–lysosome fusion and antimicrobial activities in human monocytes/macrophages.39 TLR2-mediated microbial recognition and downstream cellular activation result in the induction of human DEFB2 expression,40 reflecting a link between TLR-sensing systems and efferent effector arms in innate immune responses. Recent studies have revealed that the induction of DEFB4 expression requires both TLR2/1-mediated IL-1β and vitamin D receptor activation and contributes to antimicrobial activity against intracellular mycobacteria.41 Moreover, knockdown of both DEFB4 and cathelicidin ablates TLR2/1-mediated antimicrobial responses, suggesting that both DEFB4 and cathelicidin expression are required for optimal antimicrobial activity against intracellular mycobacterial infection.41 These observations suggest that DEFB4 and LL-37 may have important roles in innate immune responses against Mabc infection through TLR2/dectin-1-dependent signaling.
The multidomain scaffold protein p62 functions as a signaling adaptor molecule in cellular signaling pathways, particularly in the regulation of nuclear factor-κB through interaction with TNF receptor-associated factor 6.33, 42 The biological functions of p62 have been studied widely in osteoclastogenesis, inflammation, differentiation and obesity.43 However, the exact mechanisms by which p62 mediates the activation of caspase-1 and IL-1β maturation have not been clarified. Our data suggest that Mabc-induced p62 expression is required for caspase-1 activation and the secretion of mature IL-1β in human macrophages. The polyubiquitin-binding protein p62/SQSTM1 is a common component of protein aggregates and yields protein bodies after polymerization.13 p62 is involved in linking protein aggregates to the autophagic machinery, autophagosomes and lysosomal structures, and thus reduces the toxicity of mutant protein aggregates in the cytosol.13, 15, 30 The current study does not examine whether Mabc infection induces autophagy in human monocytes. Indeed, numerous bacteria can utilize several virulence factors or strategies to evade the autophagy system.44 We have found that p62 levels were increased significantly in human MDMs after Mabc infection (see Figure 6a and Supplementary Figure S4a), indicating that Mabc may cause cytoplasmic accumulation of p62 protein aggregates and thus may modify or inhibit autophagosome formation in human macrophages. Defective autophagy resulted in the upregulation of p62, which is responsible for increased nuclear factor-κB regulation, and promoted pathophysiological conditions, including cellular proliferation, tumorigenesis and chronic inflammation.15, 45, 46 When autophagy is inhibited, p62-positive inclusion-protein aggregates may lead to inflammasome activation and IL-1β secretion. Defective autophagy results in the enhanced generation of mitochondrial reactive oxygen species (ROS), which in turn leads to the activation of the NLRP3 inflammasome and IL-1β secretion.47 Future studies will clarify whether Mabc induces the inhibition of macroautophagy, the accumulation of p62 protein and increased cellular stress including mitochondrial ROS, thus contributing to the activation of the NLRP3 inflammasome machinery and caspase-1 activation.
Using c.f.u. assays, we demonstrated that NLRP3/ASC is required for the antimicrobial response to Mabc infection. Accumulating evidence indicates the pivotal role of inflammasome activation in host defense against several types of infection.48 Indeed, M. tuberculosis is known to prevent inflammasome activation and IL-1β secretion.34 The M. tuberculosis zmp1 gene, which encodes a putative Zn2+ metalloprotease, is required for activation of the inflammasome and enhanced antimicrobial responses in infected lungs of mice and in macrophages.31 However, recent studies using wild-type and ASC-deficient mice have demonstrated that ESAT-6 system-1 (Esx-1)-dependent inflammasome activation exacerbates mycobacterial infection without restricting bacterial growth, suggesting a detrimental effect of Esx-1-mediated inflammation.49 ESAT, a 6-kDa mycobacterial virulence factor, promotes IL-1β secretion and caspase-1 activation through the perturbation of host cell membranes and the formation of an infection-inducible inflammasome complex.50, 51 Additionally, neither NLRP3 nor caspase-1 significantly affected the restriction of M. tuberculosis in vivo using an experimental tuberculosis mice model.52 Moreover, caspase-1- and ASC-deficient mice were not noticeably susceptible to M. tuberculosis infection, whereas IL-1β-deficient mice showed a high mortality with increased pulmonary bacterial burden after M. tuberculosis infection.53 Therefore, neither NLRP3 nor ASC are critical for defense against M. tuberculosis during in vivo infection, or multiple redundant pathways may work in concert to provide host defense in vivo. Future studies will clarify the protective role of inflammasome activation against a variety of atypical mycobacteria, including Mabc, during in vivo infection.
