Abstract
Antiphospholipid syndrome is an autoimmune disease characterized by the presence of circulating antiphospholipid antibodies (aPL) that promote thrombosis, pregnancy complications and cardiovascular diseases. Alterations in the function of vascular cells induced by aPL underlie these outcomes. This review will discuss recent findings that indicate a novel mechanism by which aPL antagonize endothelial cell production of nitric oxide and thereby promote thrombosis.
Introduction
The antiphospholipid syndrome (APS) is an autoimmune disease characterized by the production of antiphospholipid antibodies (aPL) that promote vascular thrombosis and pregnancy loss1, 2. In addition to its occurrence in individuals without an underlying disorder, APS afflicts a significant number of patients with systemic lupus erythematosus (SLE), with as many as 34% of lupus patients having circulating aPL1. Along with arterial and venous thrombosis and pregnancy complications, patients with APS have an increased risk of coronary artery disease, myocardial infarction, and stroke3. A link between APS and premature atherosclerosis has also been reported4, 5.
Human studies as well as work in cell culture and in animal models indicate that actions of aPL on endothelial cells likely play a major role in the vascular disease phenotypes in APS6, 7. There is evidence of endothelial cell activation in APS patients, with plasma levels of soluble adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and von Villebrand factor (vWF) increased in APS patients compared to healthy controls8–10. In addition, elevations in circulating endothelial microparticles and in circulating mature endothelial cells, which are markers of endothelial activation and damage, have been observed in APS patients8, 11. Several studies also indicate that flow-mediated vasodilation is blunted in APS patients compared to healthy subjects8, 10, 12. Consistent with these findings in humans, the exposure of cultured endothelial cells to aPL isolated from APS patients causes VCAM-1, ICAM-1 and E-selectin upregulation, and it also increases the expression of tissue factor (TF). Similarly, in mouse models the administration of aPL causes increased expression of adhesion molecules and it enhances endothelial cell-leukocyte interaction13, 14. Furthermore, mirroring the human condition, the injection of aPL in rodent models leads to enhanced thrombus formation15, 16
Over the past two decades our knowledge of the pathogenetic mechanisms underlying APS have been expanded through studies of the molecular pathways by which aPL alter the function of endothelium and platelets7, 17. This review will focus on recent findings that indicate a novel mechanism by which aPL antagonize endothelial cell production of nitric oxide (NO) and thereby promote thrombosis.
Endothelial NO Synthase Antagonism by aPL
One of the key signaling molecules that has a beneficial impact on vascular health by preventing thrombosis and endothelial cell-leukocyte interaction is NO18, 19. The primary source of NO in the vascular wall under normal conditions is the endothelial isoform of NO synthase (eNOS). The NO generated by eNOS downregulates adhesion molecule expression, and it also inhibits platelet aggregation by increasing cGMP production in platelets18, 20. Thus, the activation of eNOS and subsequent production of NO modulate a number of the vascular processes that are known to be adversely affected by aPL. Studies in both mouse models and humans have suggested that there is a potential link between aPL and changes in bioavailable NO. In mice the administration of aPL reduces plasma concentrations of NO metabolites and it also reduces acetylcholine (Ach)-induced relaxation in isolated aortic rings, which is an endothelium-dependent, NO-dependent process21, 22. In humans, plasma aPL levels are inversely correlated with urinary NO metabolite excretion, and APS patients have lower levels of plasma nitrites compared to control subjects23, 24. Thus, there are data both in mouse models and in humans that support a potential role for impaired NO production in the pathogenesis of APS.
