Abstract
Heat-shock proteins (Hsps) have been invoked in the pathogenesis of a variety of autoimmune diseases. The mycobacterial heat-shock protein 65 (Bhsp65) has been studied extensively as one of the antigenic triggers of autoimmunity in experimental models of, as well as patients with, rheumatoid arthritis. As Hsps are highly conserved and immunogenic, it is generally anticipated that self Hsps might serve as the endogenous targets of the immune response initiated by the homologous foreign Hsps. Contrary to this expectation, studies in the rat adjuvant arthritis (AA) model have revealed that priming of the self (rat) hsp65 (Rhsp65)-directed T cells in the Lewis rat leads to protection against AA instead of disease induction or aggravation. The arthritis-protective attribute of the self hsp65 is also evident following spontaneous priming of the anti-Rhsp65 T cells during the natural course of AA. Furthermore, immunization of rats with human hsp60, or with Bhsp65 peptides that are crossreactive with the corresponding self hsp65 peptides, leads to protection against AA. Importantly, high levels of T cell reactivity against self hsp60 in patients with juvenile idiopathic arthritis positively correlate with a favorable outcome of the disease. Thus, immune response against self hsp65 in autoimmune arthritis is protective rather than being pathogenic.
Keywords: Adjuvant arthritis, Autoimmunity, Heat-shock protein 65, Immunoregulation, Juvenile Idiopathic Arthritis
The immune system of a healthy individual is endowed with the ability to control aberrant self-directed immune responses. This is accomplished via diverse mechanisms of central and peripheral tolerance that cooperate effectively to suppress the initiation and progression of a potentially harmful anti-self response 1-5. However, the efficacy of this protective arsenal can be compromised under certain conditions involving the convergence of a highly susceptible genetic background, a defective tolerogenic mechanism, and a potent environmental trigger for autoreactivity 6-9. One of the frequent culprits in this regard is the microbial agent that provides ample ligands for the activation of the innate and adaptive immune responses 10, 11. These immune effector responses might inadvertently target self components that fortuitously mimic the microbial antigens, leading to the initiation of autoimmunity 10, 11. The eventual outcome of this initial breach of self tolerance is dependent in large part on the responsiveness of the host’s immune regulatory mechanisms. The adjuvant-induced arthritis (AA) model of human rheumatoid arthritis (RA) recapitulates several of the features of induction of autoimmunity highlighted above. AA can be induced in the Lewis (RT.1l) rat by immunization with heat-killed M. tuberculosis H37Ra (Mtb) 12. The mycobacterial heat-shock protein 65 (Bhsp65) is one of the major targets of the immune response of arthritis rats 13-16. The region 180-188 of Bhsp65 harbors an arthritogenic T cell determinant 13. The T cells primed by Mtb/Bhsp65 are believed to induce autoimmune arthritis via recognition of cartilage-resident self proteins. Unlike the Lewis rat, the Wistar Kyoto (WKY) (RT.1l) rat, the Brown Norway (BN) (RT.1n) rat, and the Fischer F344 (RT.1lvl) rat are relatively resistant to the induction of AA following Mtb challenge 15-17.
AA is a self-limiting disease. However, the immunological basis of the spontaneous regression of autoimmune inflammation in AA is not fully defined. The results of our studies based on self (rat) hsp65 (Rhsp65), the self homologue of Bhsp65, provide a conceptual framework for linking the inflammation and regulation of autoimmunity 15, 18, 19. (For uniformity of nomenclature, we have referred to the rat hsp60 as rat hsp65.) Inflammation is an integral component of the processes involved in the initiation of autoimmune arthritis. However, with the progression of arthritis, inflammation both upregulates the cellular expression of self Hsps, including Rhsp65, and modulates the antigen processing and presentation events, culminating in the triggering of regulatory immune responses 15, 18, 19(Fig. 1). The self hsp65-directed T cell repertoire can also be recruited by Bhsp65, which possesses several disease-regulating epitopes spanning different regions of the protein 14-16, 20, 21. The regulatory attribute of self Hsp unravels the ‘brighter’ side of these proteins in contrast to their ‘darker’ side, exemplified by their role as self antigenic targets in autoimmune pathology 22, 23. The dual role of self hsps in autoimmunity 22, 23 parallels that of several other biological molecules such as cytokines 24, many of which possess both pro-inflammatory and anti-inflammatory properties that manifest under distinct sets of conditions. We present below the results of studies by others and us that highlight the regulatory aspect of self Hsp65 in AA. Also discussed in brief are studies by other investigators in patients with juvenile idiopathic arthritis (JIA) that support the concept of self hsp65-mediated regulation of autoimmune arthritis.
Figure 1. Regulation of adjuvant arthritis (AA) by self hsp65-reactive T cells.
