Abstract
The striatum receives glutamatergic inputs from two main thalamostriatal systems that originate either from the centre median/parafascicular complex (CM/PF-striatal system) or the rostral intralaminar, midline, associative and relay thalamic nuclei (non-CM/PF-striatal system). These dual thalamostriatal systems display striking differences in their anatomical and, most likely, functional organization. The CM/PF-striatal system is topographically organized, and integrated within functionally segregated basal ganglia-thalamostriatal circuits that process sensorimotor, associative and limbic information. CM/PF neurons are highly responsive to attention-related sensory stimuli, suggesting that the CM/PF-striatal system, through its strong connections with cholinergic interneurons, may play a role in basal ganglia-mediated learning, behavioral switching and reinforcement. In light of evidence for prominent CM/PF neuronal loss in Parkinson’s disease, we propose that the significant CM-striatal system degeneration, combined with the severe nigrostriatal dopamine loss in sensorimotor striatal regions, may alter normal automatic actions, and shift the processing of basal ganglia-thalamocortical motor programs towards goal-directed behaviors.
Keywords: Centromedian, Parafascicular, set shifting, striatum, learning, cholinergic interneuron
In traditional models of the basal ganglia circuitry, the cerebral cortex is considered as the prime source of excitatory glutamatergic afferents to the striatum, while the thalamus is recognized as the main target of basal ganglia outflow [1–3]. However, it has long been known that the thalamus is also a predominant source of excitatory inputs to the striatum [4, 5], but due to the limited knowledge about the functional role of this system, its integration into the functional circuitry of the basal ganglia has long been neglected. However, converging data from recent studies have highlighted the potential role of the thalamostriatal system from the caudal intralaminar nuclei in alertness and behavioral switching. These findings, combined with evidence that the caudal intralaminar nuclei undergo significant degeneration in Parkinson’s disease, and may serve as a potential target for deep brain stimulation in movement disorders, have set the stage for significant advances in our understanding of the anatomical and functional organization of the thalamostriatal system. In this review, we will highlight these recent developments, and provide a comprehensive analysis of the anatomical substrate through which the thalamostriatal systems could mediate their effects upon behavioral switching and attention shifts in normal and parkinsonian states.
1. Anatomy of the dual thalamostriatal systems
Although the caudal intralaminar nuclei (centromedian/parafascicular complex, CM/PF), represent the main source of thalamic inputs to the striatum, it is important to recognize that thalamostriatal projections also arise from several other thalamic nuclei, including the rostral intralaminar, midline and specific relay nuclear groups [6–21]. Based on their dual thalamic origin, and distinctive anatomical features, the thalamostriatal networks can be divided into two segregated subsystems: (1) the CM/PF-striatal projection and (2) the non CM/PF-striatal projections.
1.1. CM/PF-striatal projection and basal ganglia-thalamostriatal circuits
The intralaminar nuclei of the thalamus are located lateral to the mediodorsal nucleus within the dense axonal meshwork of the internal medullary lamina. They are divided into a rostral group –the central medial, paracentral and central lateral nuclei– and a caudal group which, in primates, consists of the centromedian (or centre median, CM) and the parafascicular (PF) nuclei, that together form the CM/PF complex [22]. Because the CM/PF complex is the main source of thalamic inputs to the striatum [18, 23, 24], the CM/PF-striatal projection has been the most extensively studied thalamostriatal subsystem.
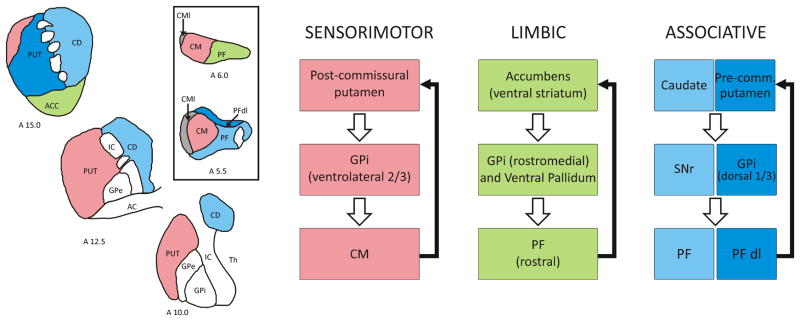
In primates, the CM/PF projects to all functional regions of the striatum in a topographic fashion: (1)The rostral third of PF innervates predominantly the nucleus accumbens (ventral “limbic” striatum); (2) the caudal two thirds of PF project to the caudate nucleus (“associative” striatum); (3) the dorsolateral PF (PFdl) projects to the anterior putamen (“associative” striatum); (4) the medial two thirds of CM innervate the post-commissural putamen (“sensorimotor” striatum); and (5) the lateral third of CM (CMl) provides inputs to the primary motor cortex [18, 23, 24] (Fig. 1). Through these extensive projections, the CM/PF gains access to the whole striatal complex, thereby making the CM/PF-striatal system a functionally organized network that could have broad influences upon motor and non-motor basal ganglia functions.
Fig. 1.
Segregated basal ganglia-thalamostriatal circuits. On the left, the illustration shows the pattern of distribution of color-coded thalamic inputs from the CM/PF complex to three rostrocaudal levels (stereotaxic coordinates at the bottom left) of the striatal complex in monkeys. Apart from the lateral 1/3 of the CM (CMl) which projects mainly to the motor cortex, the rest of the complex is tightly linked in a topographical fashion with the dorsal and ventral striatum. On the right, the functional circuits are indicated. The sensorimotor GPi (ventrolateral 2/3) projects to the CM. The limbic GPi (rostromedial and ventral pallidum) innervate the rostral PF, and the associative GPi (dorsal 1/3) provides inputs to the dorsolateral PF (PFdl). In turn, CM/PF neurons project back to the corresponding functional territories of the striatum (black arrows). The substantia nigra reticulata (SNr) innervates PF neurons that project to the caudate nucleus. Additional abbreviations: A, anterior; AC, anterior commissure; ACC, Accumbens; CD, caudate; GPe, globus pallidus, external segment; GPi, globus pallidus internal segment; IC, internal capsule; PF, parafascicular nucleus; PFdl, dorslateral parafasccular nucleus; Pre-comm., Pre-commissural; Put, Putamen; Th, thalamus.
In addition to these massive and tight connections with the striatum, the CM/PF complex is integrated within the basal ganglia circuitry via direct inputs from the two main output nuclei of the basal ganglia, the internal globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). These topographic and functionally organized projections originate from axon collaterals of GPi and SNr afferents to the ventral anterior/ventral lateral (VA/VL) nuclear complex [24–27]. Through these connections, the CM/PF is part of functionally segregated basal ganglia-thalamostriatal loops that process sensorimotor, associative and limbic information in primates (Fig. 1).
Although the overall organization of these projections has also been described in rodents, it is important to recognize that the caudal intralaminar nuclear complex in these species is solely made up of a single nuclear mass called PF of which the lateral sector is considered as the homologue of the primate CM that projects to the sensorimotor striatum, while the medial sector corresponds to the primate PF proper, connected with associative and limbic striatal regions [7, 28].
1.2. Non CM/PF-striatal system
The existence of thalamostriatal projections from thalamic nuclei other than the CM/PF has long been recognized [5, 29]. Studies using retrograde and anterograde labeling have identified thalamic inputs to the striatum from the midline nuclei, rostral intralaminar nuclei, VA/VL, mediodorsal nucleus, and the pulvinar [6, 7, 10, 13, 15, 17, 22, 28, 30–39].
All areas of the striatum receive modest thalamic glutamatergic projections from non CM/PF nuclei, with a certain degree of topographical organization and specificity. In primates and non-primates, the nucleus accumbens (ventral striatum) receives inputs from dorsal midline thalamic nuclei [7, 17, 22, 31], while the rostral intralaminar group provides afferents to the dorsal striatum [22, 30, 33, 34, 38].
In primates, regions of the VL interconnected with specific areas of the motor cortex project to sectors of the sensorimotor striatum that also receive inputs from the same cortical regions, suggesting convergence and interactions of functionally related corticostriatal and thalamostriatal systems [14]. A similar pattern of connectivity has also been suggested for relationships between VA, associative frontal cortices and related target sites in the caudate nucleus and anterior putamen [14, 15].
1.3. Anatomical Differences between the CM/PF-striatal and other thalamostriatal systems
In addition to their origin, the CM/PF- and non CM/PF-striatal systems display other striking anatomical differences, as illustrated in Figure 2.