The current study describes Mabc-mediated activation of the NLRP3 inflammasome via dectin-1/Syk-dependent signaling and p62 in human macrophages. Given the important role of the activation of the NLRP3 inflammasome in Mabc infection in macrophages, future studies will be required to clarify the exact roles and mechanisms of the inflammasomes induced by various atypical mycobacteria. These efforts may optimize the development of vaccines or therapeutics against NTM infection.
METHODS
Isolation and culture of human MDMs and cell lines
Human MDMs and the THP-1 cell line (ATCC TIB-202; Manassas, VA, USA) were cultured as described previously.39, 48 Cells were maintained in RPMI 1640 complete medium (Gibco-BRL, Grand Island, NY, USA), with 10% fetal bovine serum (Gibco-BRL), 1% L-glutamine, 50 U l–1 penicillin and 50 mg ml–1 streptomycin (Invitrogen, Carlsbad, CA, USA). For macrophage differentiation, adherent monocytes obtained using Lymphoprep (Axis-Shield, Oslo, Norway) were incubated for 1 h at 37 °C, and nonadherent cells were then removed. Adherent monocytes were further incubated for 5 days with 10% pooled human plasma (macrophage differentiation) as described previously.39 THP-1 cells were differentiated by treatment with 20 nM phorbol-12-myristate13-acetate (PMA; Sigma-Aldrich, St Louis, MO, USA) for 24 h. This study was approved by the Institutional Research and Ethics Committee at Chungnam National University Hospital and Konyang University Hospital. Informed consent was obtained from individual donors, in accordance with the Declaration of Helsinki (as revised in Edinburgh 2000).
Bacterial culture
Mabc-type strain ATCC 19977 was cultured as previously described.23 Bacteria were grown at 37 °C on Middlebrook 7H10 agar medium (Difco Laboratories, Detroit, MI, USA) supplemented with 10% OADC (oleic acid, albumin, dextrose, catalase) (BD Pharmingen, San Diego, CA, USA) and 0.05% Tween 80 (Sigma-Aldrich). Mabc were collected by centrifugation and resuspended in Middlebrook 7H9 medium supplemented with 0.2% glycerol, 0.05% Tween 80 and 10% OADC. Frozen aliquots were stored at −70 °C. Representative vials were thawed and the numbers of c.f.u. viable on Middlebrook 7H10 agar were counted. Single-cell suspensions of mycobacteria were prepared as described previously.23
Reagents
LPS (Escherichia coli serotype 055:B5), ATP disodium salt and DMSO (dimethyl sulfoxide; added to the cultures at 0.1% (v/v) as a solvent control) were from Sigma-Aldrich. For the experiments employing elevated potassium levels (150 mM; Sigma), sodium was replaced by potassium at an equivalent molar concentration.54 BAPTA-AM and z-YVAD-fmk were from Tocris Bioscience (Ellisville, MO, USA). For western blot analysis, Syk (N-19), p62/SQSTM1 (H-290), MyD88 (N-19), LL-37 (H-40) and β-actin (I-19) Abs were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Human caspase-1 p45 (sc-622) and p10 (sc-515) were purchased from Santa Cruz Biotechnology. Human IL-1β was from Cell Signaling Technology (Danvers, MA, USA). Mouse anti-human dectin-1/CLEC7A mAb was from R&D Systems (Minneapolis, MN, USA) and IC mAb (IgG2a) was purchased from eBioscience (San Diego, CA, USA).
shRNA transduction
Human pLKO.1 lentiviral constructs from Open Biosystems (Huntsville, AL, USA) that were used to target NLRP3 (NM_001079821), ASC (NM_013258), LL-37 (NM_004345) and p62 (NM_001142298) were supplied by Sigma-Aldrich as glycerol stocks. Virus production was performed as described previously.39 Briefly, lentiviruses were transfected into HEK 293T cells with pLKO puro.1 or target shRNA plasmids and the packaging plasmids pMDL-RRE (5 μg), pRSV-REV (2.5 μg) and pVSV-g (3 μg). After 48 h, HEK 293T cell supernatants with target-containing virus were collected. The nontarget pLKO.1 shRNA (shNS) or target lentiviral particles were mixed with 8 μg ml–1 Polybrene (Sigma-Aldrich) in HEKs, according to the manufacturer's protocol. After 3 days, the transduction efficiency was determined by RT–PCR.