To directly test this possibility and determine the underlying processes, Ramesh et al. recently determined if aPL alter eNOS activation in cultured endothelial cells25. Human or bovine aortic endothelial cells were pretreated with polyclonal aPL or normal human IgG (NHIgG) isolated from APS patients or healthy individuals, respectively, and eNOS activation by vascular endothelial growth factor (VEGF) was evaluated. In the presence of NHIgG, VEGF treatment led to a predictable increase in eNOS activity. In contrast, aPL caused complete attenuation of eNOS activation by VEGF. The activation of the enzyme by other agonists was also blunted by aPL. To test whether these mechanisms are operative in vivo, the investigators evaluated carotid vascular conductance responses to Ach in mice, which indicate increases in blood flow invoked by endothelium-derived NO26. Ach-mediated increases in carotid artery vascular conductance were tested at baseline and following the IV administration of either NHIgG or aPL. Whereas NHIgG did not alter the response to Ach, the administration of aPL caused marked attenuation of the vasodilatory response. These findings indicate that aPL have a dramatic inhibitory effect on eNOS both in cell culture and in vivo in mice.
Since NO plays an important role in preventing leukocyte adhesion to endothelium and it is antithrombotic20, Ramesh et al. further determined the implications of eNOS antagonism of aPL in studies of leukocyte-endothelial cell adhesion and thrombus formation using intravital microscopy of the mesenteric microcirculation. Wild-type (eNOS+/+) or eNOS deficient (eNOS−/−) mice were treated with NHIgG or aPL, and 24 hours later endothelial cell-leukocyte adhesion and thrombosis were studied. In eNOS+/+ mice aPL caused a marked increase in leukocyte adhesion and thrombus formation. In contrast, in eNOS−/− mice aPL did not affect either leukocyte adhesion or thrombosis. These combined results indicate that eNOS antagonism underlies the increase in both leukocyte-endothelial cell adhesion and thrombus formation caused by aPL.
Ramesh et al. further determined how aPL blunt the stimulation of eNOS, which is caused by most agonists via the activating phosphorylation of Ser1177 of the enzyme (Ser1177 in human eNOS, Ser1179 in bovine eNOS)27, 28. Whereas in the presence of control NHIgG VEGF caused increased phosphorylation of eNOS at Ser1177, aPL completely suppressed VEGF-induced Ser1177 phosphorylation. It was additionally determined that this process does not involve alterations in VEGF-stimulated Akt kinase activation, which is responsible for Ser1177 phosphorylation. Alternatively, the impaired phosphorylation of eNOS caused by aPL was mediated by the protein phosphatase 2A (PP2A), which is the principal phosphatase that dephosphorylates eNOS at Ser117729, 30 This distal event in the actions of aPL on eNOS is depicted in Figure 1(c).
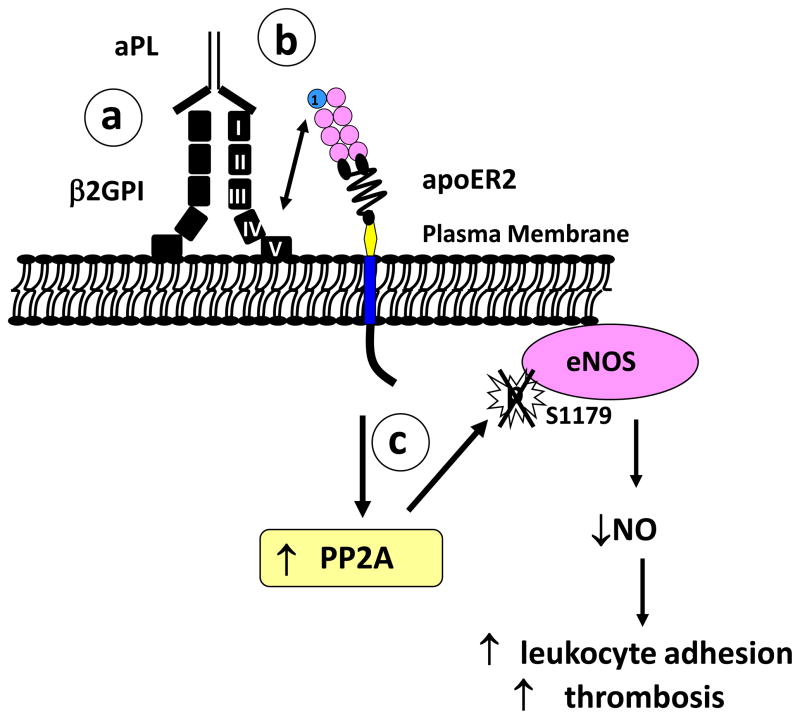
Figure 1. aPL promote leukocyte adhesion and thrombosis by antagonizing eNOS viaβ2GPI, apoER2 and the phosphatase PP2A.
