Abstract
Stress often has deleterious effects on cognition. We show that moderate stress enhanced late reversal learning in a mouse touchscreen-based choice task. Ventromedial prefrontal cortex (vmPFC) lesions mimicked the effects of stress, while orbitofrontal (OFC) and dorsolateral striatal (DLS) lesions impaired reversal. Stress-facilitation of reversal was prevented by BDNF infusion into the vmPFC. These findings suggest a mechanism in which stress-induced vmPFC dysfunction disinhibits learning by alternate (e.g., striatal) systems.
Although the negative effects of stress on cognitive function are often emphasized, a more nuanced view is emerging in which stress can either impair or enhance cognition depending upon the nature of the stressor, characteristics of the subject and the specific cognitive process being examined1–4. For example, chronic stress promotes habit-like behavior in concert with vmPFC dendritic retraction and DLS hypertrophy5. However, it remains unclear whether more limited stress exposure impacts corticostriatal mediation of cognitive functions such as reversal learning, and which mechanisms underlie such effects.
Here, we investigated stress effects in a reversal assay in which male C57BL/6J mice were trained to proficiently (≥85% accuracy) discriminate between two visual stimuli projected on a computer screen by touching one of the stimuli for food reward. Mice were then subjected to 10 -min swim stress once daily for 3 days6. Reversal testing, in which the stimulus-reward designation for each mouse was switched, commenced the following day and proceeded to (≥85% accuracy) criterion (Fig. 1a). Errors (touching non-rewarded stimulus) and correction errors (an error following another error) were measured (Supporting Materials for details). Based on previous analyses of reversal and prefrontal lesions in mice, rats and monkeys7–9, we segregated performance into an early stage, when accuracy was <50% and dominated by perseveration at the previously rewarded stimulus, and a late stage, when accuracy was ≥50% and primarily driven by learning the new association. Experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee.
Figure 1. Stress facilitates late reversal.
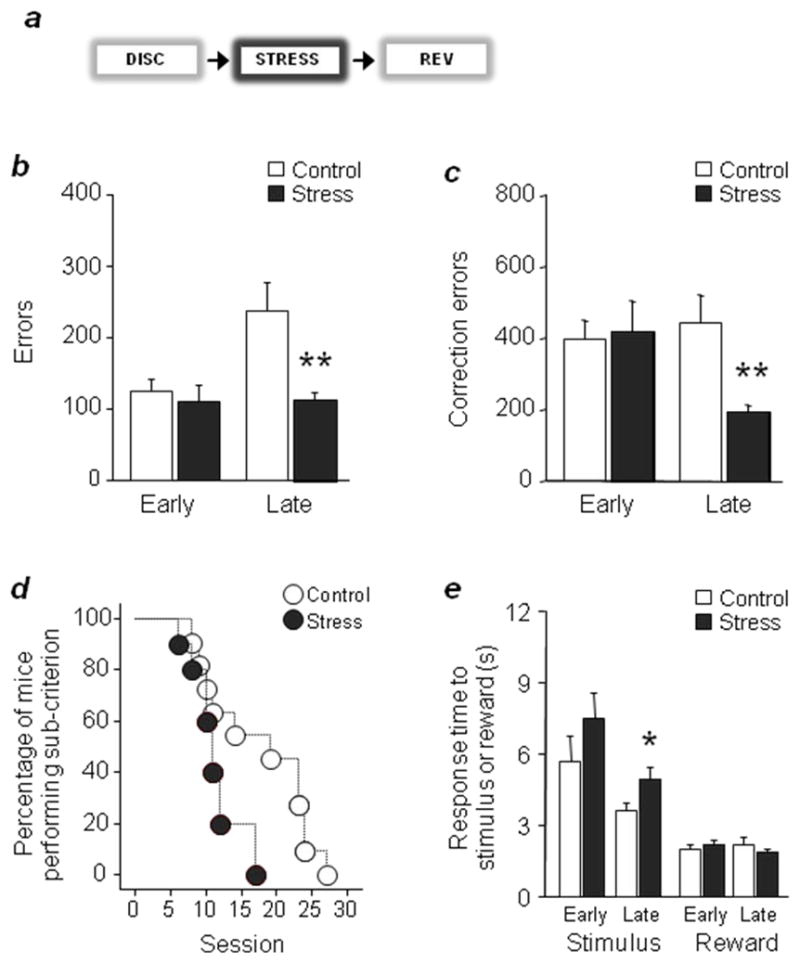
(a) Swim stress was applied once daily for 3 days prior to reversal. Stress decreased errors (b) and correction errors (c). (d) The percentage of mice performing below learning criterion decreased more rapidly across sessions after stress. (e) Stress increased stimulus but not reward response times during late reversal. n=10–11/stress treatment. Data are Means ±S EM. **P<.01, *P<.05 versus controls