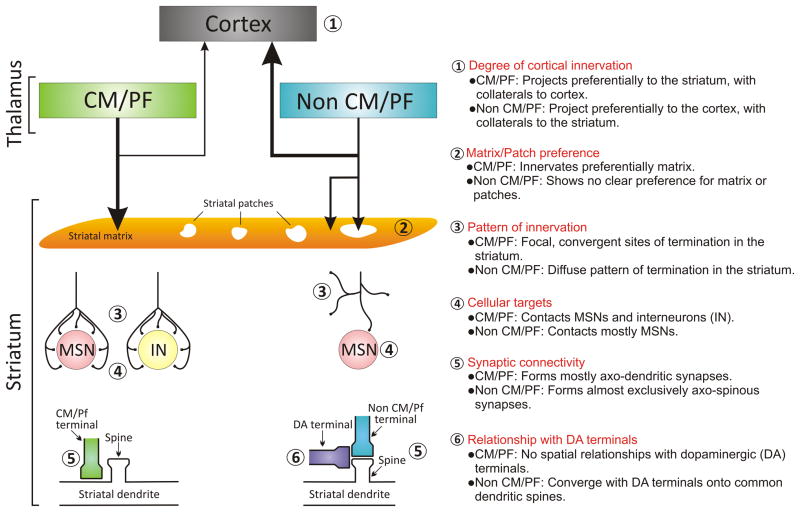
Fig. 2.
Dual thalamostriatal systems from CM/PF and non CM/PF nuclei. The thalamus provides striatal cells with two independent and anatomically different systems. The main anatomical differences between the two systems are summarized.
For instance, in contrast to most thalamic nuclei that provide major inputs to specific cortical regions, and a more modest diffuse projection to the striatum [11, 12, 15, 28], CM/PF neurons provide massive topographically organized striatal projections with only sparse and diffuse collateral projections to the cerebral cortex [6, 20, 40].
The pattern of striatal innervation is also quite different between the two systems. While the CM/PF complex provides dense, focal and highly convergent inputs to the striatum, striatal projections from other thalamic nuclei are more diffuse and sparsely distributed, thereby implying that terminals from individual CM/PF axonal projections may provide a more massive focused innervation of a restricted pool of striatal neurons than inputs from non CM/PF thalamic nuclei [10, 20, 30, 41]. Such a different pattern of termination may impact the synaptic strength of these two systems on striatal neuronal activity.
CM/PF terminals target both MSNs and striatal interneurons. In fact, with the exception of calretinin-positive cells, all types of striatal interneurons are contacted by CM/PF terminals [42, 43]. The cholinergic interneurons, in particular, receive a dense innervation from CM/PF terminals in rats and monkeys [42, 44, 45]. The functional significance of this tight relationship between the CM/PF-striatal system and cholinergic interneurons will be discussed in more detail in section 2.3. Although both direct and indirect striatal projection neurons receive CM/PF inputs, there is a slight preference for CM/PF-striatal afferents towards direct pathway neurons that project to GPi in monkeys [41]. However, non CM/PF-striatal terminals contact almost exclusively MSNs [30, 46], without any specific discrimination between direct or indirect pathway neurons in rats [47].
At the ultrastructural level, the majority of CM and PF terminals form asymmetric synapses with dendrites of striatal projection neurons and interneurons [41, 42, 46, 48, 49]. In contrast, most thalamic terminals from non-CM/PF sources display a pattern of synaptic connectivity similar to that of glutamatergic corticostriatal afferents, i. e., they contact almost exclusively dendritic spines of projection neurons [21, 46].
Dopaminergic terminals from the substantia nigra compacta (SNc), known as key regulators of glutamatergic transmission in the striatum, exhibit different spatial relationships with thalamic terminals from CM/PF versus other thalamic nuclei on the surface of striatal projection neurons. Non-CM/PF thalamic terminals contact dendritic spines in close proximity to dopaminergic boutons, while CM/PF terminals show no spatial closeness to dopaminergic afferents [46, 50, 51]. Nevertheless, despite their distant location, it is possible that dopaminergic and CM/PF inputs terminate on the same striatal neurons, and that the non-synaptic volume transmission of dopamine mediates functional interactions between these neural systems [51, 52].
The striatum is a non-homogenous structure made up of at least two different compartments referred to as “extrastriosomal matrix” (or matrix) and “patches” (or striosomes [53, 54]) that are recognized by their differential anatomical, neurochemical and, most likely, functional characteristics. These compartments also differ in their main sources of thalamic innervation. Afferents from the CM/PF tend to terminate in the matrix, [49, 55], while inputs from other thalamic nuclei are less selective and more widely distributed across both compartments, although terminals from some midline nuclei target selectively the patches [7, 39, 46, 56].
Thus, in light of these striking anatomical differences between striatal inputs from CM/PF versus non CM/PF thalamic nuclei, the thalamostriatal network must be seen as a composite system made up of two major sets of axonal projections that display a unique anatomical organization and a differential pattern of synaptic connectivity within the striatal microcircuitry depending on their thalamic origin. The anatomical and functional organization of this dual thalamostriatal system will now be examined and compared with the massive corticostriatal network, which provides the bulk of glutamatergic excitatory drive to the striatum.
1.4. Thalamostriatal versus corticostriatal projections: Their differences and commonalities
While both cortical and thalamic projections provide glutamatergic inputs to the striatum, these neural systems differ significantly in various anatomical, neurochemical and functional grounds. In this section, the specific features that characterize thalamic versus cortical projections to the striatum will be highlighted in light of the functional roles these neural networks may play in the physiology and pathophysiology of the basal ganglia in normal and diseased conditions.
First, these striatal afferent projections can be differentiated by the segregated expression of vesicular glutamate transporter type 1 (vGlut1) in cortical terminals and vesicular glutamate transporter type 2 (vGlut2) in thalamic terminals [57, 58]. In both rodent and primate striata, double electron microscopy immunocytochemistry demonstrated that vGlut1 is specifically associated with cortical glutamatergic terminals, while vGlut2 is confined to thalamic afferents; with less than 5% of total glutamatergic striatal terminals co-expressing both vGluTs [46, 59]. These studies also allowed to quantify the relative prevalence of cortical over thalamic glutamatergic terminals in the rat and monkey striatum, revealing that vGlut1- and vGlut2-positive terminals represent about 50% and 20% respectively of total glutamatergic terminals in the monkey striatum [59], while these percentages are about 35% and 25% for vGlut1 and vGlut2 respectively in the rat striatum [60]. A considerable proportion of putative glutamatergic terminals do not express either vGlut1 or vGlut2 in rat and monkey, suggesting the presence of another vesicular glutamate transporter, yet to be identified [59, 60].
Besides their useful application as markers of cortico- or thalamostriatal boutons, the selective expression of vGlut1 or vGlut2 may confer unique functional properties to the cortical and thalamic striatal afferent systems. In other brain regions, the presence of vGlut1 or vGlut2 in axon terminals is associated with low and high probability of transmitter release respectively and a different degree of synaptic plasticity [58, 61]. Consistent with these descriptions, recent in vitro electrophysiological data have shown that thalamostriatal synapses exhibit higher probability of glutamate release than corticostriatal synapses, and differ in their short-term synaptic plasticity [62]. These studies also revealed that thalamic and cortical inputs to the striatum differ in ratio and composition of NMDA and AMPA glutamate receptors [62, 63].
In regard to their synaptology, cortical inputs to the striatum target dendritic spines of medium spiny projection neurons, with very rare incidence of axo-dendritic synapses, a pattern reminiscent of the non CM/PF-striatal system, but strikingly different from the CM/PF-striatal projection (see previous section) [46, 48–50, 64, 65]. There is evidence that thalamic and cortical projections differ in the degree of innervation of specific populations of striatal interneurons. For instance, although CM/PF terminals provide a strong input to the proximal dendrites and cell bodies of cholinergic interneurons, cortical terminals only contribute scarce inputs to the distal dendrites of these neurons in primates and non-primates [42, 44, 45, 66]. These data are supported by slice electrophysiology data showing that thalamic, but not cortical, stimulation evokes patterned responses in cholinergic interneurons [67]. The functional importance of the CM/PF-striatal system in regulating cholinergic interneurons activity in learning and behavioral switching/reinforcement is discussed in section 2.3. In contrast to cholinergic cells, striatal GABAergic parvalbumin-positive interneurons (putatively fast-spiking interneurons [68]) appear to receive a significantly stronger cortical than thalamic innervation in rats [43], though such may not be the case in primates [42].
A recent study has revealed a neurochemical feature that appears to be specific for the CM/PF-striatal system. In rats, PF-striatal terminals express immunoreactivity for a protein called cerebellin 1, which was found to play an important role in shaping dendritic structure of striatal MSNs [69]. So far, there is no evidence that cortical terminals contain cerebellin 1 or related proteins.
2. Role of the CM/PF-striatal system in attention and behavioral switching
As described above, the CM/PF is, by far, the main source of thalamic inputs to the striatum. Although our understanding of the functional significance of the thalamostriatal systems remains limited, the recent interest towards the possible role of CM/PF neurons in attention and its importance in regulating cholinergic interneurons activity, combined with evidence for significant CM/PF degeneration in PD patients, have set the stage for a deeper understanding of the importance of the CM/PF-striatal system in mediating basal ganglia responses to unpredicted stimuli, and the potential consequences of the degeneration of this system towards behavioral switching deficits in PD. Despite significant anatomical evidence for thalamostriatal projections that originate outside the CM/PF (see above), the paucity of information on the functional role of these non-CM/PF-striatal projections limits considerably our interpretation of the importance of these systems in the functional circuitry of the basal ganglia. Thus, the following discussion will be entirely devoted to the CM/PF-striatal system, and its possible implication in attention and behavioral switching in normal and parkinsonian conditions. We will conclude with a brief overview of future studies one should consider to move this field forward.