(a) aPL binding to domain I of β2GPI induces β2GPI dimerization and (b) interaction between domain V of β2GPI and the first LDL binding domain of apoER2. Through yet-to-be-determined mechanism(s), the interaction of β2GPI with apoER2 causes (c) increased activation of PP2A. This promotes the dephosphorylation of Ser1177 of eNOS yielding decreased enzyme activity and a decline in bioavailable NO, which results in increased leukocyte adhesion and increased thrombosis. Reprinted with permission 25.
Role of β2-Glycoprotein I
Although initially thought to directly recognize anionic phospholipids on the surface of target cells, it has become apparent that pathologically relevant aPL are directed against phospholipid-binding proteins, particularly β2-glycoprotein I (β2GPI) 31, 32. β2GPI is a plasma protein composed of five distinctive domains (domains I-V), and it has been shown that it binds to phospholipids on the surface of platelets through domain V32. In vitro studies have shown that antibody recognition of phospholipid-bound β2GPI causes its dimerization, which further increases its affinity for negatively-charged phospholipids on the cell surface33. Anti-β2GPI-specific monoclonal antibodies increase adhesion molecule expression and the synthesis of cytokines, endothelin-1, and TF in cultured endothelial cells, and in isolated platelets they enhance the production of thromboxane B2, adhesion to collagen and aggregation 14, 34. In APS patients there is a strong relationship between the levels of circulating anti-β2GPI antibodies specific for domain I and the incidence of thrombosis35, 36.
Ramesh and colleagues evaluated the involvement of β2GPI in aPL antagonism of eNOS in cultured endothelial cells25. When cells were deprived of serum, which is the source of β2GPI in culture, exposure to aPL did not cause eNOS inhibition, indicating that β2GPI is required for aPL action. The role of β2GPI was further confirmed by testing the effect of anti-β2GPI monoclonal antibodies. Monoclonal anti-β2GPI antibodies that recognize domain I inhibited VEGF-induced eNOS activation, mimicking the effect of the polyclonal aPL that is isolated from APS patients; in contrast, a monoclonal antibody that recognizes domain II of β2GPI did not. These findings indicate that β2GPI is required for aPL actions on eNOS and that aPL recognition of domain I of β2GPI mediates the process. Further experiments revealed that it is not simply the recognition of domain I of β2GPI by aPL that initiates the process; the resulting dimerization of plasma membrane-associated β2GPI is also required (Figure 1(a)).
Role of apolipoprotein E receptor 2
Previous in vitro studies found that dimerized β2GPI binds to multiple members of the LDL receptor family in purified form34, 37. One such receptor is apolipoprotein E receptor 2 (apoER2, also known as LRP8), which plays a critical role in brain development as a signal tranducer for the glycoprotein Reelin38. Although apoER2 is predominantly expressed in brain, a splice variant of apoER2 designated apoER2' has been identified in platelets and megakaryocytic cell lines34. Recently it has been demonstrated that aPL induce β2GPI binding to apoER2' in isolated platelets, and that the binding causes platelet activation ex vivo34.
Recognizing that apoER2 is an abundant plasma membrane receptor in endothelial cells and that it interacts with dimerized β2GPI, Ramesh and coworkers tested the requirement for apoER2 in aPL-induced eNOS antagonism25. In cultured endothelial cells, the knockdown of apoER2 expression by small interference RNA (RNAi) had no effect on the promotion of monocyte-endothelial cell adhesion prompted by lipopolysaccharide (LPS) treatment of the endothelium. However, it fully prevented the enhancement of adhesion caused by anti-β2GPI antibody. eNOS antagonism by aPL was also fully prevented by apoER2 knockdown. Studies with a soluble peptide based on the sequence of the first LDL-binding domain of apoER2’ (BD1) that binds to β2GPI39 further demonstrated that the eNOS antagonism caused by antibody recognition of β2GPI requires interaction between β2GPI and BD1 of the receptor. As such, apoER2 is the linchpin that couples antibody recognition of β2GPI and its dimerization on the endothelial cell surface to the antagonism of intracellular eNOS and the changes in endothelial cell behavior that ensue (Figure 1(b)).