Mice exposed to stress made significantly fewer errors (t(19)=2.50, P<.01) and correction errors (t(19)=2.92, P<.01) than non-stressed controls during the late, but not early, reversal stage (Fig. 1b,c). In addition, a survival analysis measuring the percentage of mice that continued to perform at sub-criterion, showed that the stressed group attained criterion in fewer sessions than controls (χ2=2.17, df=1, P<.05) (Fig. 1d). Stress did not affect latency to retrieve reward but slowed stimulus-reaction time during late reversal (t(19)=2.17, P<.05) (Fig. 1e). In separate experiments, exposure to 3 days of 10-min swim stress did not affect either discrimination learning (errors: controls=101.1 ±17.5, stressed=104.2 ±31.3; correction errors: controls=234.9 ±46.6, stressed=233.6 ±67.6, n=10–12), acquisition of a simple instrumental learning task (sessions to criterion: controls=13.3 ±3.1, stressed=18.0 ±2.2, n=5–6) or sensorimotor gating (percent prepulse inhibition of startle: controls=101.5 ±11.6, stressed=97.6 ±10.2, n=13–15)10 (Fig. 1f). Thus, this moderate stressor selectively facilitated reversal without broad effects on other cognitive processes. By contrast, a single swim stress exposure prior to reversal failed to affect learning (Supplementary Fig. 1a–e), demonstrating that a minimum degree of stress was necessary to alter this behavior. We also found that 3x daily swim stress prior to reversal and then continuing following each reversal session mimicked the reversal-facilitating effect of pre-test-only 3x stress (Supplementary Fig. 1f–j), although the impact of the prolonged stress regimen was if anything slightly less impressive, perhaps due to a degree of stress-habituation.
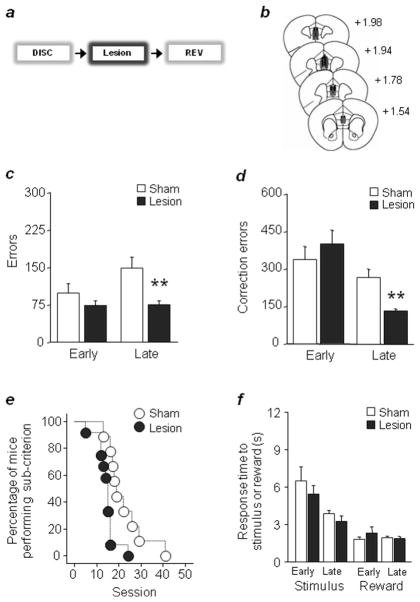
Stress can impair vmPFC functions2,3 and the same stressor employed here produces significant vmPFC dendritic retraction and associated deficits in fear extinction6. To test whether vmPFC damage produced effects on our reversal paradigm similar to stress, we trained mice to discrimination criterion and then made excitotoxic vmPFC lesions prior to reversal (Fig. 2a,b). Lesioned mice made significantly fewer errors (t(19)=3.39, P<.01) and correction errors (t(19)=4.13, P<.01) during late but not early reversal (Fig. 2c,d), and completed the task in fewer sessions (χ2=7.90, df=1, P<.01) (Fig. 2e), as compared to sham controls (reaction and reward-retrieval latencies were unaffected (Fig. 2f). Thus, vmPFC damage closely mimics stress-induced reversal facilitation.
