Abstract
Blood-brain-barrier disruption occurs with a high incidence after traumatic brain injury, and is an important contributor to many pathological processes, including brain edema, inflammation, and neuronal cell death. Therefore, blood-brain-barrier integrity is an important potential therapeutic target in the treatment of the acute phase of brain trauma. In this short communication, we report our data showing that neuregulin-1 (NRG1), a growth factor with diverse functions in the CNS, ameliorates pathological increases in endothelial permeability and in BBB permeability in experimental models of injury. For in-vitro studies, rat brain endothelial cells were incubated with the inflammatory cytokine IL-1β, which caused an increase in permeability of the cell layer. Co-incubation with NRG1 ameliorated this permeability increase. For in-vivo studies, C57Bl mice were subjected to controlled cortical impact (CCI) under anesthesia, and BBB permeability was assessed by measuring the amount of Evans blue dye extravasation at 2h. NRG1 administered by tail-vein injection 10 minutes after CCI resulted in a decrease in Evans blue dye extravasation by 35%. Since Evans blue extravasation may result from an increase in BBB permeability or from bleeding due to trauma, hemoglobin ELISA was also performed at the same time point. There was a trend towards lower levels of hemoglobin extravasation in the NRG1 group, but the results did not reach statistical significance. MMP-9 activity was not different between groups at 2h. These data suggest that NRG1 has beneficial effects on endothelial permeability and BBB permeability following experimental trauma, and may have neuroprotective potential during CNS injury.
Keywords: neuregulin-1, endothelial, blood-brain barrier, brain trauma, IL-1β, permeability
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in the developed world. In the US, the annual incidence of TBI is over 1.4 million, resulting in over 50,000 deaths annually and leaving many survivors with long-term disabilities [1-3]. Blood-brain-barrier (BBB) disruption occurs with a high incidence after traumatic brain injury, and contributes to many neurological pathologies after brain injury [4, 5]. These include acute processes such as brain edema, inflammation, and loss of neuronal function or viability [5]. BBB disruption has also been implicated as a factor in long-term sequelae commonly seen after head trauma, such as cognitive impairment, memory loss, motor deficits, and seizures [5-8]. Due to its association with acute and long-term pathological processes after brain injury, the integrity of the blood-brain-barrier constitutes a potential therapeutic target in the treatment of brain trauma.
In this short communication, we report our data showing that neuregulin-1 (NRG1) ameliorates pathological increases in endothelial permeability and in BBB permeability in experimental models of injury. NRG1 is an endogenous growth factor with diverse functions in neurons and glia [9, 10]. Recent investigations from our group have shown that NRG1 signaling is also active in brain microvascular endothelial cells [11]. Here we extend the investigation of NRG1 effect on brain endothelial cells by examining its effect on endothelial permeability in an in-vitro model and on BBB permeability in an in-vivo model. In-vitro studies were conducted using IL-1β to induce an increase in permeability in RBE.4 rat brain endothelial cells. In-vivo studies were performed in a controlled cortical impact model of TBI in mice.
Materials and Methods
Endothelial cell permeability assay
A rat brain microvascular endothelial cell line (RBE.4) was cultured in complete growth media EGM-2 MV (Lonza, Walkersville, MD). RBE.4 cells were seeded onto the inner surface of collagen-coated transwell inserts (6.5mm diameter, 3.0 μm pore size polycarbonate filter; Corning, Corning, NY), which were placed in wells of a 24-well plate with complete EGM-2 growth media. When the monolayer of cells was confluent, confirmed by testing that it is impermeable to media, the cells were serum starved for 8h with EBM media without growth supplement, then incubated with IL-1β (10ng/ml) for 24h. 30 min prior to addition of IL-1β, NRG1 (12.5nM or 37.5nM) or vehicle (PBS) was added. After 24h, media in both upper and lower chambers was removed and replaced with fresh media. Permeability was measured by adding 0.1 mg/ml of Fluorescein isothiocyanate (FITC)-labeled dextran (MW, 40,000; Sigma, St. Louis, MO) to the upper chamber. After incubation for 10 min, 100ul of sample from the lower compartment was measured for fluorescence at excitation 490nm and emission 520nm. All independent experiments were performed in duplicate or triplicate.