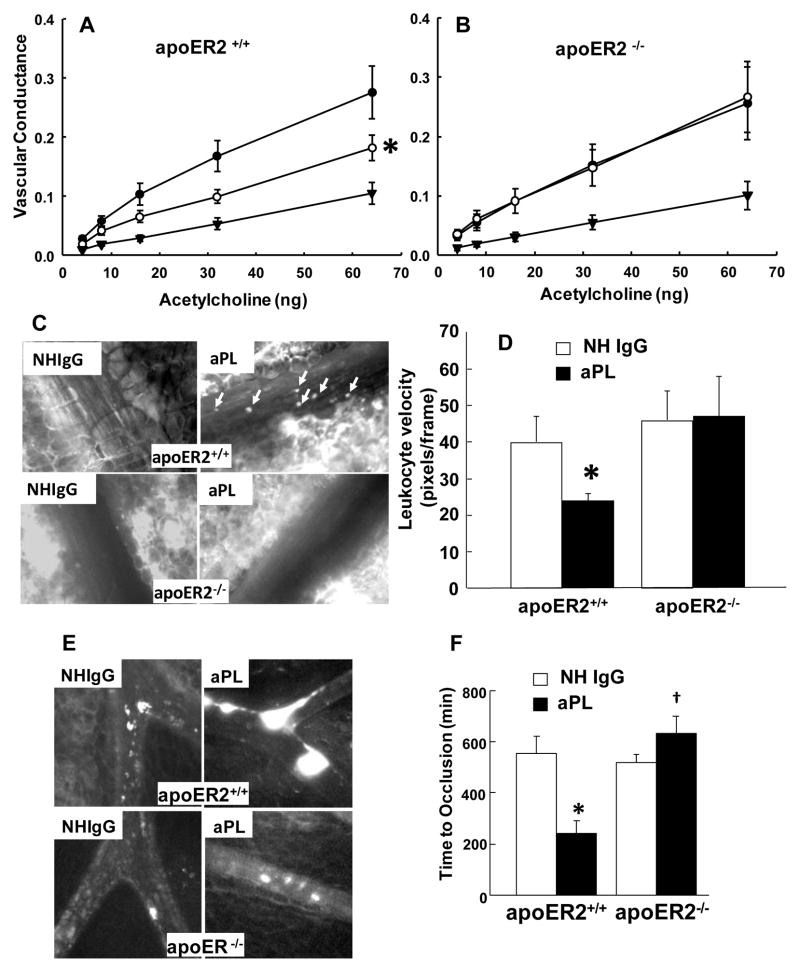
Based on these observations in cell culture, Ramesh et al. evaluated the role of apoER2 in aPL actions in vivo in a series of experiments in apoER2+/+ and apoER2−/− mice (Figure 2). The requirement for the receptor in aPL-mediated antagonism of eNOS in vivo was tested in studies of carotid vascular conductance (Figure 2A, B). aPL caused marked attenuation of the vasodilatory response to Ach in apoER2+/+ mice, shifting the dose-response curve downward and to the right. In contrast, Ach-induced vasodilation was identical before and after aPL treatment in apoER2−/− mice. The involvement of apoER2 in aPL-induced increases in leukocyte adhesion to endothelium and in thrombus formation was also evaluated (Figure 2C-F; representative still images in 2C and 2E, quantitative data in 2D and 2F). ApoER2+/+ or apoER2−/− mice were treated with NHIgG or aPL, and 24 hours later endothelial cell-leukocyte adhesion or thrombus formation was evaluated using intravital microscopy. In apoER2+/+ mice, aPL increased leukocyte adhesion to endothelium resulting in a decrease in leukocyte velocity (Figure 2C, D). In contrast, aPL had no effect on leukocyte-endothelial cell adhesion in apoER2−/− mice. Similarly, in apoER2+/+ mice, compared to NHIgG, aPL enhanced thrombus formation and thereby decreased the time to total vessel occlusion (Figure 2E, F). In contrast, aPL did not alter thrombus formation in apoER2−/− mice. Romay-Panabad et al. recently confirmed the role of apoER2 in aPL-induced thrombosis40. They found that human polyclonal aPL or murine anti-β2GPI monoclonal antibody enhances TF production in the carotid artery and increases thrombus formation in wild-type mice, and that these pathologic effects of antibody are reduced in apoER2−/− mice. Furthermore, they showed that the blocking peptide that interferes with β2GPI-apoER2 interaction attenuates these pathologic events invoked by aPL or anti-β2GPI antibody in wild-type mice. These cumulative observations indicate that aPL recognition of β2GPI causes eNOS antagonism, that this process is mediated by apoER2, and that it underlies the vascular disease phenotypes of APS.