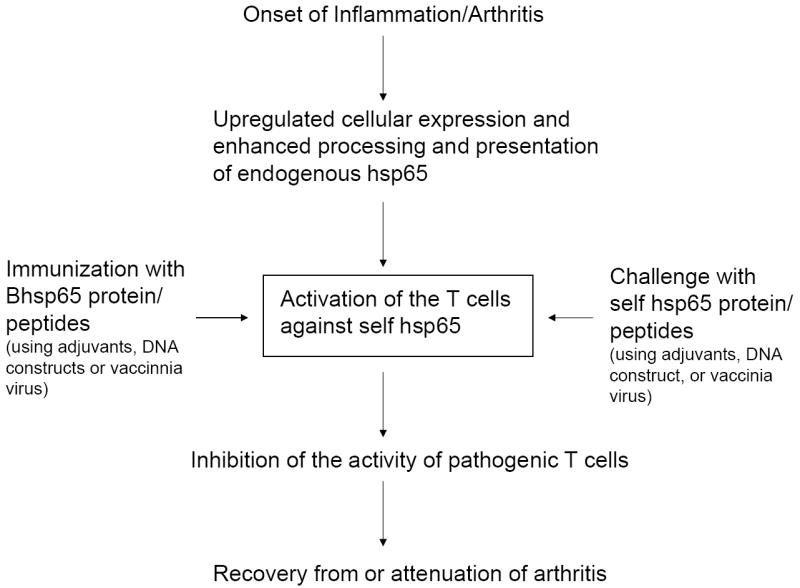
The onset of AA (inflammation) leads to upregulation of the cellular expression of self hsp65 as well as enhancement of the processing and presentation of this self hsp. This in turn leads to priming of the self hsp65-reactive T cells available in the mature repertoire (‘incomplete’ tolerance). This subset of T cells can also be activated following immunization with self hsp65 or by the crossreactive homologous Bhsp65. The T cell reactivity against self hsp65 is focused on the C-terminal epitopes of the protein. The in vivo priming of the T cells against self hsp65 or its C-terminal epitopes leads to downregulation of AA. Furthermore, challenge with Bhsp65 or its peptides (e.g., certain C-terminal epitopes and peptide 256-265) results in activation of the T cells that are crossreactive with self hsp65 as well as capable of inhibiting the progression of AA. Thus, despite the appreciable sequence homology (approximately 50%) between bacterial and self homologues of hsp65, the self hsp65 is involved in immune modulation rather than induction of AA.
I. Shaping of the T cell repertoire against self hsp65 and the role of these T cells in the pathogenesis of AA
The development of mature T cell repertoire occurs via positive and negative selection events in the thymus, and efficient induction of self tolerance is critical for the prevention of autoimmunity 1-5. Like other self antigens, self Hsps are also expected to influence the thymic selection of the T cell repertoire. The experiments conducted in our laboratory in the AA model have revealed that subsets of T cells specific for self hsp65 (Rhsp65) escape tolerance induction in the thymus and make it into the periphery of naive Lewis rats, demonstrating that self tolerance to Rhsp65 is incomplete. In this regard, Rhsp65 is different from certain other self proteins that are efficient tolerogens 25-27. The T cell response of Lewis rats immunized with Rhsp65 is targeted predominantly to the C-terminal epitopes of that protein. The in vivo-primed T cells specific for the C-terminal epitopes of Rhsp65 can be restimulated efficiently by the epitopes generated from endogenous hsp within heat-stressed APC 18. Furthermore, the pretreatment of Lewis rats with Rhsp65 affords protection against AA and this protection also is attributable primarily to the epitopes in the C-terminal region of the protein (Table 1). The protection against AA can be adoptively transferred to naïve syngeneic recipients via the T cells primed by the C-terminal epitopes of Rhsp65. Thus, we have offered experimental support both for in vivo priming of the T cells by the C-terminal epitopes of Rhsp65 during AA and for the likely role of these T cells in the natural recovery from the acute disease 15, 18, 19. On the basis of the above observations, we have proposed a model for the role of self hsp65 in regulation of AA 15, 18, 19, 28. We suggested that inflammation following the onset of AA upregulates the expression as well as the processing and presentation of Hsps. This in turn leads to activation of the T cells against the C-terminal epitopes of Rhsp65 and subsequent recovery from acute AA (Fig. 1) 15, 18, 19. Our results not only provide evidence for the self hsp65-mediated regulation of acute AA, but also offer novel insights for developing better antigen-specific immunotherapeutic approaches for human RA.
Table 1.
The involvement of self hsp60-directed T cell repertoire in regulation of adjuvant arthritis (AA) in rats
| Origin of hsp60/Epitope region | Reference | Remarks |
|---|---|---|
| (I) Rat hsp65 (Rhsp65) | 18 | Immunization of Lewis rats (= rats) with native Rhsp65 in IFA s.c. induces protection against AA |
| (II) Rhsp65 determinants: | ||
| 5 C-terminal determinants | 18 | Pre-treatment of rats with a mixture of peptides (in IFA) representing these 5 determinants affords protection against AA |
| 465-479 | 19, 44 | This peptide primes a predominantly Th1-type (IFN-γ) of response, which suppresses subsequent AA |
| 256-270 | 20 | Treatment of rats with Bhsp65 256-270 downmodulates AA; this peptide is crossreactive with its self hsp65 homologue, (Rhsp65 256-270), which does not have AA-regulatory activity |
| 61-80 | 16 | Injection of rats with this peptide reduces the severity of AA; sera of young Brown-Norway rats, old Lewis rats and post-arthritic Lewis rats possess antibodies reactive against this peptide. |
| (III) Human hsp60 (Hhsp60) | 14, 29, 30 | DNA vaccination of rats with the construct encoding Hhsp60, or injection with vaccinia virus expressing Hhsp60 induces protection against AA |
| (IV) Hhsp60 determinant: | ||
| Hu3 | 21 | DNA vaccine containing a DNA fragment encoding the epitope 31-50 suppresses the development of AA; it involves a Th2/Th3 cytokine shift in response to the arthritogenic epitope Bhsp65 176-190; this epitope has the same sequence in Rhsp65 |
IFA= incomplete Freund’s adjuvant; s.c.= subcutaneously; Th1= T helper 1
II. Vaccination with human hsp60 (Hhsp60) induces protection against AA
The protective effect of Rhsp65 in arthritis is further supported by studies showing the inhibition of AA in rats by DNA vaccination with Hhsp60, which is over 97% homologous to Rhsp65 (Table 1). Quintana et al. 14 tested the efficacy of DNA vaccination using plasmids encoding self (Hhsp60) or foreign (Bhsp65) Hsp. Their results showed that Hhsp60 was more effective than Bhsp65 in suppressing AA. Although both Hsps could prime self hsp60-reactive T cells, the immunization of rats with Hhsp60 activated T cells that produced much higher amounts of IL-10 and/or TGF-β compared to that produced by the T cells activated by Bhsp65. This difference in cytokine production constituted an important factor that rendered Hhsp60 superior to Bhsp65 in protection against AA. In another study, vaccination of rats with a plasmid carrying the gene for Hhsp60 followed by mapping of the T cell epitopes using overlapping hsp60 peptides showed that the T cell response was directed predominantly against the regulatory peptide, Hu3 21. The Hu3-reactive T cells secreted mostly IL-10. Immunization of rats with the Hu3 peptide or the adoptive transfer of splenocytes derived from Hu3 peptide-vaccinated rats into naïve syngeneic rats prior to Mtb injection inhibited the development of AA 21. However, the mycobacterial counterpart of Hu3 lacked arthritis-regulating properties. Thus, the protective effect of self hsp60 can be induced by immunization with the whole protein or with the functionally relevant peptide epitope of the protein (Table 1).