Transfection of siRNA into THP-1 cells
RNA interference experiments were performed as described previously.39 Briefly, the psiRNA–h7SKGFPzeo plasmids for human TLR2 and TLR4 were purchased from InvivoGen (San Diego, CA, USA). Human Syk-targeting siRNA (sc-29501; a mixture of three target-specific 20–25 nt siRNAs) and control nontargeting siRNA (sc-37007) were purchased from Santa Cruz Biotechnology. Cells were transfected with 20 nM of scrambled siRNA or specific siRNA (dectin-1, Syk and MyD88; Santa Cruz Biotechnology) with the transfection reagent Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. Transfected cells were stimulated with Mabc after being harvested for western blot analysis.
RNA extraction and RT–PCR analysis
RNA extraction from monocytes and THP-1 cells was performed as described previously.34, 39 Briefly, total RNA was extracted used TRIzol reagent (Invitrogen). PCR was performed with a Peltier Thermal Cycler-200 (Watertown, MA, USA) using 35 cycles with 45 s of annealing, and temperatures as follows: 52 °C for p62 and β-actin, 54 °C for TLR2 and TLR4, 58 °C for ASC and NLRP3, 59 °C for LL-37, 60 °C for dectin-1, 65 °C for DEFB4 and 70 °C for IL-1β. The primer pairs are shown in Supplementary Table 1.
Western blot and ELISA analysis
Western blot analysis using whole-cell lysates and cell supernatants was performed as described previously.46, 55 Briefly, after stimulation, nuclei and cell debris were removed by centrifugation (20 min; 14 000 g; 4 °C). The post-nuclear supernatants were further cleared by centrifugation at 100 000 g for 60 min at 4 °C. To concentrate the secreted IL-1β from cell culture media, 10 μl of StrataClean resin (Stratagene, La Jolla, CA, USA) were added to the each sample (200 μl), which were then incubated for 30 min at 4 °C, according to the manufacturer's protocol. After resin-bound protein, samples were washed twice with phosphate-buffered saline by centrifugation, resuspended with 1 × sample buffer and analyzed by western blot analysis. Cytokine levels were measured by ELISA (for IL-1β, TNF-α and IL-8 (BD Pharmingen)), as described previously.49
Intracellular Ca2+ measurements
Intracellular Ca2+ measurements were performed as described previously.56 Briefly, THP-1 cells grown on coverslips were loaded with the Ca2+ indicator Fluo-4/AM (10 mM, 30 min for kinetic measurements) in HBSS (Hank's balanced salt solution) buffer, according to the manufacturer's protocol (Molecular Probes, Carlsbad, CA, USA). The cells were washed twice with HBSS buffer and infected with Mabc. For confocal measurements, images were obtained with an LSM510 confocal microscope (Zeiss, Thornwood, NY, USA).
Quantification of mycobacterial growth
Briefly, the capacity of intracellular survival was examined using Mabc-induced human MDMs. After 3 days of culture, cells were trypsinized and lysed with 0.025% Triton X-100 to collect intracellular bacteria. The lysates were then resuspended and sonicated in a preheated 37 °C water bath sonicator (Elma, Singen, Germany) for 5 min. Aliquots of the sonicates were serially diluted in 7H9 broth, plated separately on 7H10 agar plates for colony counting in triplicate and incubated at 37 °C with 5% CO2 for 7 days.