Figure 2. aPL-induced eNOS antagonism, endothelial cell-leukocyte adhesion and thrombosis in mice are mediated by apoER2.
(A, B) ApoER2+/+ and apoER2−/− mice were instrumented and changes in carotid vascular conductance in response to acetylcholine were compared before and after treatment with aPL. Dose-responses to acetylcholine were performed sequentially at baseline (●), 60 min after aPL treatment (○), and 10 min after L-NAME administration (▼). n=6/group. *p<0.05 vs. baseline dose-response. (C-F) ApoER2+/+ and apoER2−/− mice were injected IP with NHIgG or aPL, and 24 hours later prepared for intravital microscopy. Leukocytes or platelets were fluorescence-labeled by injecting Rhodamine 6G or anti-mouse GPIbβ antibody, respectively. The mesentery was exposed for the observation and recording of images of leukocyte rolling or thrombus formation. Representative still images are shown (C, E). (In C, leukocytes are indicated by arrows). Quantitative data are shown as velocity of leukocyte rolling (D) and time required for complete vessel occlusion (F) in apoER2+/+ and apoER2−/− mice. In D and F, values are mean±SEM, n=5–7/group, *p<0.05 vs. NHIgG, †p<0.05 vs. apoER2+/+. Reprinted with permission25 .
Conclusions
Patients with APS suffer from arterial and venous thrombosis, and they are at increased risk of myocardial infarction and stroke. Recent experiments in cell culture and in mice have provided new knowledge of the underlying events, revealing that aPL inhibit eNOS via recognition of the cell surface protein β2GPI and its interaction with the plasma membrane receptor apoER2. However, numerous aspects of the molecular mechanisms of aPL action remain unknown. How does apoER2 activate the phosphatase PP2A? Does a known adaptor molecule of apoER2, such as Disabled-1, play a role in the signal transduction38, 41? Do similar processes occur in platelets, which express the splice variant of apoER2, apoER2’, and eNOS, which attenuates platelet activation in an autocrine manner 42? Perhaps most importantly, how does aPL recognition of β2GPI and resulting signaling by apoER2 occur in a manner that would explain the episodic nature of the thrombotic diathesis that characterizes APS? Because APS patients most often do not display clinical symptoms even in the persistent presence of circulating aPL, it has been hypothesized that additional stimuli are required for the occurrence of thrombosis episodes (designated the “two-hit hypothesis”)43. If impaired NO production and subsequent endothelial dysfunction induced by aPL represent "the first hit", what is the “second hit” that triggers the thrombotic events in APS? The “second hit” may involve a proinflammatory stimulus or infection because rats administered aPL have spontaneous thromboses if they also receive LPS44. Furthermore, how are the “first hit” and the “second hit” mechanistically coupled? As the molecular mechanisms responsible for the “first hit” are further revealed, the nature of the other required processes that ultimately lead to the thrombotic events will become more approachable.