2.1. CM/PF neurons: afferents and functions
In order to critically examine the mechanisms by which the CM/PF-striatal system may contribute to the regulation of behavioral switching in response to attention-related stimuli, it is important to recognize the main sources of inputs that contribute to the regulation of CM/PF neuronal activity. In addition to the prominent basal ganglia GABAergic projections from the GPi and SNr (see section 1.1.), the CM receives inputs from motor, premotor and somatosensory cortices [70–76], while cortical inputs to PF originate preferentially from the frontal and supplementary eye fields [77, 78], and associative areas of the parietal cortex [79, 80].The CM/PF complex also receives strong inputs from various subcortical sources, including the pedunculopontine tegmental nucleus [81–83], the superior colliculus [84–88], the cerebellum [89, 90], the raphe nuclei and locus coeruleus [89, 91, 92], and from brainstem regions of the mesencephalic, pontine and medullary reticular formation [89, 93–99]. Because of these significant ascending connections from the reticular formation and various brainstem regions, combined with the traditional view that the CM/PF and other intralaminar thalamic nuclei are the sources of widely distributed “nonspecific” thalamocortical projections, the intralaminar nuclei were considered part of the ascending “reticular activating system” that regulates arousal and attention (as reviewed in [22]). In line with this concept, functional imaging studies in humans have demonstrated a significant increase of activity in CM/PF during processing of attention-related stimuli [100–102]. Studies in behaving monkeys have provided direct evidence that one of the main roles of CM/PF neurons is, indeed, to process sensory stimuli related to shifts in attention and action bias [103–105].
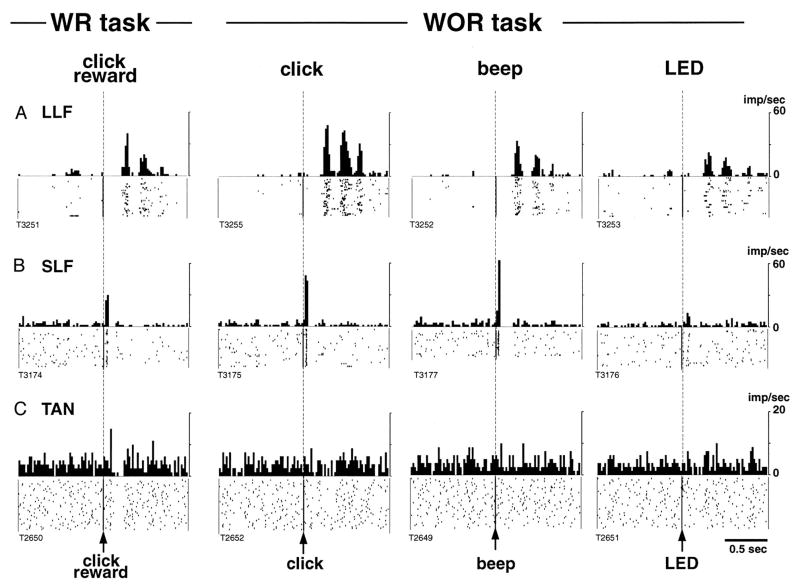
CM/PF neurons which, at rest, have low firing rates and burst-like discharge pattern, increase their activity in response to a wide variety of sensory stimuli (visual, auditory or tactile), and habituate rapidly after repeated presentation of the stimulus [103]. On the basis of the response latency to sensory stimuli, Kimura and colleagues have classified CM/PF neurons in short-latency or long latency facilitation (SLF or LLF respectively, [103–105]). SLF neurons are found more frequently in PF, whereas LLF cells lay preferentially in CM. Responses of both types of neurons are independent of the rewarding attributes of the stimuli (Fig. 3A, B).
Fig. 3.
Sensory responses of two types of CM/PF neurons, and a striatal TAN recorded during the presentation of a stimulus with reward (WR) and stimulus without reward (WOR). Spike raster and histograms aligned to the time of presentation of the stimulus. A: representative activity of a CM neuron with long-latency facilitation following stimulus presentation (LLF). B: activity of a PF neuron showing short-latency facilitation after stimulus (SLF). C: activity of a TAN. Note that thalamic responses occur in both WR and WOR tasks, whereas TAN responses occur only in the WR task (from Matsumoto et al, 2001)
CM/PF inactivation impairs performance of monkeys trained in attention-related tasks [104], and most CM neurons are activated when task conditions demand a change in response type after unpredicted events. In rats, performance in a reversal learning task, which requires shifting choice patterns and behavioral flexibility, is impaired after pharmacological inactivation of PF [106]. Based on these evidences, Kimura and colleagues have proposed that the CM/PF complex is particularly relevant for re-directing attention or behavior from biased actions [105, 107]. Thus, CM/PF neurons play an important role in attention re-directing and shifting behavioral choices under unexpected conditions.
2.2. Control of striatal activity by CM/PF thalamic inputs
Although CM/PF projections to the striatum are massive, their impact on the activity of striatal neurons remains poorly understood. However, significant effort using in vitro and in vivo preparations in rats and primates has been devoted to characterize the physiological effects of thalamic inputs upon striatal neurons activity. Early studies in anesthetized cats and rats, using in vivo intracellular recording methods, described short latency (likely monosynaptic) excitatory postsynaptic potentials in striatal neurons following electrical stimulation of the CM/PF complex [108–110]. Wilson and colleagues further characterized these effects, and demonstrated that both striatal projection neurons and tonically active neurons (TANs; putative cholinergic interneurons) often display early excitatory responses to electrical stimulation of intralaminar nuclei. However, they also described prolonged inhibitory and long latency excitatory responses following these stimulations [111, 112], suggesting polysynaptic responses to thalamic stimulation that might induce complex patterns of striatal activity in response to intralaminar nuclei activation.
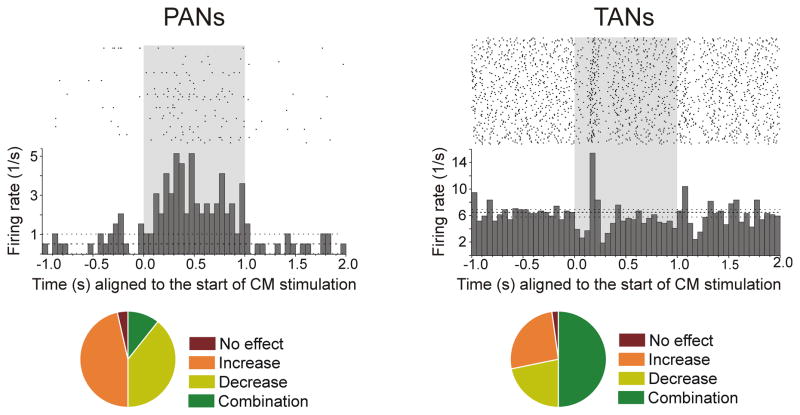
Recent data from rhesus monkeys, using in vivo extracellular single unit recording methods, further demonstrated the intricate nature of the physiological responses CM/PF stimulation elicits in striatal neurons [113]. Following CM stimulation, a large proportion of phasically active neurons (PANs, putative MSNs) increase their firing rate, while TANs (putative cholinergic interneurons) display complex responses that include short- and long-latency increases and decreases in activity (Fig. 4) [113]. These complex events are correlated with evidence that CM or PF stimulation results either in a glutamate-mediated increase in striatal acetylcholine (ACh) [114], or a reduction in ACh levels that is abolished by intrastriatal pharmacological blockade of GABA-A receptors [113, 115]. Therefore, activation of CM/PF connections to the striatum can induce either increase or decrease in ACh release; the former being most likely mediated by direct, monosynaptic, glutamatergic afferents from the CM/PF onto cholinergic interneurons (see section 1.3.), while the latter probably results from CM/PF-induced activation of MSNs or GABAergic interneurons that, in turn, inhibit cholinergic interneurons. Based on these findings, we propose that the CM exerts strong modulation of both striatal projection neurons and interneurons, mediated by a complex interplay between direct monosynaptic CM-striatal glutamatergic inputs and multisynaptic influences through CM-mediated activation of GABAergic MSNs and interneurons which, via intrinsic microcircuits, could indirectly reduce striatal activity.
Fig. 4.
Responses of PANs (putatively MSNs) and TANs (cholinergic interneurons) to electrical stimulation of CM, in rhesus monkeys. The stimulation (100 Hz, 100 pulses) is indicated by the shaded area. Right: Example of a PAN responding with increased firing to CM stimulation. Left: Example of a TAN responding with a brief decrease followed by an increase in firing. The histograms and rasters are aligned to the start of stimulation trains. Bottom: Summary of responses. While the majority of PANs presented increases in firing rate, most TANs presented combinatory (increases and decreases) responses (from Nanda et al, 2009).
2.3. CM/PF-striatal effects upon TANs
As described in section 1.3, CM/PF neurons provide strong synaptic inputs to striatal cholinergic interneurons [42] considered to be the tonically active neurons (TANs) in functional studies. In the presence of reward, or reward-related sensory stimuli, TANs display a stereotypic response consisting of a short burst followed by a clear pause and a later excitation (Fig. 3C) [103, 116–120]. However, the responses of TANs are diminished when the reward is delivered in a consistent predictable manner [121], while they are enhanced when the timing of rewards is not predictable [122]. Apicella has proposed that the sensitivity of TANs to changes in the sequence of stimuli indicates these cells might be involved in processing temporal sequence, and participate in the formation of automatic actions [123].