Figure 2. vmPFC lesions mimic stress facilitation of reversal.
(a) Lesions were conducted after discrimination, prior to reversal. (b) Lesion estimates (black=minimum, grey=maximum). vmPFC lesions decreased errors (c) and correction errors (d) during late reversal (e) The percentage of mice performing below criterion decreased more rapidly across sessions after lesioning. (f) Stimulus and reward response latencies were unaffected. n=9–10/lesion. (Data are Means ±SE M. **P<.01 versus sham.
Reversal is likely mediated by multiple corticostriatal regions and it remains possible that stress facilitated this learning via effects on other prefrontal regions, such as OFC. However, we found that bilateral OFC lesions caused marked reversal impairment, as predicted from prior studies11 and opposite to vmPFC lesions (Supplementary Fig. 2). Moreover, bilateral lesions of DLS also significantly impaired reversal (Supplementary Fig. 3). This is notable in demonstrating that our reversal paradigm, like other operant tasks requiring slow, incremental and eventually habitual responding12, is DLS-mediated. Indeed, when we trained a set of non-lesioned mice through reversal and then devalued reward by pairing with malaise, neither performance vigor nor accuracy was affected (Supplementary Fig. 4), indicating habit formation. Collectively, these data suggest a ‘competing systems’ model in which stress-induced vmPFC dysfunction could facilitate reversal by removing a brake on learning by subcortical areas, including DLS. This would explain the stress-related effects on habit and DLS hypertrophy recently found in rats5, and suggests that DLS lesions would mitigate the impact of stress.
Brain-derived neurotrophic factor (BDNF) is strongly implicated in aberrant behavioral responses to stress. For example, stress alters vmPFC BDNF levels and vmPFC BDNF infusions facilitate stress-sensitive behaviors13–15. We therefore tested the ability of BDNF infusions directly into vmPFC to rescue the stress effect on reversal. Mice trained to discrimination criterion and implanted with guide cannula bilaterally directed at vmPFC (Fig. 3a,b) were stressed as above and given a single (0.08 μg) BDNF (or vehicle) infusion after the final stressor (reversal training commenced the next day).
Figure 3. vmPFC BDNF infusions rescue stress-induced facilitation of reversal.
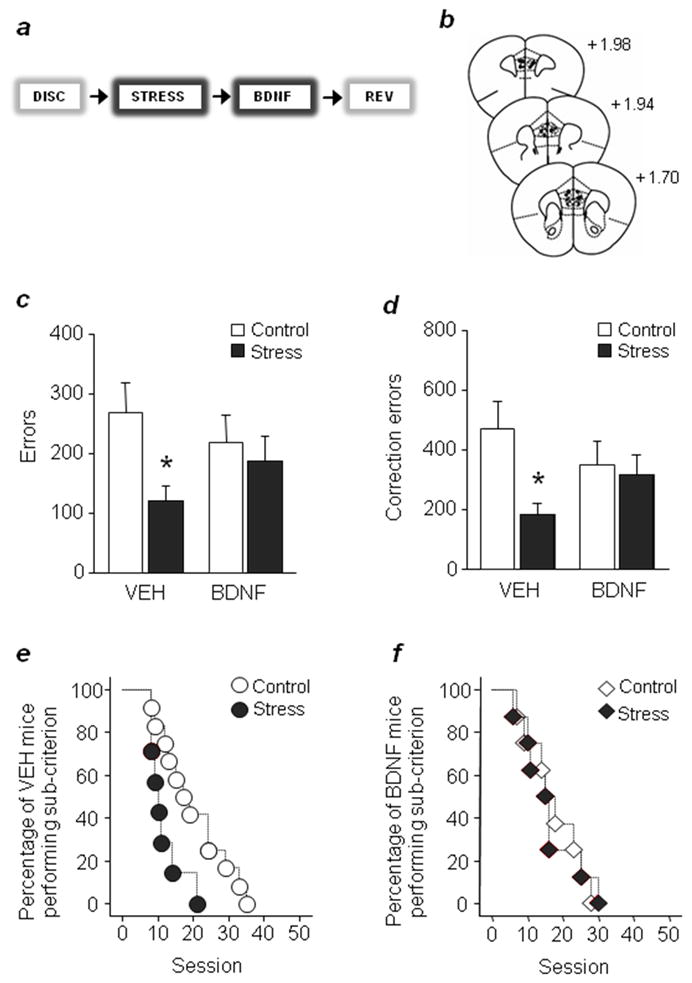
(a) A single BDNF infusion was delivered immediately after the third stress session. (b) Cannula placements. Vehicle-, but not BDNF-infused, stressed mice made fewer errors (c) or correction errors. (d) The percentage of mice performing below criterion decreased more rapidly across sessions after stress in vehicle- (e) but not BDNF- (f) infused mice. n=7–12/stress treatment/infusion group. Data are Means ± SEM. *P<.05 versus controls of same infusion group