Currently patients with APS are treated chronically with anticoagulation medications such as heparin or warfarin. Despite long-term use of the anticoagulants, recurrent thrombosis can occur in APS patients. Furthermore, use of anticoagulants is fraught with complications including bleeding episodes and osteopenia45–47. With the recent new knowledge that has been gained regarding the processes that underlie aPL actions on endothelium, we anticipate that novel interventions can be developed that directly target the pathogenetic mechanisms, thereby affording greater efficacy and fewer complications in the management of this potentially life-threatening disorder.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002 Mar 7;346(10):752–63. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, de Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006 Feb;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 3.Soltesz P, Szekanecz Z, Kiss E, Shoenfeld Y. Cardiac manifestations in antiphospholipid syndrome. Autoimmun Rev. 2007 Jun;6(6):379–86. doi: 10.1016/j.autrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Ames PR, Margarita A, Alves JD. Antiphospholipid antibodies and atherosclerosis: insights from systemic lupus erythematosus and primary antiphospholipid syndrome. Clin Rev Allergy Immunol. 2009 Aug;37(1):29–35. doi: 10.1007/s12016-008-8099-5. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura E, Lopez LR. Autoimmune-mediated atherothrombosis. Lupus. 2008 Oct;17(10):878–87. doi: 10.1177/0961203308093553. [DOI] [PubMed] [Google Scholar]
- 6.Giannakopoulos B, Passam F, Rahgozar S, Krilis SA. Current concepts on the pathogenesis of the antiphospholipid syndrome. Blood. 2007 Jan 15;109(2):422–30. doi: 10.1182/blood-2006-04-001206. [DOI] [PubMed] [Google Scholar]
- 7.Pierangeli SS, Chen PP, Raschi E, Scurati S, Grossi C, Borghi MO, Palomo I, Harris EN, Meroni PL. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost. 2008 Apr;34(3):236–50. doi: 10.1055/s-0028-1082267. [DOI] [PubMed] [Google Scholar]
- 8.Cugno M, Borghi MO, Lonati LM, Ghiadoni L, Gerosa M, Grossi C, De AV, Magnaghi G, Tincani A, Mari D, Riboldi P, Meroni PL. Patients with antiphospholipid syndrome display endothelial perturbation. J Autoimmun. 2010 Mar;34(2):105–10. doi: 10.1016/j.jaut.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Kaplanski G, Cacoub P, Farnarier C, Marin V, Gregoire R, Gatel A, Durand JM, Harle JR, Bongrand P, Piette JC. Increased soluble vascular cell adhesion molecule 1 concentrations in patients with primary or systemic lupus erythematosus-related antiphospholipid syndrome: correlations with the severity of thrombosis. Arthritis Rheum. 2000 Jan;43(1):55–64. doi: 10.1002/1529-0131(200001)43:1<55::AID-ANR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 10.Stalc M, Poredos P, Peternel P, Tomsic M, Sebestjen M, Kveder T. Endothelial function is impaired in patients with primary antiphospholipid syndrome. Thromb Res. 2005 Nov 4; doi: 10.1016/j.thromres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Pericleous C, Giles I, Rahman A. Are endothelial microparticles potential markers of vascular dysfunction in the antiphospholipid syndrome? Lupus. 2009 Jul;18(8):671–5. doi: 10.1177/0961203309103062. [DOI] [PubMed] [Google Scholar]
- 12.Bilora F, Sartori MT, Zanon E, Campagnolo E, Arzenton M, Rossato A. Flow-mediated arterial dilation in primary antiphospholipid syndrome. Angiology. 2009 Feb;60(1):104–7. doi: 10.1177/0003319708315304. [DOI] [PubMed] [Google Scholar]
- 13.Meroni PL, Raschi E, Testoni C. Endothelium as a target for anti-phospholipid antibodies and for therapeutical intervention. Autoimmun Rev. 2002 Feb;1(1–2):55–60. doi: 10.1016/s1568-9972(01)00014-3. [DOI] [PubMed] [Google Scholar]