Controlled Cortical Impact
The trauma protocol was approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and complied with the NIH Guide for the Care and Use of Laboratory Animals. Male C57Bl/6 mice (10-12 weeks of age), weighing 22 to 26 kg, were used. They were given food and water ad libidum and were housed in pathogen free facilities with 12h day/night cycles. The controlled cortical impact (CCI) model was used as previously described with minor modifications [12]. Mice were anesthetized with 4% isoflurane (Anaquest, Memphis, TN) in 70% N2O and 30% O2 using a Fluotec 3 vaporizer (Colonial Medical, Amherst, NH) and positioned in a stereotaxic frame. Anesthesia was maintained using 2-3% isoflurane. A right craniotomy was made using a portable drill and 5mm trephine over the right parieto-temporal cortex, and the bone flap was removed. Mice were subjected to CCI using a pneumatic cylinder with a 3mm flat-tip impounder, velocity 6m/s, depth of 0.6mm, and 150ms impact duration. Following CCI, the scalp was sutured closed and mice were returned to their cages to recover from anesthesia. Vehicle (PBS) or NRG1 at a dose of 2.5ng/kg and 50ng/kg body weight was administered by tail-vein injection 10 min after trauma. An injection volume of 150ul was used for all injections. Investigators were blinded to treatment type (vehicle vs. NRG1) during surgery and data acquisition. Vehicle- and drug-treated mice (n=9-12 per group) were subjected to CCI concomitantly, with the investigator alternating between groups when performing CCI.
Determination of Evans Blue dye extravasation after CCI
Evans Blue dye (2% wt/vol in PBS) in a volume of 4ml/kg was given by tail vein injection 30 min after CCI and allowed to circulate for 1h 30min. 2h after CCI, the mice were sacrificed under deep anesthesia by intra-cardiac perfusion with PBS, at the end of which clear PBS was seen to return to the right atrium in all the animals. The brains were removed; the hemispheres were separated, then homogenized in 50% trichloroacetic acid (wt/vol) solution and centrifuged at 10,000rpm for 20 min. The supernatant, which contains the extracted dye, was diluted with ethanol (1:3), and 100ul from each sample was read using a fluorescence plate reader (ex/em: 620 nm/680 nm). Calculations were based on Evans blue external standards (25-2000 ng/ml) in the solvent 50% TCA /ethanol (1:3). The amount of Evans blue was quantified according to a linear standard curve and is expressed as nanograms of Evans blue per gram of brain tissue.
Hemoglobin ELISA
Vehicle (PBS) or NRG1 at a dose of 50ng/kg body weight was administered by tail-vein injection 10 min after CCI. 2h after CCI, animals were sacrificed under deep anesthesia by intra-cardiac perfusion with PBS. Brains were removed; hemispheres were separated and homogenized in cell lysate buffer (Cell Signaling Technologies, Danvers, MA) containing Halt Protease Inhibitor Cocktail (Thermo Scientific, Waltham, MA) and centrifuged at 14,000rpm for 20 min. Bio-Rad Protein Assay was used to determine the protein concentration and 10ug of protein was used for the Mouse Hemoglobin ELISA (Kamiya Biomedical Company, Seattle, WA).
Gelatin zymography
Brain homogenates were prepared in the same fashion as for the hemoglobin ELISA. 50ug of brain homogenate from each hemisphere was loaded and separated by 10% Tris-glycine gel with 0.1% gelatin as substrate. After separation by electrophoresis, the gel was re-naturated and then incubated with developing buffer at 37°C for 24h as described previously [13]. After developing, the gel was stained with 0.5% Coomassie Blue R-250 for 1.5h and then distained appropriately. In all gels, human MMP-2 standard and human MMP-9 standards (Calbiochem, EMD Chemicals, Darmstadt, Germany) were used as positive controls. Clear bands were quantified using Image-J and integrated band intensities expressed as a percentage of the loaded MMP-9 and MMP-2 controls.
Statistical Analysis
Quantitative data for experiments were analyzed using ANOVA followed by Tukey HSD for multiple comparisons when there were more than 2 groups, and using students’ t-test when there were only 2 groups. Statistical significance was set at p<0.05.