Using the AA model, Lopez-Guerrero et al 29 have reported the immunomodulatory effects of recombinant vaccinia virus expressing the Hhsp60 gene (Table 1). The challenge of rats with this virus on day 7 after the induction of AA reduced the severity of arthritis but induced both T cell and antibody responses to Hhsp60. In another study, the disease-protective effects of vaccinia virus expressing Bhsp65 or Hhsp60 were compared 30. Both Hsps were effective in the prevention of AA. However, in the therapeutic regimen, Hhsp60 induced higher protection against AA than that offered by Bhsp65, although both constructs induced optimal T cell responses against the respective protein 30. The results of another vaccinia virus-based study provided support to the protective effect of Bhsp65 31. In that study, soluble Bhsp65 aggravated the disease development 31. Taken together, the above findings provide a convincing rationale for the possible therapeutic use of Hhsp60 in autoimmune arthritis.
III. Arthritis-protective effects of the crossreactive T cells directed against self and bacterial hsp65
The initial studies on the pathogenesis of AA revealed that arthritis can be induced in Lewis rats by the passive transfer of T cells derived from syngeneic arthritic rats 32, 33, and that the pathogenic T cells include subsets of T cells reactive against Bhsp65. Subsequently, it has been demonstrated that a T cell clone (A2b) specific for the epitope region 180-188 of Bhsp65 could adoptively transfer AA to naïve recipients 13, 34 and that this T cell clone was crossreactive with an epitope of the cartilage proteoglycan 35. However, Bhsp65 per se failed to induce the disease (AA) in rats, although the T cells from arthritic rats responded to Bhsp65 upon in vitro restimulation. Surprisingly, immunization of naïve rats with Bhsp65 afforded protection against subsequent induction of AA by Mtb injection. The disease-protective effect of Bhsp65 has also been documented in other models of arthritis besides AA 13, 36-38, namely streptococcal cell wall-induced arthritis in rats 39, Pristane-induced arthritis 40 and dimethyl dioctadecyl ammonium bromide (DDA)-induced arthritis 41.
Reports from different laboratories have further highlighted the protective effects of Bhsp65-derived peptides in AA 15, 19, 20, 42, 43. There is increasingly realization that the disease-protective effect of Bhsp65 is mediated in part via T cells that are crossreactive with self hsp65 (Table 1). Van Eden et al. showed that a T cell line directed against the Bhsp65 epitope 256-270 was capable of transferring protection against AA and that these T cells are crossreactive with the mammalian hsp60 homologue 20. Furthermore, the pretreatment of rats with Bhsp65 peptide 256-270 afforded protection against AA and the T cells from these rats responded to heat-stressed syngeneic antigen presenting cells (APC) expressing endogenous (rat) hsp65.
We have previously reported the spreading of T cell response to Bhsp65 C-terminal determinants (BCTD) during the course of AA, as well as the protection against AA following pretreatment of rats with a mixture of peptides comprising BCTD 15, 18, 19. Moreover, the epitope spreading to Bhsp65 was accompanied by spontaneous induction of T cell response to the corresponding C-terminal epitopes of the homologous self hsp65 (Rhsp65) 15. We have also shown that the T cells activated by peptides comprising BCTD could adoptively transfer protection against AA 19, and that these T cells can be restimulated by the naturally processed C-terminal epitopes of recombinant self hsp65 as well as the endogenous hsp65 expressed within heat-stressed APC. These findings led us to suggest that the diversification of T cell response to BCTD within Bhsp65 during the course of inflammatory AA was the result of upregulation of the display of BCTD coupled with spontaneous induction of the T cell response to the crossreactive C-terminal epitopes of self hsp65 15, 18, 19. Thus, the activation of T cells reactive against the C-terminal determinants of Bhsp65/Rhsp65 constitutes a critical mechanism of protection/recovery in AA.
The arthritis-regulating activity of hsp65-primed T cells has been attributed to the production of predominantly anti-inflammatory/immunoregulatory Th2 (IL-10)/Th3 (TGF-β) cytokines 14, 16, 20, 21, 42. In our study on the AA-protective effect of the Rhsp65 peptide 465-479 (R465), we observed that the regulatory effect of this peptide was mediated primarily via the Th1 cytokine IFN-γ 44. These results are supported by similar findings of protection mediated via IL-12/IFN-γ in various other models of autoimmunity 24, 45, 46. The control of Th1-mediated autoimmunity by IFN-γ highlights the complexities as well as limitations of the simplified Th1-Th2 concept of cytokine-based regulation of autoimmunity 47, 48. In addition to cytokines, CD4+CD25+ T regulatory cells (Treg) 3-5 also mediate disease regulation in autoimmunity. In this regard, Hsps have been shown to activate Treg 49, 50. However, the precise role of Treg in Hsp65 peptide-induced downmodulation of AA or in natural recovery from AA is not yet defined.