- 14.Pierangeli SS, Vega-Ostertag M, Harris EN. Intracellular signaling triggered by antiphospholipid antibodies in platelets and endothelial cells: a pathway to targeted therapies. Thromb Res. 2004;114(5–6):467–76. doi: 10.1016/j.thromres.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Gharavi AE, Pierangeli SS, Colden-Stanfield M, Liu XW, Espinola RG, Harris EN. GDKV-induced antiphospholipid antibodies enhance thrombosis and activate endothelial cells in vivo and in vitro. J Immunol. 1999 Sep 1;163(5):2922–7. [PubMed] [Google Scholar]
- 16.Jankowski M, Vreys I, Wittevrongel C, Boon D, Vermylen J, Hoylaerts MF, Arnout J. Thrombogenicity of beta 2-glycoprotein I-dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood. 2003 Jan 1;101(1):157–62. doi: 10.1182/blood-2002-05-1310. [DOI] [PubMed] [Google Scholar]
- 17.Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol. 2011 Jun;7(6):330–9. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 18.Loscalzo J. Nitric oxide and vascular disease. N Engl J Med. 1995 Jul 27;333(4):251–3. doi: 10.1056/NEJM199507273330410. [DOI] [PubMed] [Google Scholar]
- 19.Voetsch B, Jin RC, Loscalzo J. Nitric oxide insufficiency and atherothrombosis. Histochem Cell Biol. 2004 Oct;122(4):353–67. doi: 10.1007/s00418-004-0675-z. [DOI] [PubMed] [Google Scholar]
- 20.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001 Apr 27;88(8):756–62. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 21.Belizna C, Lartigue A, Favre J, Gilbert D, Tron F, Levesque H, Thuillez C, Richard V. Antiphospholipid antibodies induce vascular functional changes in mice: a mechanism of vascular lesions in antiphospholipid syndrome? Lupus. 2008 Mar;17(3):185–94. doi: 10.1177/0961203307086931. [DOI] [PubMed] [Google Scholar]
- 22.Delgado AJ, Mason LJ, Ames PR, Chen PP, Rauch J, Levine JS, Subang R, Isenberg DA. Antiphospholipid antibodies are associated with enhanced oxidative stress, decreased plasma nitric oxide and paraoxonase activity in an experimental mouse model. Rheumatology (Oxford) 2005 Oct;44(10):1238–44. doi: 10.1093/rheumatology/keh722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ames PR, Tommasino C, Alves J, Morrow JD, Iannaccone L, Fossati G, Caruso S, Caccavo F, Brancaccio V. Antioxidant susceptibility of pathogenic pathways in subjects with antiphospholipid antibodies: a pilot study. Lupus. 2000;9(9):688–95. doi: 10.1191/096120300677692516. [DOI] [PubMed] [Google Scholar]
- 24.Ames PR, Batuca JR, Ciampa A, Iannaccone L, Delgado AJ. Clinical relevance of nitric oxide metabolites and nitrative stress in thrombotic primary antiphospholipid syndrome. J Rheumatol. 2010 Dec;37(12):2523–30. doi: 10.3899/jrheum.100494. [DOI] [PubMed] [Google Scholar]
- 25.Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, Herz J, Urbanus RT, de Groot PG, Thorpe PE, Salmon JE, Shaul PW, Mineo C. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J Clin Invest. 2010 Dec 1; doi: 10.1172/JCI39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mineo C, Gormley AK, Yuhanna IS, Osborne-Lawrence S, Gibson LL, Hahner L, Shohet RV, Black S, Salmon JE, Samols D, Karp DR, Thomas GD, Shaul PW. FcgammaRIIB mediates C-reactive protein inhibition of endothelial NO synthase. Circ Res. 2005 Nov 25;97(11):1124–31. doi: 10.1161/01.RES.0000194323.77203.fe. [DOI] [PubMed] [Google Scholar]
- 27.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther. 2001 Dec;299(3):818–24. [PubMed] [Google Scholar]
- 28.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 29.Greif DM, Kou R, Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry (Mosc) 2002 Dec 31;41(52):15845–53. doi: 10.1021/bi026732g. [DOI] [PubMed] [Google Scholar]
- 30.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001 May 25;276(21):17625–8. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 31.Galli M, Luciani D, Bertolini G, Barbui T. Anti-beta 2-glycoprotein I, antiprothrombin antibodies, and the risk of thrombosis in the antiphospholipid syndrome. Blood. 2003 Oct 15;102(8):2717–23. doi: 10.1182/blood-2002-11-3334. [DOI] [PubMed] [Google Scholar]