Chemical inactivation of CM/PF abolishes the characteristic reward-related responses of TANs, showing the importance of CM-striatal connections in mediating TANs activity changes in response to reward-related stimuli [103]. In rats, reversal learning is associated with an increase in striatal ACh, which is blocked by PF inactivation [106]. In addition, dopaminergic innervation to the striatum is also an essential regulator of TANs responses to reward-predicting stimuli [124, 125]. This has led to the suggestion that CM/PF provide TANs with information regarding salient events to activate conditional responses, and this information is integrated by TANs with dopaminergic signals from the SNc [103].
Based on findings gathered from a recent in vitro study, Surmeier and colleagues proposed a cellular mechanism by which thalamic regulation of cholinergic interneurons could influence corticostriatal signaling to mediate attentional shifts in response to salient environmental stimuli [67]. Although partly speculative, the authors proposed the interesting working hypothesis that, in response to the presentation of a salient stimulus, the thalamostriatal system can activate cholinergic interneurons which, in turn, regulate corticostriatal signaling and MSNs activity to elicit attention-related behavioral responses [67]. Therefore, because of its critical role in the regulation of striatal microcircuitry in response to attention-related sensory events, inactivation of the CM/PF complex results in contralateral sensory neglect and learning deficits in sensory-related attentional tasks in nonhuman primates [103, 104].
In spite of their significant interest, the main limitation of the in vitro slice experiments described above, and other recent studies [62, 63, 67, 126], is their reliance on non-specific electrical stimulation of the thalamus that most likely involves both the PF-striatal and the non PF-striatal systems (see section 1.2.). Future studies using more specific thalamic stimulation methods that could allow selective activation of the CM/PF-striatal projection are warranted to further address these issues.
In summary, CM/PF projections to the striatum are located to subserve an important control over putative cholinergic interneurons, through which they can regulate their role in attentional direction, behavioral flexibility and formation of automatic actions.
3. How does the CM/PF-striatal system degeneration contribute to set shifts and behavioral switching problems in Parkinson’s Disease?
Parkinson’s Disease (PD) is clinically identified by the motor signs of akinesia, rigidity and tremor at rest. In addition to these characteristic motor signs, most PD patients suffer cognitive deficits, such as impairment in attention tasks, working memory, set shifting and cognitive flexibility related to difficulty in planning, organizing and regulating goal-directed behavior [127, 128]. At the same time, PD patients have a decreased capacity to engage in normal automatic (habitual) control of actions, and become increasingly dependent on a goal-directed mode of action, which impede their normal daily activities [129].
Although degeneration of the nigrostriatal dopaminergic system remains the key pathological feature of PD, it is clear that many other neural systems are also affected [130, 131], including the CM/PF-striatal projection. Evidence from postmortem human brain studies demonstrates that the CM/PF complex presents a 30 to 40% cell loss in PD [132–134]. This thalamic degeneration appears to be specific to CM/PF because neighboring thalamic nuclei remain intact [133]. A similar pattern of degeneration was recently found in the CM/PF complex of parkinsonian monkeys chronically treated for many months with low doses of the toxin MPTP [135]. Furthermore, studies of the synaptic organization of vGlut2-positive (thalamostriatal) terminals in the putamen of MPTP-treated monkeys showed a decrease in the relative prevalence of vGlut2-positive axo-dendritic synapses which, for the most part, originate in CM (see section 1.3.) [59]. This observation is consistent with the possibility that CM inputs to the sensorimotor striatum are partly lost in parkinsonism. Loss of PF neurons has also been reported in some rodent models of parkinsonism ([136–138] but see [139]).
Presumably, the degeneration of the CM/PF-striatal system significantly contributes to both motor and non-motor deficits in PD. Based on the findings about the anatomy and functions of the CM/PF-striatal system discussed above, we propose that CM/PF degeneration could underlie set shifting deficits and inability to restore habitual behaviors in PD patients.
3.1. Set shift impairments
PD patients have deficits in set shifting, that is they have problems to alter ongoing behavior in response to sudden changes in their environment, an impairment that can be mediated by loss of dopamine in the associative striatum and/or prefrontal cortex [127] [140]. In light of the recent functional data related to the responses of CM/PF neurons to salient sensory stimuli, we suggest that degeneration of the CM/PF-striatal connection may also contribute to the set shifting problem described in PD. As we have discussed (section 2), CM/PF plays, indeed, a particular important role in redirecting attention to salient stimuli, behavioral flexibility and changing behavior in responses to unpredicted stimuli [107] [106]. Degeneration of caudal intralaminar nuclei, with corresponding loss of modulation over the activity of TANs and instrastriatal circuitry, could result in deficiencies to switch attention and reselect a proper action under changing circumstances. In this manner, the loss of CM/PF neurons could be one of the contributing factors to the set shifting inability in PD.
3.2. Habit vs Goal directed behavior
In a recent review, Redgrave et al (2010) have proposed that the basal ganglia have a prominent role in selecting between goal-directed (voluntary) and a habitual (automatic) control of behavior [129]. A large body of evidence obtained from human, rodent and primate studies indicates that the ventromedial (associative) striatum regulates goal-directed behavior, while the dorsolateral (sensorimotor) striatum is in charge of habitual control [reviewed in 129]. It is well recognized that PD patients show impairments in tasks that are normally controlled automatically, and have difficulties when learning new habits or performing automatic components of movement sequences [141–147]. Due to the reduced capacity of selecting habitual actions, PD patients have to rely on the more time consuming goal-directed action control system. The diminished capacity of basal ganglia to select habitual control system over goal-directed behaviors in PD patients, may be related to the heterogeneous loss of striatal dopamine, which is more severe in the sensorimotor striatum, recognized as the striatal control site for habitual behaviors [129].
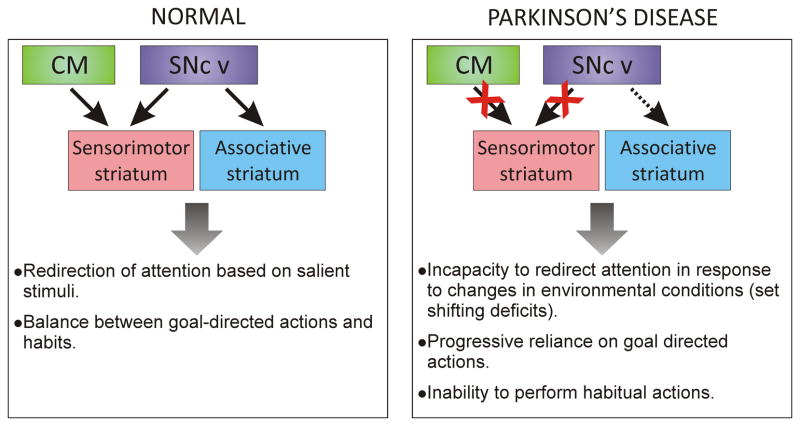
We propose that, in addition to the prominent loss of dopamine in the sensorimotor striatal sector, the degeneration of CM neurons that project mostly to the sensorimotor region of the striatum could also play an important role in the reduced capacity to use habitual actions in PD. In section 2.3., we have discussed how animal studies suggest an involvement of TANs in habit formation. The available evidence indicates a strong regulation of TANs by CM/PF projections to the striatum. Although the exact mechanisms remain unknown, the CM/PF degeneration in PD could result in altered control of TANs, and therefore of striatal microcircuitry, with a concomitant deficit of habit formations or expression. Figure 5 presents a summary of the ideas proposed in this section of the manuscript. In PD, the sensorimotor striatum would be particularly affected by the combination of a severe loss of SNc dopamine inputs and a significant degeneration of glutamatergic inputs from the CM [133] [135]. In contrast, the associative striatum would retain a relatively higher level of dopaminergic innervation. In the sensorimotor striatum, the reduced dopamine and glutamatergic CM inputs would result in complex changes in the intrinsic GABAergic and cholinergic striatal microcircuitry, which would underlie the development of attention and set-shift deficits along with an inadequate balance of habitual versus goal-directed behaviors.
Fig. 5.
Schematic illustrating the possible roles of CM and SNc degeneration towards the development of deficits in habitual actions in PD. The massive dopaminergic denervation from the ventral tier of the SNc (SNc v) to the sensorimotor striatum combined with extensive CM cell loss, over the less affected dopaminergic innervation of associative striatal regions, may be the source of attention-related deficits PD patients display in performing habit behaviors (see text for details).
4. Open questions and future studies
Although recent years have witnessed important advances in our understanding of the functional anatomy of the thalamostriatal systems, many unresolved issues remain, that will necessitate careful scrutiny in order to dissect out the significance of these systems in mediating normal basal ganglia function, and their relevance towards the development of various behavioral deficits in PD and other basal ganglia circuitry disorders.