IV. Antibodies against hsp65 are protective against AA
There is increasing evidence supporting the role of antibodies in the pathogenesis of AA, which is generally believed to be a predominantly T cell-mediated disease. Ulmansky et al. 16, 17 showed that the resistance to induction of AA in naïve BN rats, or in Lewis rats recovered from arthritis, was due to the presence of antibodies against hsp65. Moreover, the resistance to AA could be transferred to naïve susceptible rats by infusion of these antibodies. These antibodies are produced naturally in BN rats, but acquired during AA in Lewis rats. The kinetics of antibodies against Bhsp65 epitopes was different in resistant versus susceptible rat strains: arthritis-susceptible rats had antibodies to a few epitopes within Bhsp65 and acquired more epitope reactivities during the disease progression, whereas resistant rats had those antibodies naturally throughout. These results suggest that similar to epitope spreading involving the T cell epitopes, spreading also targets the antibody epitopes during the course of AA. The vaccination of Lewis rats with peptide 6 (a.a. 31-46) or peptide 7 (a.a. 37-52) of Bhsp65, or peptide 5 of self hsp65 (a.a. 61-80) (rat homologue of bacterial peptide 6) (Table 1), led to the production of antibodies against both bacterial peptide 6 and Bhsp65 as well as protection against AA. These three peptides share a common amino acid motif (VEWGP), which might contribute to the AA-protective effect of these peptides 16. Antibodies to peptide 6 were shown to induce production of IL-10 by the mononuclear cells 16. In an independent study, we reported the kinetics, epitope specificity and the functional attributes of antibodies directed against Bhsp65, self hsp65 and peptides representing different regions of these two proteins in Lewis versus WKY rats. We observed that the AA-resistant WKY rats mounted a vigorous antibody response to both Bhsp65 and self hsp65 after immunization with Mtb, whereas the AA-susceptible Lewis rats developed antibodies against relatively fewer epitopes of Bhsp65. In Lewis rats, antibodies to self hsp65 did not appear until the recovery phase of AA 51. The functional significance of anti-hsp65 antibodies was evident following serum adoptive transfer experiments. Serum from arthritic Lewis rats in the late phase of AA afforded protection against AA to the recipient rats. Studies by other investigators 29, 30, showing that protection induced by vaccinia virus expressing Hhsp60 is associated with the generation of cell-mediated and humoral immune response to self hsp, have provided indirect support for the protective effect of antibodies to self hsp65 in AA.
V. Modulation of AA by other Hsp family members
We have elaborated above the role of self/foreign hsp65 in protection against AA. The protective effects of other hsp family members such as mycobacterial hsp70 (Bhsp70) 52-54, mycobacterial hsp90 (Bhsp90) 55 and mycobacterial hsp10 (Bhsp10) 56 have also been documented. Wendling et al. 54 reported that the conserved Bhsp70 sequence 111-125 was found to be immunogenic and that it induced T cells that are crossreactive with the homologous rat sequence. The T cells reactive against peptide 111-125 produced IL-10, and the nasal administration of this peptide induced protection from subsequent induction of AA. Similar results were obtained by Tanaka et al. 53, who reported that pretreatment with peptide 234-252 of Bhsp70 produced high amounts of IL-10 and suppressed the development of AA. The transfer of a T cell line specific for peptide 234-252 also inhibited AA, suggesting that the T cells recognizing the conserved hsp70 peptide play a critical role in protection against arthritis.
VI. The disease-regulating attributes of human heat-shock protein 60 (Hhsp60) in juvenile idiopathic arthritis (JIA)
JIA is comprised of a heterogeneous group of clinical disorders characterized by chronic arthritis with disease onset before the age of 16 years 57. Based on the International League of Associations for Rheumatology (ILAR) criteria, JIA is currently classified into 7 different disease categories, including systemic arthritis (SA), oligoarthritis (OA), rheumatoid factor (RF)-positive polyarthritis (PA), RF-negative PA, enthesitis-related arthritis, psoriatic arthritis and undifferentiated arthritis 57. Most of the studies examining the role of Hhsp60 in JIA 58-65 are focused mainly on OA, PA and SA (Table 2). The overall results support the notion that Hhsp60 is regulatory in the OA category of JIA (OA-JIA). The regulatory function of Hhsp60 is associated with cell-mediated immune response characterized by cytokine deviation to a Th2-type. However, in a couple of studies, no correlation was observed between the T cell response to Hhsp60 and disease activity in JIA patients 66, 67. Furthermore, the Hhsp60-specific humoral response may not be specific for JIA and the significance of the role of humoral response in the pathogenesis of JIA is not yet clear. In regard to adult rheumatoid arthritis (RA), the role of Hhsp60 in regulation of the disease activity is rather inconclusive because both positive 68, 69 and negative 70, 71 associations have been reported for the specificity of Hhsp60-induced T cell activation, with or without Th2 cytokine deviation. It is a pleasure to contribute this paper to this special issue of the Journal of Autoimmunity devoted to the lifetime achievements of Dr. Noel Rose to autoimmunology 72-77, to his longstanding dedication to patient care, including the development of American Autoimmune Related Disease Association (AARDA) 78. This volume is part of the Journal’s commitment to recognize outstanding contributions in the field of autoimmunity 79-81.
Table 2.