- 32.Miyakis S, Giannakopoulos B, Krilis SA. Beta 2 glycoprotein I--function in health and disease. Thromb Res. 2004;114(5–6):335–46. doi: 10.1016/j.thromres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 33.de Groot PG, van Lummel M, Pennings M, Urbanus R, Bas dL, Lenting PJ, Derksen RH. Beta2-glycoprotein I and LDL-receptor family members. Thromb Res. 2004;114(5–6):455–9. doi: 10.1016/j.thromres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Urbanus RT, Derksen RH, de Groot PG. Platelets and the antiphospholipid syndrome. Lupus. 2008 Oct;17(10):888–94. doi: 10.1177/0961203308096344. [DOI] [PubMed] [Google Scholar]
- 35.de Laat B, de Groot PG. Autoantibodies directed against domain I of beta2-glycoprotein I. Curr Rheumatol Rep. 2011 Feb;13(1):70–6. doi: 10.1007/s11926-010-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Laat B, Derksen RH, van LM, Pennings MT, de Groot PG. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood. 2006 Mar 1;107(5):1916–24. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 37.Pennings MT, van Lummel M, Derksen RH, Urbanus RT, Romijn RA, Lenting PJ, de Groot PG. Interaction of beta2-glycoprotein I with members of the low density lipoprotein receptor family. J Thromb Haemost. 2006 Aug;4(8):1680–90. doi: 10.1111/j.1538-7836.2006.02036.x. [DOI] [PubMed] [Google Scholar]
- 38.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006 Nov;7(11):850–9. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 39.Pennings MT, Derksen RH, van Lummel M, Adelmeijer J, VanHoorelbeke K, Urbanus RT, Lisman T, de Groot PG. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: glycoprotein Ibalpha and apolipoprotein E receptor 2'. J Thromb Haemost. 2007 Feb;5(2):369–77. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 40.Romay-Penabad Z, guilar-Valenzuela R, Urbanus RT, Derksen RH, Pennings MT, Papalardo E, Shilagard T, Vargas G, Hwang Y, de Groot PG, Pierangeli SS. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011 Jan 27;117(4):1408–14. doi: 10.1182/blood-2010-07-299099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urbanus RT, Pennings MT, Derksen RH, de Groot PG. Platelet activation by dimeric beta2-glycoprotein I requires signaling via both glycoprotein Ibalpha and apolipoprotein E receptor 2'. J Thromb Haemost. 2008 Aug;6(8):1405–12. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 42.Freedman JE, Loscalzo J, Barnard MR, Alpert C, Keaney JF, Michelson AD. Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin Invest. 1997 Jul 15;100(2):350–6. doi: 10.1172/JCI119540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meroni PL, Borghi MO, Raschi E, Ventura D, Sarzi Puttini PC, Atzeni F, Lonati L, Parati G, Tincani A, Mari D, Tedesco F. Inflammatory response and the endothelium. Thromb Res. 2004;114(5–6):329–34. doi: 10.1016/j.thromres.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 44.Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R, Bossi F, Ziller F, Sblattero D, Meroni P, Tedesco F. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005 Oct 1;106(7):2340–6. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 45.Deruelle P, Coulon C. The use of low-molecular-weight heparins in pregnancy--how safe are they? Curr Opin Obstet Gynecol. 2007 Dec;19(6):573–7. doi: 10.1097/GCO.0b013e3282f10e33. [DOI] [PubMed] [Google Scholar]
- 46.Giannakopoulos B, Krilis SA. How I treat the antiphospholipid syndrome. Blood. 2009 Sep 3;114(10):2020–30. doi: 10.1182/blood-2009-05-220756. [DOI] [PubMed] [Google Scholar]
- 47.Pierangeli SS, Erkan D. Antiphospholipid syndrome treatment beyond anticoagulation: are we there yet? Lupus. 2010 Apr;19(4):475–85. doi: 10.1177/0961203310361489. [DOI] [PubMed] [Google Scholar]