While in vitro studies have provided important insights into the possible cellular mechanisms by which thalamic afferents might regulate corticostriatal signaling to control MSNs and TANs activity [62, 67], these studies must be expanded, using specific stimulation of the caudal intralaminar thalamic complex, perhaps with the aid of optogenetic techniques [148, 149], to clearly assess the role of the CM/PF stimulation upon striatal activity. Similarly, the substrate underlying the complex pattern of responses recorded from TANs and MSNs in vivo following CM stimulation [113] must be elucidated. A careful analysis of the possible engagement of intrastriatal GABAergic microcircuits in mediating these effects is warranted [150, 151]. The loss of neurons in the CM/PF complex in PD (and other degenerative diseases, such as progressive supranuclear palsy, Huntington’s disease and Lewy body disease [132, 152, 153]) must be considered in our interpretation of the basal ganglia pathophysiology and learning dysfunctions that characterize these disorders. To do so, the line of research led by Kimura and his colleagues aimed at characterizing the physiological responses of CM/PF neurons to attention-related stimuli must be pursued and expanded to nonhuman primate models of PD. Animal studies have, indeed, provided evidence that CM neurons display abnormal physiological activity in parkinsonism [81, 136, 154–156], but the exact nature of these alterations, and their importance in CM/PF-mediated attention task regulation requires further consideration. The suggestion that CM neuronal loss is a compensatory response to the parkinsonian insult is of interest [157], but awaits further evidence that such a process takes place in the complex scheme of PD pathophysiology.
Our suggestion that the CM/PF neuronal loss may be an important contributor to the deficits in behavioral switching and habit behaviors in PD patients, must be further assessed through careful electrophysiological and behavioral studies in the MPTP-treated nonhuman primate model of PD. We have recently demonstrated that rhesus monkeys chronically treated over a period of 20–26 weeks with low doses of MPTP display brain pathological features that extend beyond the nigrostriatal dopaminergic system to involve other monoaminergic systems, and the CM/PF [158–160]. We believe that the use of this model represents a highly valuable asset to determine the potential role of the CM/PF-striatal system in motor, cognitive and limbic dysfunctions associated with PD.
Finally, it is important to recognize that the functional significance of the non-CM/PF thalamostriatal system is another obscure piece of the puzzle that remains to be clarified, if one hopes to fully understand the possible roles of the dual thalamostriatal systems in the functional circuitry of the basal ganglia. The integration of these connections within functional basal ganglia-thalamostriatal loops reminiscent of those proposed in this review for the various components of the CM/PF-striatal system (see Fig. 1) should be considered (see also [129, 161]). The anatomical and functional relationships between the non CM/PF-striatal connections and related corticostriatal afferents must be examined in great detail to elucidate the mechanisms by which these two neural systems may interact to regulate striatal activity and resulting basal ganglia function. The neurochemical, pharmacological and plastic properties of axo-spinous thalamostriatal versus corticostriatal excitatory synapses must be carefully assessed to determine the substrate through which these systems mediate their effects. Modern transgenic approaches combined with optogenetic stimulation methods could in principle be used to activate or silence specific subpopulations of non-CM/PF thalamostriatal neurons and assess their effects upon striatal activity. Such methods are being successfully used to study other brain systems in rodents (i.e. [162, 163]).
In conclusion, despite the long and unfruitful attempts at characterizing the role of the thalamostriatal systems in the functional organization of the basal ganglia, evidence discussed in this review highlights an interesting path that could shed light into this enigma. The development of proper animal models combined with their use in attention-related tasks that rely on the integrity of subcortical basal ganglia-thalamostriatal loops through the CM/PF complex may guide us towards a deeper understanding of the functional importance of the CM/PF-striatal system in altering the functional balance between the selection of habit and goal-directed behaviors in PD.
Acknowledgments
Supported by NIH grants R01NS062876, P50NS071669 and NIH/NCRR RR-00165 (Yerkes National Primate Research Center)
Abbreviations
- ACh
Acetylcholine
- CM
Centromedian nucleus of the thalamus
- CM/PF
Centromedian/Parafascicular complex of the thalamus
- CMl
Lateral seciton of the CM
- GPi
Globus Pallidus, internal segment
- LLF
Long latency facilitation neurons of the CM/PF
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MSN
Medium spiny neuron of the striatum
- PD
Parkinson’s Disease
- PF
Parafascicular nucleus of the thalamus
- Pfdl
Dorsolateral section of the parafascicular nucleus
- SLF
Short latency facilitation neurons of the CM/PF
- SNc
Substantia Nigra paras compacta
- SNr
Substantia Nigra paras reticulata
- TAN
Tonically active neuron of the striatum
- VA
Ventral anterior nucleus of the thalamus
- vGluT1
Vesicular Glutamate Transporter type 1
- vGluT2
Vesicular Glutamate Transporter type 2
- VL
Ventral lateral nucleus of the thalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 3.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 4.Vogt C, Vogt O. Thalamusstudien I-III. I. Zur Einfurung, II. Homogenitat und Grenzgestaldung der Grisea des Thalamus, III. Griseum centrale (centrum medianum Luys) J Physiol Neurol. 1941;50:31–154. [Google Scholar]
- 5.Powell TPS, Cowan WM. A study of thalamo-striate relations in the monkey. Brain. 1956;79:364–390. doi: 10.1093/brain/79.2.364. [DOI] [PubMed] [Google Scholar]
- 6.Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus) Neuroscience. 1986;18:347–371. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- 7.Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- 8.Fenelon G, Francois C, Percheron G, Yelnik J. Topographic distribution of the neurons of the central complex (centre median-parafascicular complex) and of other thalamic neurons projecting to the striatum in macaques. Neuroscience. 1991;45:495–510. doi: 10.1016/0306-4522(91)90244-i. [DOI] [PubMed] [Google Scholar]
- 9.Francois C, Percheron G, Parent A, Sadikot AF, Fenelon G, Yelnik J. Topography of the projection from the central complex of the thalamus to the sensorimotor striatal territory in monkeys. J Comp Neurol. 1991;305:17–34. doi: 10.1002/cne.903050104. [DOI] [PubMed] [Google Scholar]
- 10.Deschenes M, Bourassa J, Parent A. Two different types of thalamic fibers innervate the rat striatum. Brain Res. 1995;701:288–292. doi: 10.1016/0006-8993(95)01124-3. [DOI] [PubMed] [Google Scholar]
- 11.Deschenes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur J Neurosci. 1996;8:329–343. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 12.Deschenes M, Bourassa J, Parent A. Striatal and cortical projections of single neurons from the central lateral thalamic nucleus in the rat. Neuroscience. 1996;72:679–687. doi: 10.1016/0306-4522(96)00001-2. [DOI] [PubMed] [Google Scholar]
- 13.Mengual E, de las Heras S, Erro E, Lanciego JL, Gimenez-Amaya JM. Thalamic interaction between the input and the output systems of the basal ganglia. J Chem Neuroanat. 1999;16:187–200. doi: 10.1016/s0891-0618(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 14.McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarland NR, Haber SN. Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. J Comp Neurol. 2001;429:321–336. doi: 10.1002/1096-9861(20000108)429:2<321::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erro EM, Lanciego JL, Gimenez-Amaya JM. Re-examination of the thalamostriatal projections in the rat with retrograde tracers. Neurosci Res. 2002;42:45–55. doi: 10.1016/s0168-0102(01)00302-9. [DOI] [PubMed] [Google Scholar]
- 18.Smith Y, Galvan A, Raju D, Wichmann T. Anatomical and Functional Organization of the Thalamostriatal Systems. In: Steiner H, Tseng K, editors. Handbook of Basal Ganglia Structure and Function. Academic Press; 2010. [Google Scholar]
- 19.Castle M, Aymerich MS, Sanchez-Escobar C, Gonzalo N, Obeso JA, Lanciego JL. Thalamic innervation of the direct and indirect basal ganglia pathways in the rat: Ipsi- and contralateral projections. J Comp Neurol. 2005;483:143–153. doi: 10.1002/cne.20421. [DOI] [PubMed] [Google Scholar]
- 20.Parent M, Parent A. Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. J Comp Neurol. 2005;481:127–144. doi: 10.1002/cne.20348. [DOI] [PubMed] [Google Scholar]