Results
NRG1 ameliorates the IL-1β-induced increase in endothelial permeability in rat brain endothelial cells
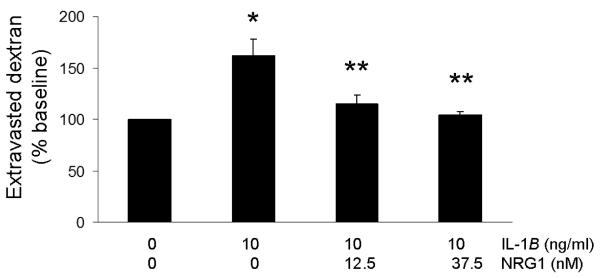
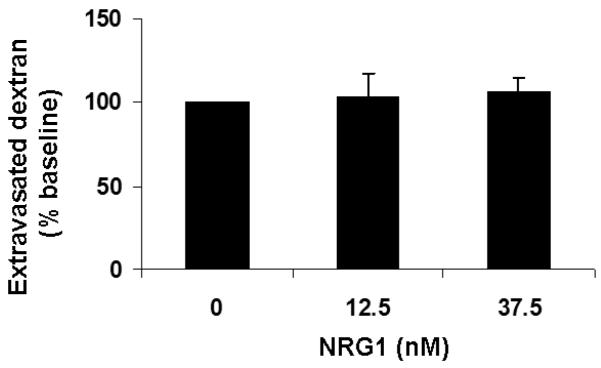
Incubation of RBE.4 cells with IL-1β (10ng/ml) in this model of cytokine-induced endothelial permeability does not result in cell death, based on MTT and LDH assays (data not shown). After incubation with 10ng/ml of IL-1β for 24h, RBE.4 cells exhibited increased permeability averaging 1.5-fold of baseline (p<0.01), as measured by the extravasation of FITC-labeled dextran-40 through the transwell. Incubation with IL-1β in the presence of NRG1 (12.5nM and 37.5nM, equivalent to 100ng/ml and 300ng/ml) resulted in a reduction of the amount of extravasated dextran-40 to near-baseline levels (p<0.01) (Fig.1a). NRG1 alone did not affect endothelial permeability (Fig.1b).
Fig. 1. NRG1 reduces endothelial permeability induced by IL-1β.
Fig. 1a 24h incubation with IL-1β (10ng/ml) resulted in an increase in permeability of the endothelial monolayer, measured as extravasated dextran-40, averaging 1.5-fold that of the baseline level (*p<.01). The presence of NRG1 during IL-1β incubation reduced endothelial permeability to baseline (**p<.01, comparing incubation with IL-1β vs. IL-1β and NRG1). Values are expressed as % of baseline. n = 3 experiments.
Fig.1b NRG1 alone did not alter permeability.
NRG1 decreases acute blood-brain barrier injury in a controlled cortical impact model of traumatic brain injury
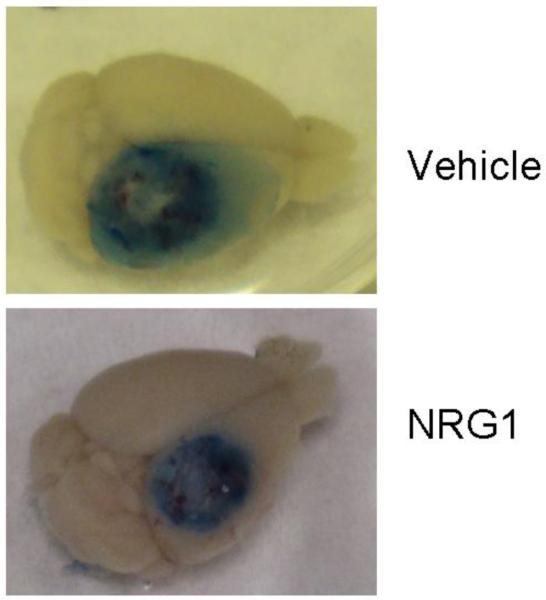
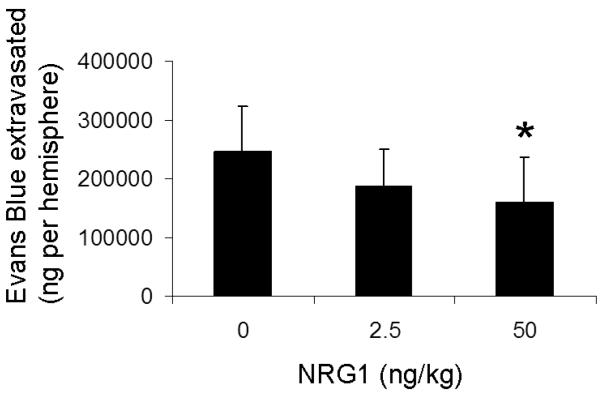
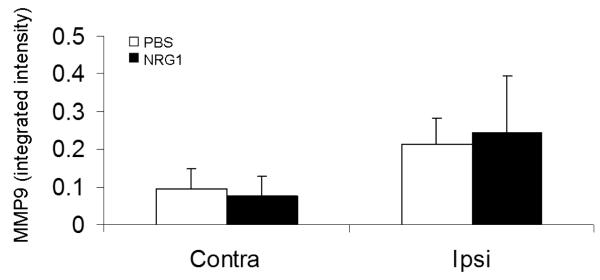
In a CCI model of TBI in mice, acute BBB permeability was assessed by measuring Evans blue dye extravasation 2h after trauma (Fig. 2a). Evans blue extravasation was reduced by 35% in mice given 50ng/ml NRG1 (p<0.05) (Fig. 2b). Hemoglobin ELISA was also performed at the same time point. There was a trend towards lower levels of hemoglobin extravasation in the NRG1 group, but the results did not reach statistical significance (p=0.24, Fig. 3). At the same time point (2h after TBI), MMP-9 activity was measured to determine its possible role as a mediator of barrier disruption. There was an approximately 2-fold increase in MMP-9 activity in the ipsilateral hemisphere compared to the contralateral hemisphere in both the PBS- and NRG-treated groups, but no difference was observed between the 2 groups (Fig. 4a, b).