The immune response to human heat-shock protein 60 in juvenile idiopathic arthritis
| JIA classification* | Experimental parameter of interest | Experimental evidence | Association with the disease activity | Notable observations | Ref |
|---|---|---|---|---|---|
| Oligoarthritis JIA | T cell response | Positive | Predicts disease remission with favorable prognosis |
|
58-61 |
| 58, 59 | |||||
| 61, 62 | |||||
| 62 | |||||
| Correlates with the duration of current arthritis |
|
60 | |||
| Negative | - |
|
67 | ||
| Antibody response | Negative | - |
|
64, 65 | |
| Synovial expression of Hhsp60 | Positive | Unknown |
|
59, 60, 63 | |
| Polyarthritis, mainly RF-negative JIA | T cell response | Positive | Unknown |
|
61, 67 |
| 61, 62 | |||||
| 62 | |||||
| Antibody response | Positive | Possibly no correlation with the disease activity |
|
65 | |
| 65 | |||||
| Negative | - |
|
64 | ||
| Synovial expression of Hhsp60 | Positive | Unknown |
|
60 | |
| Systemic arthritis | T cell response | Negative | - |
|
67 |
| JIA | Antibody response | Positive | Unknown |
|
64 |
| Negative | - |
|
64, 65 |
The International League of Associations for Rheumatology (ILAR) classification for JIA
Abbreviations: ELISA: enzyme-linked immunosorbent assay; Hhsp60: heat-shock protein 60; JIA: juvenile idiopathic arthritis; PBMCs: peripheral blood mononuclear cells; RF: rheumatoid factor; SF: synovial fluid; SFMCs: synovial fluid mononuclear cells; WB: Western blotting.
Acknowledgments
My lab members and I are thankful to Noel Rose for his advice, encouragement and support over the years. Noel’s creative contributions to the field of autoimmunity and his generous sharing of ideas have had a deep imprint on our thinking and experimental work. We also thank Rajesh Rajaiah, Yinghua Yang, Steva Nkrumah-Komeh and Hua Yu for their helpful critique and suggestions. This work was supported by grants from the Arthritis Foundation (Atlanta, GA) and the National Institutes of Health (Bethesda, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 2.Sprent J, Kishimoto H. The thymus and negative selection. Immunol Rev. 2002;185:126–35. doi: 10.1034/j.1600-065x.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersson J, Stefanova I, Stephens GL, Shevach EM. CD4+CD25+ regulatory T cells are activated in vivo by recognition of self. Int Immunol. 2007;19:557–66. doi: 10.1093/intimm/dxm021. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee R, Chaturvedi P, Qin HY, Singh B. CD4+CD25+ regulatory T cells generated in response to insulin B:9-23 peptide prevent adoptive transfer of diabetes by diabetogenic T cells. J Autoimmun. 2003;21:221–37. doi: 10.1016/s0896-8411(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 6.Rose NR. Mechanisms of autoimmunity. Semin Liver Dis. 2002;22:387–94. doi: 10.1055/s-2002-35708. [DOI] [PubMed] [Google Scholar]
- 7.Rose NR. Prediction and prevention of autoimmune disease: a personal perspective. Ann N Y Acad Sci. 2007;1109:117–28. doi: 10.1196/annals.1398.014. [DOI] [PubMed] [Google Scholar]
- 8.Shoenfeld Y, Blank M, Abu-Shakra M, Amital H, Barzilai O, Berkun Y, et al. The mosaic of autoimmunity: prediction, autoantibodies, and therapy in autoimmune diseases--2008. Isr Med Assoc J. 2008;10:13–9. [PubMed] [Google Scholar]
- 9.Moudgil KD, Deng H, Nanda NK, Grewal IS, Ametani A, Sercarz EE. Antigen processing and T cell repertoires as crucial aleatory features in induction of autoimmunity. J Autoimmun. 1996;9:227–34. doi: 10.1006/jaut.1996.0028. [DOI] [PubMed] [Google Scholar]
- 10.Blank M, Barzilai O, Shoenfeld Y. Molecular mimicry and auto-immunity. Clin Rev Allergy Immunol. 2007;32:111–8. doi: 10.1007/BF02686087. [DOI] [PubMed] [Google Scholar]
- 11.Oldstone MB. Molecular mimicry, microbial infection, and autoimmune disease: evolution of the concept. Curr Top Microbiol Immunol. 2005;296:1–17. doi: 10.1007/3-540-30791-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson CM. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956:95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- 13.van Eden W, Thole JE, van der Zee R, Noordzij A, van Embden JD, Hensen EJ, et al. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988;331:171–3. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- 14.Quintana FJ, Carmi P, Mor F, Cohen IR. Inhibition of adjuvant arthritis by a DNA vaccine encoding human heat shock protein 60. J Immunol. 2002;169:3422–8. doi: 10.4049/jimmunol.169.6.3422. [DOI] [PubMed] [Google Scholar]
- 15.Moudgil KD, Chang TT, Eradat H, Chen AM, Gupta RS, Brahn E, et al. Diversification of T cell responses to carboxy-terminal determinants within the 65-kD heat-shock protein is involved in regulation of autoimmune arthritis. J Exp Med. 1997;185:1307–16. doi: 10.1084/jem.185.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulmansky R, Cohen CJ, Szafer F, Moallem E, Fridlender ZG, Kashi Y, et al. Resistance to adjuvant arthritis is due to protective antibodies against heat shock protein surface epitopes and the induction of IL-10 secretion. J Immunol. 2002;168:6463–9. doi: 10.4049/jimmunol.168.12.6463. [DOI] [PubMed] [Google Scholar]
- 17.Ulmansky R, Naparstek Y. Immunoglobulins from rats that are resistant to adjuvant arthritis suppress the disease in arthritis-susceptible rats. Eur J Immunol. 1995;25:952–7. doi: 10.1002/eji.1830250415. [DOI] [PubMed] [Google Scholar]