- 21.Lacey CJ, Bolam JP, Magill PJ. Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. J Neurosci. 2007;27:4374–4384. doi: 10.1523/JNEUROSCI.5519-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 23.Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Sidibe M, Pare JF, Smith Y. Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey. J Comp Neurol. 2002;447:286–299. doi: 10.1002/cne.10247. [DOI] [PubMed] [Google Scholar]
- 26.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 27.Sidibe M, Bevan MD, Bolam JP, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: I. Topography and synaptic organization of the pallidothalamic projection. J Comp Neurol. 1997;382:323–347. [PubMed] [Google Scholar]
- 28.Groenewegen HJ, Berendse HW. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci. 1994;17:52–57. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 29.Cowan WM, Powell TP. The projection of the midline and intralaminar nuclei of the thalamus of the rabbit. J Neurol Neurosurg Psychiatry. 1955;18:266–279. doi: 10.1136/jnnp.18.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichinohe N, Iwatsuki H, Shoumura K. Intrastriatal targets of projection fibers from the central lateral nucleus of the rat thalamus. Neurosci Lett. 2001;302:105–108. doi: 10.1016/s0304-3940(01)01666-4. [DOI] [PubMed] [Google Scholar]
- 31.Gimenez-Amaya JM, McFarland NR, de las Heras S, Haber SN. Organization of thalamic projections to the ventral striatum in the primate. J Comp Neurol. 1995;354:127–149. doi: 10.1002/cne.903540109. [DOI] [PubMed] [Google Scholar]
- 32.Beckstead RM. The thalamostriatal projection in the cat. J Comp Neurol. 1984;223:313–346. doi: 10.1002/cne.902230302. [DOI] [PubMed] [Google Scholar]
- 33.Parent A, Mackey A, De Bellefeuille L. The subcortical afferents to caudate nucleus and putamen in primate: a fluorescence retrograde double labeling study. Neuroscience. 1983;10:1137–1150. doi: 10.1016/0306-4522(83)90104-5. [DOI] [PubMed] [Google Scholar]
- 34.Nakano K, Hasegawa Y, Tokushige A, Nakagawa S, Kayahara T, Mizuno N. Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in the Japanese monkey, Macaca fuscata. Brain Res. 1990;537:54–68. doi: 10.1016/0006-8993(90)90339-d. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka D, Jr, Isaacson LG, Trosko BK. Thalamostriatal projections from the ventral anterior nucleus in the dog. J Comp Neurol. 1986;247:56–68. doi: 10.1002/cne.902470104. [DOI] [PubMed] [Google Scholar]
- 36.Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7:315–324. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- 37.de las Heras S, Mengual E, Velayos JL, Gimenez-Amaya JM. Re-examination of topographic distribution of thalamic neurons projecting to the caudate nucleus. A retrograde labeling study in the cat. Neurosci Res. 1998;31:283–293. doi: 10.1016/s0168-0102(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 38.de las Heras S, Mengual E, Gimenez-Amaya JM. Double retrograde tracer study of the thalamostriatal projections to the cat caudate nucleus. Synapse. 1999;32:80–92. doi: 10.1002/(SICI)1098-2396(199905)32:2<80::AID-SYN2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 39.Ragsdale CW, Jr, Graybiel AM. Compartmental organization of the thalamostriatal connection in the cat. J Comp Neurol. 1991;311:134–167. doi: 10.1002/cne.903110110. [DOI] [PubMed] [Google Scholar]
- 40.Sadikot AF, Parent A, Francois C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992;315:137–159. doi: 10.1002/cne.903150203. [DOI] [PubMed] [Google Scholar]
- 41.Sidibe M, Smith Y. Differential synaptic innervation of striatofugal neurones projecting to the internal or external segments of the globus pallidus by thalamic afferents in the squirrel monkey. J Comp Neurol. 1996;365:445–465. doi: 10.1002/(SICI)1096-9861(19960212)365:3<445::AID-CNE8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Sidibe M, Smith Y. Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience. 1999;89:1189–1208. doi: 10.1016/s0306-4522(98)00367-4. [DOI] [PubMed] [Google Scholar]
- 43.Rudkin TM, Sadikot AF. Thalamic input to parvalbumin-immunoreactive GABAergic interneurons: organization in normal striatum and effect of neonatal decortication. Neuroscience. 1999;88:1165–1175. doi: 10.1016/s0306-4522(98)00265-6. [DOI] [PubMed] [Google Scholar]
- 44.Meredith GE, Wouterlood FG. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol. 1990;296:204–221. doi: 10.1002/cne.902960203. [DOI] [PubMed] [Google Scholar]
- 45.Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- 46.Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol. 2006;499:231–243. doi: 10.1002/cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doig NM, Moss J, Bolam JP. Cortical and Thalamic Innervation of Direct and Indirect Pathway Medium-Sized Spiny Neurons in Mouse Striatum. J Neurosci. 2010;30:14610–14618. doi: 10.1523/JNEUROSCI.1623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dube L, Smith AD, Bolam JP. Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J Comp Neurol. 1988;267:455–471. doi: 10.1002/cne.902670402. [DOI] [PubMed] [Google Scholar]
- 49.Sadikot AF, Parent A, Smith Y, Bolam JP. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J Comp Neurol. 1992;320:228–242. doi: 10.1002/cne.903200207. [DOI] [PubMed] [Google Scholar]
- 50.Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344:1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- 51.Moss J, Bolam JP. A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J Neurosci. 2008;28:11221–11230. doi: 10.1523/JNEUROSCI.2780-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice ME. Distinct regional differences in dopamine-mediated volume transmission. Prog Brain Res. 2000;125:277–290. doi: 10.1016/S0079-6123(00)25017-6. [DOI] [PubMed] [Google Scholar]
- 53.Graybiel AM, Ragsdale CW, Jr, Yoneoka ES, Elde RP. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience. 1981;6:377–397. doi: 10.1016/0306-4522(81)90131-7. [DOI] [PubMed] [Google Scholar]
- 54.Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- 55.Herkenham M, Pert CB. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature. 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- 56.Fujiyama F, Unzai T, Nakamura K, Nomura S, Kaneko T. Difference in organization of corticostriatal and thalamostriatal synapses between patch and matrix compartments of rat neostriatum. Eur J Neurosci. 2006;24:2813–2824. doi: 10.1111/j.1460-9568.2006.05177.x. [DOI] [PubMed] [Google Scholar]
- 57.El Mestikawy S, Wallén-Mackenzie Å, Fortin GM, Descarries L, Trudeau L-E. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- 58.Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y. Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur J Neurosci. 2008;27:1647–1658. doi: 10.1111/j.1460-9568.2008.06136.x. [DOI] [PubMed] [Google Scholar]
- 60.Lacey CJ, Boyes J, Gerlach O, Chen L, Magill PJ, Bolam JP. GABA(B) receptors at glutamatergic synapses in the rat striatum. Neuroscience. 2005;136:1083–1095. doi: 10.1016/j.neuroscience.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- 62.Ding J, Peterson JD, Surmeier DJ. Corticostriatal and Thalamostriatal Synapses Have Distinctive Properties. J Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smeal RM, Keefe KA, Wilcox KS. Differences in excitatory transmission between thalamic and cortical afferents to single spiny efferent neurons of rat dorsal striatum. Eur J Neurosci. 2008;28:2041–2052. doi: 10.1111/j.1460-9568.2008.06505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung JW, Hassler R, Wagner A. Degeneration of two of nine types of synapses in the putamen after center median coagulation in the cat. Exp Brain Res. 1977;28:345–361. doi: 10.1007/BF00235716. [DOI] [PubMed] [Google Scholar]
- 65.Kemp JM, Powell TP. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond B Biol Sci. 1971;262:429–439. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- 66.Thomas TM, Smith Y, Levey AI, Hersch SM. Cortical inputs to m2-immunoreactive striatal interneurons in rat and monkey. Synapse. 2000;37:252–261. doi: 10.1002/1098-2396(20000915)37:4<252::AID-SYN2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 67.Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Kusnoor SV, Parris J, Muly EC, Morgan JI, Deutch AY. Extracerebellar role for Cerebellin1: modulation of dendritic spine density and synapses in striatal medium spiny neurons. J Comp Neurol. 2010;518:2525–2537. doi: 10.1002/cne.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Catsman-Berrevoets CE, Kuypers HG. Differential laminar distribution of corticothalamic neurons projecting to the VL and the center median. An HRP study in the cynomolgus monkey. Brain Res. 1978;154:359–365. doi: 10.1016/0006-8993(78)90706-0. [DOI] [PubMed] [Google Scholar]
- 71.Devito JL, Smith OA. Afferent projections to the hypothalamic area controlling emotional responses (HACER) Brain Res. 1982;252:213–226. doi: 10.1016/0006-8993(82)90389-4. [DOI] [PubMed] [Google Scholar]
- 72.Jones EG, Wise SP, Coulter JD. Differential thalamic relationships of sensory-motor and parietal cortical fields in monkeys. J Comp Neurol. 1979;183:833–881. doi: 10.1002/cne.901830410. [DOI] [PubMed] [Google Scholar]