Fig. 2. Tail-vein administration of NRG1 after brain trauma decreases acute BBB permeability.
Fig. 2a Increased Evans blue extravasation is evident in the ipsilateral hemisphere 2h after CCI.
Fig. 2b Evans blue extravasation is reduced by 35% in NRG1-treated mice (50ng/kg) (*p<.01). Values are expressed as ng of extravasated Evans Blue per gm brain tissue (+/− SD). n=9 in vehicle group; 9 in 2.5ng/kg NRG1 group; 12 in 50ng/kg NRG1 group
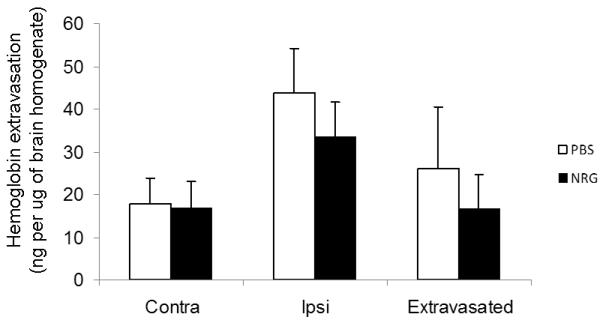
Fig. 3. Hgb ELISA did not show a significant reduction in hemoglobin extravasation.
There was a trend towards lower levels of hemoglobin extravasation in the NRG1 group, but the results did not reach statistical significance with n=5 per group (p=0.24).
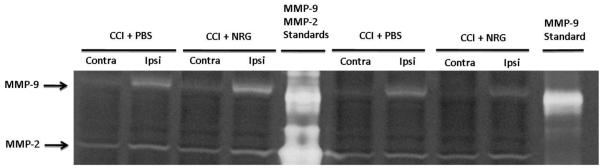
Fig. 4. Tail-vein administration of NRG1 after brain trauma does not affect MMP-9 activity at 2h after CCI.
Fig. 4a A representative zymogram shows MMP-9 and MMP-2 levels in contralateral and ipsilateral hemispheres of PBS- and NRG1-treated mice 2h after CCI. Mouse MMP-MMP-9 standard was used for calibration of the amount of MMP-9 activity
Fig. 4b Integrated band intensities were expressed as a percentage of the loaded MMP-9 control. n=9 in vehicle group; 9 in 2.5ng/kg NRG1 group; 12 in 50ng/kg NRG1 group.
Discussion
The data provide evidence that NRG1 has a beneficial effect on the barrier function of brain microvascular endothelial cells. NRG1 ameliorated the increase in endothelial permeability induced by IL-1β. Additionally, administration of NRG1 by tail-vein injection 10 min after trauma reduces acute BBB permeability in a controlled cortical impact model in mice.
Results from the in-vitro study suggest that NRG1 has a beneficial effect on endothelial barrier function during inflammation. These results are consistent with findings in the invivo study, which shows that NRG1 administered by tail-vein injection 10 min after trauma, decreases BBB permeability. Although Evans blue extravasation may also result from rupture of blood vessels due to trauma, hemoglobin extravasation was not significantly different between the PBS- and NRG1-treated groups at this early time point. However, we cannot rule out the possibility that NRG1 may have an effect on vessel integrity and post-traumatic bleeding at a later time point. Additionally, the decrease in Evans blue extravasation does not appear to be correlated with changes in MMP-9 activity at this time point after brain trauma. Our data suggest that NRG1 can ameliorate this acute BBB injury via MMP-independent mechanisms, at least at this time point.