- 18.Durai M, Gupta RS, Moudgil KD. The T cells specific for the carboxyl-terminal determinants of self (rat) heat-shock protein 65 escape tolerance induction and are involved in regulation of autoimmune arthritis. J Immunol. 2004;172:2795–802. doi: 10.4049/jimmunol.172.5.2795. [DOI] [PubMed] [Google Scholar]
- 19.Durai M, Kim HR, Moudgil KD. The regulatory C-terminal determinants within mycobacterial heat shock protein 65 are cryptic and cross-reactive with the dominant self homologs: implications for the pathogenesis of autoimmune arthritis. J Immunol. 2004;173:181–8. doi: 10.4049/jimmunol.173.1.181. [DOI] [PubMed] [Google Scholar]
- 20.Anderton SM, van der Zee R, Prakken B, Noordzij A, van Eden W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J Exp Med. 1995;181:943–52. doi: 10.1084/jem.181.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quintana FJ, Carmi P, Mor F, Cohen IR. DNA fragments of the human 60-kDa heat shock protein (HSP60) vaccinate against adjuvant arthritis: identification of a regulatory HSP60 peptide. J Immunol. 2003;171:3533–41. doi: 10.4049/jimmunol.171.7.3533. [DOI] [PubMed] [Google Scholar]
- 22.Coelho V, Broere F, Binder RJ, Shoenfeld Y, Moudgil KD. Heat-shock proteins: Inflammatory versus regulatory attributes. Cell Stress Chaperones. 2008;13:119–25. doi: 10.1007/s12192-008-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajaiah R, Moudgil KD. Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev. 2009;8:388–93. doi: 10.1016/j.autrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim EY, Moudgil KD. Regulation of autoimmune inflammation by pro-inflammatory cytokines. Immunol Lett. 2008;120:1–5. doi: 10.1016/j.imlet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moudgil KD. Determinant hierarchy: shaping of the self-directed T cell repertoire, and induction of autoimmunity. Immunol Lett. 1999;68:251–6. doi: 10.1016/s0165-2478(99)00080-2. [DOI] [PubMed] [Google Scholar]
- 26.Moudgil KD, Southwood S, Ametani A, Kim K, Sette A, Sercarz EE. The self-directed T cell repertoire against mouse lysozyme reflects the influence of the hierarchy of its own determinants and can be engaged by a foreign lysozyme. J Immunol. 1999;163:4232–7. [PubMed] [Google Scholar]
- 27.Lorenz RG, Allen PM. Direct evidence for functional self-protein/Ia-molecule complexes in vivo. Proc Natl Acad Sci U S A. 1988;85:5220–3. doi: 10.1073/pnas.85.14.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moudgil KD, Durai M. Regulation of autoimmune arthritis by self-heat-shock proteins. Trends Immunol. 2008;29:412–8. doi: 10.1016/j.it.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Guerrero JA, Lopez-Bote JP, Ortiz MA, Gupta RS, Paez E, Bernabeu C. Modulation of adjuvant arthritis in Lewis rats by recombinant vaccinia virus expressing the human 60-kilodalton heat shock protein. Infect Immun. 1993;61:4225–31. doi: 10.1128/iai.61.10.4225-4231.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Guerrero JA, Ortiz MA, Paez E, Bernabeu C, Lopez-Bote JP. Therapeutic effect of recombinant vaccinia virus expressing the 60-kd heat-shock protein on adjuvant arthritis. Arthritis Rheum. 1994;37:1462–7. doi: 10.1002/art.1780371009. [DOI] [PubMed] [Google Scholar]
- 31.Hogervorst EJ, Schouls L, Wagenaar JP, Boog CJ, Spaan WJ, van Embden JD, et al. Modulation of experimental autoimmunity: treatment of adjuvant arthritis by immunization with a recombinant vaccinia virus. Infect Immun. 1991;59:2029–35. doi: 10.1128/iai.59.6.2029-2035.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitehouse DJ, Whitehouse MW, Pearson CM. Passive transfer of adjuvant-induced arthritis and allergic encephalomyelitis in rats using thoracic duct lymphocytes. Nature. 1969;224:1322. doi: 10.1038/2241322a0. [DOI] [PubMed] [Google Scholar]
- 33.Taurog JD, Argentieri DC, McReynolds RA. Adjuvant arthritis. Methods Enzymol. 1988;162:339–55. doi: 10.1016/0076-6879(88)62089-1. [DOI] [PubMed] [Google Scholar]
- 34.Holoshitz J, Naparstek Y, Ben-Nun A, Cohen IR. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983;219:56–8. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- 35.van Eden W, Holoshitz J, Nevo Z, Frenkel A, Klajman A, Cohen IR. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985;82:5117–20. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billingham ME, Carney S, Butler R, Colston MJ. A mycobacterial 65-kD heat shock protein induces antigen-specific suppression of adjuvant arthritis, but is not itself arthritogenic. J Exp Med. 1990;171:339–44. doi: 10.1084/jem.171.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haque MA, Yoshino S, Inada S, Nomaguchi H, Tokunaga O, Kohashi O. Suppression of adjuvant arthritis in rats by induction of oral tolerance to mycobacterial 65-kDa heat shock protein. Eur J Immunol. 1996;26:2650–6. doi: 10.1002/eji.1830261116. [DOI] [PubMed] [Google Scholar]
- 38.Ragno S, Colston MJ, Lowrie DB, Winrow VR, Blake DR, Tascon R. Protection of rats from adjuvant arthritis by immunization with naked DNA encoding for mycobacterial heat shock protein 65. Arthritis Rheum. 1997;40:277–83. doi: 10.1002/art.1780400212. [DOI] [PubMed] [Google Scholar]