- 73.Kunzle H. Thalamic projections from the precentral motor cortex in Macaca fascicularis. Brain Res. 1976;105:253–267. doi: 10.1016/0006-8993(76)90424-8. [DOI] [PubMed] [Google Scholar]
- 74.Kunzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in macaca fascicularis. Brain Behav Evol. 1978;15:185–234. doi: 10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- 75.Kuypers HG, Lawrence DG. Cortical projections to the red nucleus and the brain stem in the Rhesus monkey. Brain Res. 1967;4:151–188. doi: 10.1016/0006-8993(67)90004-2. [DOI] [PubMed] [Google Scholar]
- 76.Mehler WR. The posterior thalamic region in man. Confin Neurol. 1966;27:18–29. doi: 10.1159/000103928. [DOI] [PubMed] [Google Scholar]
- 77.Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys: I. Subcortical connections. J Comp Neurol. 1986;253:415–439. doi: 10.1002/cne.902530402. [DOI] [PubMed] [Google Scholar]
- 78.Leichnetz GR, Goldberg ME. Higher centers concerned with eye movement and visual attention: cerebral cortex and thalamus. Rev Oculomot Res. 1988;2:365–429. [PubMed] [Google Scholar]
- 79.Ipekchyan NM. Comparative analysis of the quantitative characteristics of the corticothalamic projections of parietal cortex fields 5 and 7. Neurosci Behav Physiol. 2011;41:10–12. [Google Scholar]
- 80.Steriade M, Parent A, Hada J. Thalamic projections of nucleus reticularis thalami of cat: a study using retrograde transport of horseradish peroxidase and fluorescent tracers. J Comp Neurol. 1984;229:531–547. doi: 10.1002/cne.902290407. [DOI] [PubMed] [Google Scholar]
- 81.Barroso-Chinea P, Rico AJ, Conte-Perales L, Gomez-Bautista V, Luquin N, Sierra S, Roda E, Lanciego JL. Glutamatergic and cholinergic pedunculopontine neurons innervate the thalamic parafascicular nucleus in rats: changes following experimental parkinsonism. Brain Struct Funct. 2011 doi: 10.1007/s00429-011-0317-x. [DOI] [PubMed] [Google Scholar]
- 82.Pare D, Smith Y, Parent A, Steriade M. Projections of brainstem core cholinergic and non-cholinergic neurons of cat to intralaminar and reticular thalamic nuclei. Neuroscience. 1988;25:69–86. doi: 10.1016/0306-4522(88)90007-3. [DOI] [PubMed] [Google Scholar]
- 83.Parent A, Pare D, Smith Y, Steriade M. Basal forebrain cholinergic and noncholinergic projections to the thalamus and brainstem in cats and monkeys. J Comp Neurol. 1988;277:281–301. doi: 10.1002/cne.902770209. [DOI] [PubMed] [Google Scholar]
- 84.Grunwerg BS, Krauthamer GM. Sensory responses of intralaminar thalamic neurons activated by the superior colliculus. Exp Brain Res. 1992;88:541–550. doi: 10.1007/BF00228183. [DOI] [PubMed] [Google Scholar]
- 85.Krauthamer GM, Krol JG, Grunwerg BS. Effect of superior colliculus lesions on sensory unit responses in the intralaminar thalamus of the rat. Brain Res. 1992;576:277–286. doi: 10.1016/0006-8993(92)90691-2. [DOI] [PubMed] [Google Scholar]
- 86.Ichinohe N, Shoumura K. A di-synaptic projection from the superior colliculus to the head of the caudate nucleus via the centromedian-parafascicular complex in the cat: an anterograde and retrograde labeling study. Neurosci Res. 1998;32:295–303. doi: 10.1016/s0168-0102(98)00095-9. [DOI] [PubMed] [Google Scholar]
- 87.Krout KE, Loewy AD, Westby GW, Redgrave P. Superior colliculus projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2001;431:198–216. doi: 10.1002/1096-9861(20010305)431:2<198::aid-cne1065>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 88.Chevalier G, Deniau JM. Spatio-temporal organization of a branched tecto-spinal/tecto-diencephalic neuronal system. Neuroscience. 1984;12:427–439. doi: 10.1016/0306-4522(84)90063-0. [DOI] [PubMed] [Google Scholar]
- 89.Royce GJ, Bromley S, Gracco C. Subcortical projections to the centromedian and parafascicular thalamic nuclei in the cat. J Comp Neurol. 1991;306:129–155. doi: 10.1002/cne.903060110. [DOI] [PubMed] [Google Scholar]
- 90.Ichinohe N, Mori F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 2000;880:191–197. doi: 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- 91.Lavoie B, Parent A. Serotoninergic innervation of the thalamus in the primate: an immunohistochemical study. J Comp Neurol. 1991;312:1–18. doi: 10.1002/cne.903120102. [DOI] [PubMed] [Google Scholar]
- 92.Vertes RP, Linley SB, Hoover WB. Pattern of distribution of serotonergic fibers to the thalamus of the rat. Brain Struct Funct. 2010;215:1–28. doi: 10.1007/s00429-010-0249-x. [DOI] [PubMed] [Google Scholar]
- 93.Vertes RP, Martin GF. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol. 1988;275:511–541. doi: 10.1002/cne.902750404. [DOI] [PubMed] [Google Scholar]
- 94.Edwards SB, de Olmos JS. Autoradiographic studies of the projections of the midbrain reticular formation: ascending projections of nucleus cuneiformis. J Comp Neurol. 1976;165:417–431. doi: 10.1002/cne.901650403. [DOI] [PubMed] [Google Scholar]
- 95.Comans PE, Snow PJ. Ascending projections to nucleus parafascicularis of the cat. Brain Res. 1981;230:337–341. doi: 10.1016/0006-8993(81)90411-x. [DOI] [PubMed] [Google Scholar]
- 96.Cornwall J, Phillipson OT. Afferent projections to the parafascicular thalamic nucleus of the rat, as shown by the retrograde transport of wheat germ agglutinin. Brain Res Bull. 1988;20:139–150. doi: 10.1016/0361-9230(88)90171-2. [DOI] [PubMed] [Google Scholar]
- 97.Steriade M, Glenn LL. Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. J Neurophysiol. 1982;48:352–371. doi: 10.1152/jn.1982.48.2.352. [DOI] [PubMed] [Google Scholar]
- 98.Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- 99.Newman DB, Ginsberg CY. Brainstem reticular nuclei that project to the thalamus in rats: a retrograde tracer study. Brain Behav Evol. 1994;44:1–39. doi: 10.1159/000113566. [DOI] [PubMed] [Google Scholar]
- 100.Hulme OJ, Whiteley L, Shipp S. Spatially distributed encoding of covert attentional shifts in human thalamus. J Neurophysiol. 2010;104:3644–3656. doi: 10.1152/jn.00303.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Metzger CD, Eckert U, Steiner J, Sartorius A, Buchmann JE, Stadler J, Tempelmann C, Speck O, Bogerts B, Abler B, Walter M. High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Front Neuroanat. 2010;4:138. doi: 10.3389/fnana.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 103.Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophys. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- 104.Minamimoto T, Kimura M. Participation of the thalamic CM-Pf complex in attentional orienting. J Neurophys. 2002;87:3090–3101. doi: 10.1152/jn.2002.87.6.3090. [DOI] [PubMed] [Google Scholar]
- 105.Minamimoto T, Hori Y, Kimura M. Complementary process to response bias in the centromedian nucleus of the thalamus. Science. 2005;308:1798–1801. doi: 10.1126/science.1109154. [DOI] [PubMed] [Google Scholar]
- 106.Brown HD, Baker PM, Ragozzino ME. The Parafascicular Thalamic Nucleus Concomitantly Influences Behavioral Flexibility and Dorsomedial Striatal Acetylcholine Output in Rats. J Neurosci. 2010;30:14390–14398. doi: 10.1523/JNEUROSCI.2167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Minamimoto T, Hori Y, Kimura M. Roles of the thalamic CM-PF complex-Basal ganglia circuit in externally driven rebias of action. Brain Res Bull. 2009;78:75–79. doi: 10.1016/j.brainresbull.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 108.Kitai ST, Kocsis JD, Preston RJ, Sugimori M. Monosynaptic inputs to caudate neurons identified by intracellular injection of horseradish peroxidase. Brain Res. 1976;109:601–606. doi: 10.1016/0006-8993(76)90039-1. [DOI] [PubMed] [Google Scholar]
- 109.Vandermaelen CP, Kitai ST. Intracellular analysis of synaptic potentials in rat neostriatum following stimulation of the cerebral cortex, thalamus, and substantia nigra. Brain Res Bull. 1980;5:725–733. doi: 10.1016/0361-9230(80)90212-9. [DOI] [PubMed] [Google Scholar]
- 110.Kocsis JD, Sugimori M, Kitai ST. Convergence of excitatory synaptic inputs to caudate spiny neurons. Brain Res. 1977;124:403–413. doi: 10.1016/0006-8993(77)90942-8. [DOI] [PubMed] [Google Scholar]
- 111.Wilson CJ, Chang HT, Kitai ST. Origins of post synaptic potentials evoked in spiny neostriatal projection neurons by thalamic stimulation in the rat. Exp Brain Res. 1983;51:217–226. doi: 10.1007/BF00237197. [DOI] [PubMed] [Google Scholar]
- 112.Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nanda B, Galvan A, Smith Y, Wichmann T. Effects of stimulation of the centromedian nucleus of the thalamus on the activity of striatal cells in awake rhesus monkeys. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2008.06598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Consolo S, Baldi G, Giorgi S, Nannini L. The cerebral cortex and parafascicular thalamic nucleus facilitate in vivo acetylcholine release in the rat striatum through distinct glutamate receptor subtypes. Eur J Neurosci. 1996;8:2702–2710. doi: 10.1111/j.1460-9568.1996.tb01565.x. [DOI] [PubMed] [Google Scholar]