Statistical analyses
All data are presented as mean values±s.d. of independent determinations. For statistical analyses, paired t-tests with Bonferroni adjustment or analysis of variance for multiple comparisons were performed. Differences were considered significant at P<0.05.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) through the Infection Signaling Network Research Center (2011-0006228) at Chungnam National University. We are grateful to K Kim for helpful discussion and reagents; J-J Kim, T-S Kim and HS Jin for technical support.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Koh WJ, Kwon OJ, Lee KS. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J Radiol. 2002;3:145–157. doi: 10.3348/kjr.2002.3.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field SK, Cowie RL. Lung disease due to the more common nontuberculous mycobacteria. Chest. 2006;129:1653–1672. doi: 10.1378/chest.129.6.1653. [DOI] [PubMed] [Google Scholar]
- Sermet-Gaudelus I, Le Bourgeois M, Pierre-Audigier C, Offredo C, Guillemot D, Halley S, et al. Mycobacterium abscessus and children with cystic fibrosis. Emerg Infect Dis. 2003;9:1587–1591. doi: 10.3201/eid0912.020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M, Ardito F, Fiscarelli E, La Sorda M, D'Argenio P, Ricciotti G, et al. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J Clin Microbiol. 2001;39:816–819. doi: 10.1128/JCM.39.2.816-819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- Griffith D. Nontuberculous mycobacterial lung disease. Curr Opin Infect Dis. 2010;23:185–190. doi: 10.1097/QCO.0b013e328336ead6. [DOI] [PubMed] [Google Scholar]
- Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008;21:279–286. doi: 10.1097/QCO.0b013e3282f88b5d. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Delbridge LM, O'Riordan MX. Innate recognition of intracellular bacteria. Curr Opin Immunol. 2007;19:10–16. doi: 10.1016/j.coi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production. Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Dupont N, Lacas-Gervais S, Bertout J, Paz I, Freche B, Van Nhieu GT, et al. Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe. 2009;6:137–149. doi: 10.1016/j.chom.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, et al. Syk-dependent cytokine induction by dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saïd-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One. 2010;5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankkunen P, Teirilä L, Rintahaka J, Alenius H, Wolff H, Matikainen S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol. 2010;184:6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, et al. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008;10:1608–1621. doi: 10.1111/j.1462-5822.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- Xu S, Huo J, Lee KG, Kurosaki T, Lam KP. Phospholipase C gamma2 is critical for dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem. 2009;284:7038–7046. doi: 10.1074/jbc.M806650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Deretic V. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev. 2011;240:92–104. doi: 10.1111/j.1600-065X.2010.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–232. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ED, Bai X, Kartalija M, Orme IM, Ordway DJ. Host immune response to rapidly-growing mycobacteria, an emerging cause of chronic lung disease. Am J Respir Cell Mol Biol. 2010;43:387–393. doi: 10.1165/rcmb.2009-0276TR. [DOI] [PubMed] [Google Scholar]
- Lee HM, Shin DM, Choi DK, Lee ZW, Kim KH, Yuk JM, et al. Innate immune responses to Mycobacterium ulcerans via toll-like receptors and dectin-1 in human keratinocytes. Cell Microbiol. 2009;11:678–692. doi: 10.1111/j.1462-5822.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: implications for inflammatory processes. Proc Natl Acad Sci USA. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EK, Wang L, Ivashkiv LB. Calcium-activated pathways and oxidative burst mediate Zymosan-induced signaling and IL-10 production in human macrophages. J Immunol. 2010;184:5545–5552. doi: 10.4049/jimmunol.0901293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13:117–124. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Birchler T, Seibl R, Büchner K, Loeliger S, Seger R, Hossle JP, et al. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur J Immunol. 2001;31:3131–3137. doi: 10.1002/1521-4141(200111)31:11<3131::aid-immu3131>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Wooten MW. Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci. 2007;32:95–100. doi: 10.1016/j.tibs.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Campoy E, Colombo MI. Autophagy subversion by bacteria. Curr Top Microbiol Immunol. 2009;335:227–250. doi: 10.1007/978-3-642-00302-8_11. [DOI] [PubMed] [Google Scholar]
- Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol. 2010;12:863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- Lee HM, Shin DM, Yuk JM, Shi G, Choi DK, Lee SH, et al. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J Immunol. 2011;186:1248–1258. doi: 10.4049/jimmunol.1001954. [DOI] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson F, Kim J, Dumitru C, Barck KH, Carano RA, Sun M, et al. Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog. 2010;6:e1000895. doi: 10.1371/journal.ppat.1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol. 2008;10:1866–1878. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurenuma T, Kawamura I, Hara H, Uchiyama R, Daim S, Dewamitta SR, et al. The RD1 locus in the Mycobacterium tuberculosis genome contributes to activation of caspase-1 via induction of potassium ion efflux in infected macrophages. Infect Immun. 2009;77:3992–4001. doi: 10.1128/IAI.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter K, Hölscher C, Tschopp J, Ehlers S. NALP3 is not necessary for early protection against experimental tuberculosis. Immunobiology. 2010;215:804–811. doi: 10.1016/j.imbio.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, et al. Caspase-1 independent IL-1beta production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, et al. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010;12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.