- 39.van den Broek MF, Hogervorst EJ, Van Bruggen MC, Van Eden W, van der Zee R, van den Berg WB. Protection against streptococcal cell wall-induced arthritis by pretreatment with the 65-kD mycobacterial heat shock protein. J Exp Med. 1989;170:449–66. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson SJ, Francis JN, Siew LK, Webb GR, Jenner PJ, Colston MJ, et al. An immunodominant epitope from mycobacterial 65-kDa heat shock protein protects against pristane-induced arthritis. J Immunol. 1998;160:4628–34. [PubMed] [Google Scholar]
- 41.Mia MY, Durai M, Kim HR, Moudgil KD. Heat shock protein 65-reactive T cells are involved in the pathogenesis of non-antigenic dimethyl dioctadecyl ammonium bromide-induced arthritis. J Immunol. 2005;175:219–27. doi: 10.4049/jimmunol.175.1.219. [DOI] [PubMed] [Google Scholar]
- 42.Prakken BJ, van der Zee R, Anderton SM, van Kooten PJ, Kuis W, van Eden W. Peptide-induced nasal tolerance for a mycobacterial heat shock protein 60 T cell epitope in rats suppresses both adjuvant arthritis and nonmicrobially induced experimental arthritis. Proc Natl Acad Sci U S A. 1997;94:3284–9. doi: 10.1073/pnas.94.7.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Noort JM, Anderton SM, Wagenaar JP, Wauben MH, van Holten C, Boog CJ. Differential rat T cell recognition of cathepsin D-released fragments of mycobacterial 65 kDa heat-shock protein after immunization with either the recombinant protein or whole mycobacteria. Int Immunol. 1994;6:603–9. doi: 10.1093/intimm/6.4.603. [DOI] [PubMed] [Google Scholar]
- 44.Kim EY, Chi HH, Bouziane M, Gaur A, Moudgil KD. Regulation of autoimmune arthritis by the pro-inflammatory cytokine interferon-gamma. Clin Immunol. 2008;127:98–106. doi: 10.1016/j.clim.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarrant TK, Silver PB, Wahlsten JL, Rizzo LV, Chan CC, Wiggert B, et al. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon gamma, nitric oxide, and apoptosis. J Exp Med. 1999;189:219–30. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afanasyeva M, Wang Y, Kaya Z, Stafford EA, Dohmen KM, Sadighi Akha AA, et al. Interleukin-12 receptor/STAT4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-gamma-independent pathway. Circulation. 2001;104:3145–51. doi: 10.1161/hc5001.100629. [DOI] [PubMed] [Google Scholar]
- 47.Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003;4:503–5. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 48.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 49.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 50.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–32. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Kim HR, Kim EY, Cerny J, Moudgil KD. Antibody responses to mycobacterial and self heat shock protein 65 in autoimmune arthritis: epitope specificity and implication in pathogenesis. J Immunol. 2006;177:6634–41. doi: 10.4049/jimmunol.177.10.6634. [DOI] [PubMed] [Google Scholar]
- 52.Kingston AE, Hicks CA, Colston MJ, Billingham ME. A 71-kD heat shock protein (hsp) from Mycobacterium tuberculosis has modulatory effects on experimental rat arthritis. Clin Exp Immunol. 1996;103:77–82. doi: 10.1046/j.1365-2249.1996.929628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka S, Kimura Y, Mitani A, Yamamoto G, Nishimura H, Spallek R, et al. Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis. J Immunol. 1999;163:5560–5. [PubMed] [Google Scholar]
- 54.Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–7. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- 55.Quintana FJ, Carmi P, Mor F, Cohen IR. Inhibition of adjuvant-induced arthritis by DNA vaccination with the 70-kd or the 90-kd human heat-shock protein: immune cross-regulation with the 60-kd heat-shock protein. Arthritis Rheum. 2004;50:3712–20. doi: 10.1002/art.20635. [DOI] [PubMed] [Google Scholar]
- 56.Ragno S, Winrow VR, Mascagni P, Lucietto P, Di Pierro F, Morris CJ, et al. A synthetic 10-kD heat shock protein (hsp10) from Mycobacterium tuberculosis modulates adjuvant arthritis. Clin Exp Immunol. 1996;103:384–90. doi: 10.1111/j.1365-2249.1996.tb08291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–78. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 58.Prakken AB, van Eden W, Rijkers GT, Kuis W, Toebes EA, de Graeff-Meeder ER, et al. Autoreactivity to human heat-shock protein 60 predicts disease remission in oligoarticular juvenile rheumatoid arthritis. Arthritis Rheum. 1996;39:1826–32. doi: 10.1002/art.1780391108. [DOI] [PubMed] [Google Scholar]
- 59.de Graeff-Meeder ER, van Eden W, Rijkers GT, Prakken BJ, Kuis W, Voorhorst-Ogink MM, et al. Juvenile chronic arthritis: T cell reactivity to human HSP60 in patients with a favorable course of arthritis. J Clin Invest. 1995;95:934–40. doi: 10.1172/JCI117801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Graeff-Meeder ER, van der Zee R, Rijkers GT, Schuurman HJ, Kuis W, Bijlsma JW, et al. Recognition of human 60 kD heat shock protein by mononuclear cells from patients with juvenile chronic arthritis. Lancet. 1991;337:1368–72. doi: 10.1016/0140-6736(91)93057-g. [DOI] [PubMed] [Google Scholar]
- 61.Kamphuis S, Kuis W, de Jager W, Teklenburg G, Massa M, Gordon G, et al. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet. 2005;366:50–6. doi: 10.1016/S0140-6736(05)66827-4. [DOI] [PubMed] [Google Scholar]