- 115.Zackheim J, Abercrombie ED. Thalamic regulation of striatal acetylcholine efflux is both direct and indirect and qualitatively altered in the dopamine-depleted striatum. Neuroscience. 2005;131:423–436. doi: 10.1016/j.neuroscience.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 116.Kimura M. Behavioral modulation of sensory responses of primate putamen neurons. Brain Res. 1992;578:204–214. doi: 10.1016/0006-8993(92)90249-9. [DOI] [PubMed] [Google Scholar]
- 117.Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Apicella P, Scarnati E, Schultz W. Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp Brain Res. 1991;84:672–675. doi: 10.1007/BF00230981. [DOI] [PubMed] [Google Scholar]
- 119.Kimura M, Rajkowski J, Evarts E. Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci U S A. 1984;81:4998–5001. doi: 10.1073/pnas.81.15.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 121.Apicella P, Legallet E, Trouche E. Responses of tonically discharging neurons in the monkey striatum to primary rewards delivered during different behavioral states. Exp Brain Res. 1997;116:456–466. doi: 10.1007/pl00005773. [DOI] [PubMed] [Google Scholar]
- 122.Ravel S, Sardo P, Legallet E, Apicella P. Reward unpredictability inside and outside of a task context as a determinant of the responses of tonically active neurons in the monkey striatum. J Neurosci. 2001;21:5730–5739. doi: 10.1523/JNEUROSCI.21-15-05730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends Neurosci. 2007;30:299–306. doi: 10.1016/j.tins.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 124.Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–415. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- 125.Watanabe K, Kimura M. Dopamine receptor-mediated mechanisms involved in the expression of learned activity of primate striatal neurons. J Neurophysiol. 1998;79:2568–2580. doi: 10.1152/jn.1998.79.5.2568. [DOI] [PubMed] [Google Scholar]
- 126.Smeal RM, Gaspar RC, Keefe KA, Wilcox KS. A rat brain slice preparation for characterizing both thalamostriatal and corticostriatal afferents. J Neurosci Methods. 2007;159:224–235. doi: 10.1016/j.jneumeth.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 127.Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain. 2001;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- 128.Williams-Gray CH, Foltynie T, Lewis SJ, Barker RA. Cognitive deficits and psychosis in Parkinson’s disease: a review of pathophysiology and therapeutic options. CNS Drugs. 2006;20:477–505. doi: 10.2165/00023210-200620060-00004. [DOI] [PubMed] [Google Scholar]
- 129.Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 131.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 132.Henderson JM, Carpenter K, Cartwright H, Halliday GM. Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson’s disease: clinical and therapeutic implications. Brain. 2000;123 (Pt 7):1410–1421. doi: 10.1093/brain/123.7.1410. [DOI] [PubMed] [Google Scholar]
- 133.Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre median-parafascicular complex in Parkinson’s disease. Ann Neurol. 2000;47:345–352. [PubMed] [Google Scholar]
- 134.Truong L, Brooks D, Amaral F, Henderson JM, Halliday GM. Relative preservation of thalamic centromedian nucleus in parkinsonian patients with dystonia. Mov Disord. 2009;24:2128–2135. doi: 10.1002/mds.22747. [DOI] [PubMed] [Google Scholar]
- 135.Villalba R, Wichmann T, Smith Y. Neuronal loss in the caudal intralaminar nuclear group, CM/Pf, in MPTP-treated parkinsonian monkeys. Society for Neuroscience Abstracts. 2011 [Google Scholar]
- 136.Aymerich MS, Barroso-Chinea P, Perez-Manso M, Munoz-Patino AM, Moreno-Igoa M, Gonzalez-Hernandez T, Lanciego JL. Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats. Eur J Neurosci. 2006;23:2099–2108. doi: 10.1111/j.1460-9568.2006.04741.x. [DOI] [PubMed] [Google Scholar]
- 137.Freyaldenhoven TE, Ali SF, Schmued LC. Systemic administration of MPTP induces thalamic neuronal degeneration in mice. Brain Res. 1997;759:9–17. doi: 10.1016/s0006-8993(97)00045-0. [DOI] [PubMed] [Google Scholar]
- 138.Ghorayeb I, Fernagut PO, Hervier L, Labattu B, Bioulac B, Tison F. A ‘single toxin-double lesion’ rat model of striatonigral degeneration by intrastriatal 1-methyl-4-phenylpyridinium ion injection: a motor behavioural analysis. Neuroscience. 2002;115:533–546. doi: 10.1016/s0306-4522(02)00401-3. [DOI] [PubMed] [Google Scholar]
- 139.Henderson JM, Schleimer SB, Allbutt H, Dabholkar V, Abela D, Jovic J, Quinlivan M. Behavioural effects of parafascicular thalamic lesions in an animal model of parkinsonism. Behav Brain Res. 2005;162:222–232. doi: 10.1016/j.bbr.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 140.Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koerts J, Leenders KL, Brouwer WH. Cognitive dysfunction in non-demented Parkinson’s disease patients: Controlled and automatic behavior. Cortex. 2009;45:922–929. doi: 10.1016/j.cortex.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 142.Hoshiyama M, Kaneoke Y, Koike Y, Takahashi A, Watanabe S. Hypokinesia of associated movement in Parkinson’s disease: a symptom in early stages of the disease. J Neurol. 1994;241:517–521. doi: 10.1007/BF00873512. [DOI] [PubMed] [Google Scholar]
- 143.Schwab RS, Chafetz ME, Walker S. Control of two simultaneous voluntary motor acts in normals and in parkinsonism. AMA Arch Neurol Psychiatry. 1954;72:591–598. doi: 10.1001/archneurpsyc.1954.02330050061010. [DOI] [PubMed] [Google Scholar]
- 144.Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 145.Stelmach GE, Worringham CJ, Strand EA. Movement preparation in Parkinson’s disease. The use of advance information. Brain. 1986;109 (Pt 6):1179–1194. doi: 10.1093/brain/109.6.1179. [DOI] [PubMed] [Google Scholar]
- 146.Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110 (Pt 2):361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- 147.Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008;21:478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- 148.Boyden ES. A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 Biology reports. 2011:3. doi: 10.3410/B3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gonzales KK, Pare JF, Jenkins S, Wichmann T, Smith Y. International Basal Ganglia Society. X. Long Branch, NJ: 2010. Striatal cholinergic interneurons receive intrinsic GABAergic inputs from axon collaterals of direct and indirect striatofugal neurons in the primate putamen; p. P-61. [Google Scholar]
- 151.Gonzales KK, Pare JF, Wichmann T, Smith Y. Society for Neuroscience Abstracts. Vol. 37. Washington, DC: 2011. GABAergic microcircuitry of cholinergic interneurons in the monkey putamen. [Google Scholar]
- 152.Heinsen H, Rub U, Gangnus D, Jungkunz G, Bauer M, Ulmar G, Bethke B, Schuler M, Bocker F, Eisenmenger W, Gotz M, Strik M. Nerve cell loss in the thalamic centromedian-parafascicular complex in patients with Huntington’s disease. Acta Neuropathol. 1996;91:161–168. doi: 10.1007/s004010050408. [DOI] [PubMed] [Google Scholar]
- 153.Brooks D, Halliday GM. Intralaminar nuclei of the thalamus in Lewy body diseases. Brain Res Bull. 2009;78:97–104. doi: 10.1016/j.brainresbull.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 154.Ni ZG, Gao DM, Benabid AL, Benazzouz A. Unilateral lesion of the nigrostriatal pathway induces a transient decrease of firing rate with no change in the firing pattern of neurons of the parafascicular nucleus in the rat. Neuroscience. 2000;101:993–999. doi: 10.1016/s0306-4522(00)00337-7. [DOI] [PubMed] [Google Scholar]
- 155.Hirsch EC, Perier C, Orieux G, Francois C, Feger J, Yelnik J, Vila M, Levy R, Tolosa ES, Marin C, Trinidad Herrero M, Obeso JA, Agid Y. Metabolic effects of nigrostriatal denervation in basal ganglia. Trends Neurosci. 2000;23:S78–85. doi: 10.1016/s1471-1931(00)00021-5. [DOI] [PubMed] [Google Scholar]
- 156.Orieux G, Francois C, Feger J, Yelnik J, Vila M, Ruberg M, Agid Y, Hirsch EC. Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neuroscience. 2000;97:79–88. doi: 10.1016/s0306-4522(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 157.Kusnoor SV, Muly EC, Morgan JI, Deutch AY. Is the loss of thalamostriatal neurons protective in parkinsonism? Parkinsonism Relat Disord. 2009;15 (Suppl 3):S162–166. doi: 10.1016/S1353-8020(09)70806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Masilamoni GJ, Bogenpohl JW, Alagille D, Delevich K, Tamagnan G, Votaw JR, Wichmann T, Smith Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain. 2011;134:2057–2073. doi: 10.1093/brain/awr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Masilamoni GJ, Weinkle AP, Bogenpohl J, Groover OA, Wichmann T, Smith Y. A nonhuman primate model of Parkinson’s disease associated with cortical and subcortical dopaminergic, noradrenergic and serotonergic neuronal degeneration. Mov Disord. 2011:26. [Google Scholar]
- 160.Villalba RM, Smith Y. Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated parkinsonian monkeys. J Comp Neurol. 2011;519:989–1005. doi: 10.1002/cne.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 162.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]