Results showed that vehicle-infused stressed mice made fewer errors (t(17)=2.23, P<.05) and correction errors (t(17)=2.36, P<.05) than non-stressed controls during late (Fig. 3c,d) but not early (Supplementary Fig. 5) reversal, replicating our initial observation. By contrast, stressed mice receiving BDNF infusions performed no differently from BDNF-infused non-stressed controls. In addition, vehicle- but not BDNF-infused stressed mice completed reversal in fewer sessions than non-stress counterparts (χ2=5.77, df=1, P<.05) (Fig. 3e,f). These data demonstrate that post-stress infusion of BDNF into vmPFC is sufficient to prevent stress-induced facilitation in reversal.
In conclusion, current findings help recast the long-established focus on the cognition-impairing effects of stress and cortical damage, and support a more nuanced view in which stress shifts the relative contribution of parallel systems with the corticostriatal circuitry to bias towards certain (e.g., habit-like) behavioral strategies.
Supplementary Material
Acknowledgments
We thank Yavin Shaham, Greg Quirk and Geoffrey Schoenbaum for valuable discussions. Research supported by the NIAAA IRP(Z01-AA000411).
Footnotes
AUTHOR CONTRIBUTIONS
CG and AH designed the study. TJB and LMS provided behavioral software. CG, MF, ES and JLB performed experiments and analyzed data. AH, TJB and CG wrote the manuscript.
References
- 1.McEwen BS. Dialogues Clin Neurosci. 2006;8:367–81. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes A, Wellman CL. Neurosci Biobehav Rev. 2009;33:773–83. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnsten AF. Nat Rev Neurosci. 2009;10:410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shors TJ. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dias-Ferreira E, et al. Science. 2009;325:621–5. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 6.Izquierdo A, Wellman CL, Holmes A. J Neurosci. 2006;26:5733–8. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigman JL, et al. Cereb Cortex. 2010;20:1955–63. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chudasama Y, Robbins TW. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Jones B, Mishkin M. Exp Neurol. 1972;36:362–77. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- 10.Wiedholz LM, et al. Mol Psychiatry. 2008;13:631–40. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- 11.Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balleine B, Dickinson A. Q J Exp Psychol B. 1992;45:285–301. [PubMed] [Google Scholar]
- 13.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Science. 2010;328:1288–90. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gourley SL, Kedves AT, Olausson P, Taylor JR. Neuropsychopharmacology. 2009;34:707–16. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duman RS. Dialogues Clin Neurosci. 2009;11:239–55. doi: 10.31887/DCNS.2009.11.3/rsduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.