- 62.de Kleer IM, Kamphuis SM, Rijkers GT, Scholtens L, Gordon G, De Jager W, et al. The spontaneous remission of juvenile idiopathic arthritis is characterized by CD30+ T cells directed to human heat-shock protein 60 capable of producing the regulatory cytokine interleukin-10. Arthritis Rheum. 2003;48:2001–10. doi: 10.1002/art.11174. [DOI] [PubMed] [Google Scholar]
- 63.Boog CJ, de Graeff-Meeder ER, Lucassen MA, van der Zee R, Voorhorst-Ogink MM, van Kooten PJ, et al. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med. 1992;175:1805–10. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen TT, Zlacka D, Vavrincova P, Sedlacek P, Hromadnikova I. Detection of antibodies against 60-, 65- and 70-kDa heat shock proteins in paediatric patients with various disorders using Western blotting and ELISA. Clin Chem Lab Med. 2006;44:442–9. doi: 10.1515/CCLM.2006.088. [DOI] [PubMed] [Google Scholar]
- 65.de Graeff-Meeder ER, Rijkers GT, Voorhorst-Ogink MM, Kuis W, van der Zee R, van Eden W, et al. Antibodies to human HSP60 in patients with juvenile chronic arthritis, diabetes mellitus, and cystic fibrosis. Pediatr Res. 1993;34:424–8. doi: 10.1203/00006450-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Life P, Hassell A, Williams K, Young S, Bacon P, Southwood T, et al. Responses to gram negative enteric bacterial antigens by synovial T cells from patients with juvenile chronic arthritis: recognition of heat shock protein HSP60. J Rheumatol. 1993;20:1388–96. [PubMed] [Google Scholar]
- 67.Sedlackova L, Velek J, Vavrincova P, Hromadnikova I. Peripheral blood mononuclear cell responses to heat shock proteins and their derived synthetic peptides in juvenile idiopathic arthritis patients. Inflamm Res. 2006;55:153–9. doi: 10.1007/s00011-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 68.MacHt LM, Elson CJ, Kirwan JR, Gaston JS, Lamont AG, Thompson JM, et al. Relationship between disease severity and responses by blood mononuclear cells from patients with rheumatoid arthritis to human heat-shock protein 60. Immunology. 2000;99:208–14. doi: 10.1046/j.1365-2567.2000.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Roon JA, van Eden W, van Roy JL, Lafeber FJ, Bijlsma JW. Stimulation of suppressive T cell responses by human but not bacterial 60-kD heat-shock protein in synovial fluid of patients with rheumatoid arthritis. J Clin Invest. 1997;100:459–63. doi: 10.1172/JCI119553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaston JS, Life PF, van der Zee R, Jenner PJ, Colston MJ, Tonks S, et al. Epitope specificity and MHC restriction of rheumatoid arthritis synovial T cell clones which recognize a mycobacterial 65 kDa heat shock protein. Int Immunol. 1991;3:965–72. doi: 10.1093/intimm/3.10.965. [DOI] [PubMed] [Google Scholar]
- 71.Zou J, Rudwaleit M, Thiel A, Lauster R, Braun J, Sieper J. T cell response to human HSP60 and yersinia 19 kDa in ankylosing spondylitis and rheumatoid arthritis: no evidence for a causal role of these antigens in the pathogenesis. Ann Rheum Dis. 2002;61:473–4. doi: 10.1136/ard.61.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shoenfeld Y, Selmi C, Zimlichman E, Gershwin ME. The autoimmunologist: geoepidemiology, a new center of gravity, and prime time for autoimmunity. J Autoimmmun. 2008;31:325–330. doi: 10.1016/j.jaut.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Rose NR, Leskovsek N. Scleroderma: immunopathogenesis and treatment. Immunol Today. 1998;19:499–501. doi: 10.1016/s0167-5699(98)01322-x. [DOI] [PubMed] [Google Scholar]
- 74.ElRehewy M, Kong YM, Giraldo AA, Rose NR. Syngeneic thyroglobulin is immunogenic in good responder mice. Eur J Immunol. 1981;11:146–151. doi: 10.1002/eji.1830110216. [DOI] [PubMed] [Google Scholar]
- 75.Rose NR. Autoimmune diseases. Sci Am. 1981;244:80–84. 86–90. doi: 10.1038/scientificamerican0281-80. passim. [DOI] [PubMed] [Google Scholar]
- 76.Rose NR, Kong YC, Okayasu I, Giraldo AA, Beisel K, Sundick RS. T-cell regulation in autoimmune thyroiditis. Immunol Rev. 1981;55:299–314. doi: 10.1111/j.1600-065x.1981.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 77.Kaya Z, Göser S, Buss SJ, Leuschner F, Ottl R, Li J, Völkers M, Zittrich S, Pfitzer G, Rose NR, Katus HA. Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis. Circulation. 2008;118:2063–2072. doi: 10.1161/CIRCULATIONAHA.108.788711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mackay IR, Leskovsek NV, Rose NR. Cell damage and autoimmunity: a critical appraisal. J Autoimmun. 2008;30:5–11. doi: 10.1016/j.jaut.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whittingham S, Rowley MJ, Gershwin ME. A tribute to an outstanding immunologist - Ian Reay Mackay. J Autoimmun. 2008;31:197–200. doi: 10.1016/j.jaut.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Gershwin ME. Bone marrow transplantation, refractory autoimmunity and the contributions of Susumu Ikehara. J Autoimmun. 2008;30:105–107. doi: 10.1016/j.jaut.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 81.Blank M, Gershwin ME. Autoimmunity: from the mosaic to the kaleidoscope. J Autoimmun. 2008;30:1–4. doi: 10.1016/j.jaut.2007.11.015. [DOI] [PubMed] [Google Scholar]