To our knowledge, this is the first report that NRG1 affects the barrier function of brain microvascular endothelial cells in-vitro and the BBB in-vivo. IL-1β -induced endothelial permeability was used in our in-vitro model because inflammation is present after traumatic brain injury and promotes BBB permeability [14-16]. IL-1β is an inflammatory cytokine which contributes to BBB disruption after brain trauma. Alterations in systemic and intrathecal levels of IL-1β have been reported in patients following TBI. After brain injury, IL-1β is released from activated microglia and astrocytes, and can mediate rapid disruption of the BBB, increase in excitotoxicity [16], and seizure activity.
Our finding that NRG1 enhances endothelial barrier function is consistent with our previous report that NRG1 is cytoprotective for microvascular endothelial cells [11]. In a previous study [17] in which NRG1 was given by tail vein injection prior to CCI, NRG1-treated mice show an improvement in spatial memory. Our current findings suggest that NRG1 effect on BBB permeability may be one component of its neuroprotective properties.
There are several limitations to our study. Our in-vitro experiments, which utilized a cell line, do not incorporate the interactions between the different cell types that constitute the BBB. Within the in- vivo experiment, BBB permeability was evaluated only at one early time point after CCI, when peak BBB permeability is expected to occur. It is possible that a difference in MMP-9 activation between the PBS- and the NRG-treated groups may occur at a later time, since MMP-9 activation has been reported to be present even after 48h [18]. Additionally, the experiments did not delineate the mechanism by which NRG1 decreases BBB permeability in this model. Further experiments will be needed to clarify whether NRG1 acts on some or all of the cell types that constitute the BBB, including endothelial cells, astrocytes, and pericytes, as well as on the matrix that constitute the basement membrane.
Post-traumatic BBB disruption can lead to many immediate and long-term harmful sequelae. Our data suggest that TBI-BBB injury is ameliorated by NRG1. These findings may open new possibilities for investigating NRG1 in neuroprotective strategies after TBI.
Acknowledgements
This study was supported by National Institute of Health grants (K08NS057339 to JL, R01NS53560, RO1NSO61255 to MW, and R01-NS76694 to EHL).
Footnotes
The authors declare no conflicts of interest.
Bibliography
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006 Sep-Oct;21(5):375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006 Nov-Dec;21(6):544–8. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999 Dec;14(6):602–15. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A. Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. J Clin Neurophysiol. 2005 Jan-Feb;22(1):1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- 5.Tomkins O, Friedman O, Ivens S, Reiffurth C, Major S, Dreier JP, et al. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007 Feb;25(2):367–77. doi: 10.1016/j.nbd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, et al. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009 Jul 15;29(28):8927–35. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruttan L, Martin K, Liu A, Colella B, Green RE. Long-term cognitive outcome in moderate to severe traumatic brain injury: a meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Arch Phys Med Rehabil. 2008 Dec;89(12 Suppl):S69–76. doi: 10.1016/j.apmr.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008 Jan 24;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997 Sep;124(18):3501–10. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- 10.Talmage DA. Mechanisms of neuregulin action. Novartis Found Symp. 2008;289:74–84. doi: 10.1002/9780470751251.ch6. discussion -93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lok J, Sardi SP, Guo S, Besancon E, Ha DM, Rosell A, et al. Neuregulin-1 signaling in brain endothelial cells. J Cereb Blood Flow Metab. 2009 Jan;29(1):39–43. doi: 10.1038/jcbfm.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You Z, Yang J, Takahashi K, Yager PH, Kim HH, Qin T, et al. Reduced tissue damage and improved recovery of motor function after traumatic brain injury in mice deficient in complement component C4. J Cereb Blood Flow Metab. 2007 Apr 25; doi: 10.1038/sj.jcbfm.9600497. [DOI] [PubMed] [Google Scholar]
- 13.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001 Oct 1;21(19):7724–32. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari CC, Depino AM, Prada F, Muraro N, Campbell S, Podhajcer O, et al. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004 Nov;165(5):1827–37. doi: 10.1016/S0002-9440(10)63438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vezzani A, Baram TZ. New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr. 2007 Mar-Apr;7(2):45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003 Sep 24;23(25):8692–700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lok J, Wang H, Murata Y, Zhu HH, Qin T, Whalen MJ, et al. Effect of neuregulin-1 on histopathological and functional outcome after controlled cortical impact in mice. J Neurotrauma. 2007 Dec;24(12):1817–22. doi: 10.1089/neu.2007.0372. [DOI] [PubMed] [Google Scholar]
- 18.Bermpohl D, You Z, Lo EH, Kim HH, Whalen MJ. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2007 Apr 4; doi: 10.1038/sj.jcbfm.9600487. [DOI] [PubMed] [Google Scholar]