Summary
Background:
Post-surgical evaluation of the pituitary gland in MRI is difficult because of a change of anatomical conditions. It depends also on numerous other factors, including: size and expansion of a tumour before surgery, type of surgical access, quality and volume of filling material used and time of its resorption.The aim of the study was to compare MR image of the pituitary gland after surgery with clinical findings and to establish a correlation between MRI presentation of spared pituitary and its hormonal function.
Material/Methods:
124 patients after resection of pituitary adenomas – 409 MRI results in total – were studied. With a 1.5-T unit, T1-weighted sagittal and coronal, enhanced and unenhanced images were obtained.
Results:
The pituitary gland seemed to be normal in MRI in 11 patients, 8 of them had completely regular pituitary function but in 3 of them we noticed a partial hypopituitarism. In 99 patients only a part of the pituitary gland was recognised, 53 of them had hypopituitarism but 46 of them were endocrinologically healthy. 14 patients seemed to have no persistent pituitary gland in MRI, in comparison to hormonal studies: there was panhypopituitarism in 6 and hypopituitarism in 8 cases.
Conclusions:
MRI presentation of post – surgical pituitary gland doesn’t necessarily correlate with its hormonal function – there was a significant statistical difference. Some patients with partial pituitary seems normal hormonal function. In some cases the pituitary seem normal in MRI but these patients have hormonal disorders and need substitution therapy.
Keywords: pituitary tumours, MRI, surgery, hormonal function
Background
Pituitary tumours constitute approximately 10 to 15% of all primary intracranial carcinomas [1–4] and are the most common causes of pituitary function disorders and field of vision loss [5,6]. Therefore, early diagnosis and therapy is a serious clinical problem.
Introduction of modern imaging techniques, including computed tomography (CT) and magnetic resonance imaging (MRI), and of modern methods of neurosurgery and pharmacotherapy revolutionised diagnostics and therapy of pituitary tumours. Currently, magnetic resonance is a method of choice for imaging of the pituitary gland and the perisellar area, and advanced MRI techniques – spectroscopy and diffusion and perfusion – are increasingly applied [1,6–15].
If pituitary tumour presentation in MRI was described in details and usually does not present any diagnostic difficulties, post-surgical evaluation of the pituitary gland becomes commonly a serious problem. And surgical therapy is a primary therapeutic method, except for prolactin-secreting microadenomas [3,16–27]. Interpretation of MRI images taken after surgical therapy of pituitary tumours is difficult because of a change of anatomical conditions. The interpretation depends also on numerous other factors, including: size and expansion of a tumour before surgery, type of surgical access, quality and volume of filling material used and time of its resorption [2,28]. Proper evaluation of post-surgical MRI images is crucial for determination of completeness of the resection. Therefore neuro-radiologists face a responsible and difficult task of evaluation of structures present in the surgical area, and especially of differentiation of residue tumour from the filling material used and from post-surgical changes (fibrous and cicatricial), or even from a part of normal gland left at site. Moreover, monitoring of the post-surgical course is also important, especially in case of hormonally inactive tumours. Diagnosis of a proliferative process recurrence in subsequent control examinations plays a crucial role for further therapy.
Purpose of this study was to compare MRI images of a partial pituitary gland left after a surgery with evaluation of its hormonal function.
Material and Methods
The study group consisted of 124 patients with the following diagnoses: pituitary adenoma (117 cases), colloid cyst (1 case), pituitary abscess (1 case), Rathke’s pouch cyst (1 case), intra-sellary located craniopharyngioma (3 cases) and hamartoma (1 case). Patients undergoing radiotherapy after the surgery were excluded from the study group, in order to eliminate an additional factor able to alter structure and function of the pituitary gland. Types of pituitary tumours in the analysed material are presented in the Table 1. Types of pituitary adenomas diagnosed in patients undergoing surgery are presented in the Table 2.
Table 1.
Types of pituitary tumours in the analysed material.
| Types of tumours | No. of patients | |
|---|---|---|
| Adenomas | Pituitary macroadenomas | 103 |
| Pituitary microadenomas | 14 | |
| Other tumours | Intra-sellary located craniopharyngioma | 3 |
| Intra-sellary located Rathke’s pouch cyst | 1 | |
| Pituitary colloid cyst | 1 | |
| Pituitary abscess | 1 | |
| hamartoma localised in the pituitary gland | 1 | |
| Total | 124 | |
Table 2.
Type of diagnosed pituitary adenomas in individual patients.
| Lp. | Type of pituitary adenoma | No. of cases | Ratio (%) |
|---|---|---|---|
| 1 | Growth hormone (GH) secreting adenoma | 49 | 41.88% |
| 2 | Prolactin (PRL) secreting adenoma | 26 | 22.22% |
| 3 | GH and PRL secreting adenoma | 2 | 1.70% |
| 4 | Adrenocorticotrophin (ACTH) secreting adenoma | 9 | 7.69% |
| 5 | Luteotropin (LH) secreting adenoma | 1 | 0.08% |
| 6 | Hormonally inactive adenoma | 30 | 25.64% |
There were 74 women (59.7%) in the study group, age ranging from 15 to 70, mean age 41.74 years; and 50 men (40.3%), age ranging from 16 to 75, mean age 41.46 years. Overall age of the included patients ranged from 15 to 75, mean age was 41.52 years. Due to the fact that the patients were observed prospectively, patient’s age at the time of surgery was considered.
One to eight MRI examinations were performed in individual patients after surgery of the pituitary tumour. One examination was performed in 22 patients (17.74%), and the remaining 102 patients (82.26%) were examined more than once post-surgically. Total number of 409 examinations were analysed, considering also previous examinations performed in other centres. Precise number of MRI examinations included in the study in individual patients is presented in the Table 3.
Table 3.
Number of analysed MRI examinations post-surgically in individual patients.
| Number of performed MRI examinations | Number of examined patients | Ratio |
|---|---|---|
| 1 examination post-surgically | 22 | 17.74% |
| 2 examinations post-surgically | 27 | 21.17% |
| 3 examinations post-surgically | 24 | 17.26% |
| 4 examinations post-surgically | 18 | 14.51% |
| 5 examinations post-surgically | 19 | 15.32% |
| 6 examinations post-surgically | 6 | 4.83% |
| 7 examinations post-surgically | 6 | 4.83% |
| 8 examinations post-surgically | 2 | 1.61% |
| Total: 409 examinations | Total: 124 patients | Total: 100% |
Imaging was performed using the Picker Edge Eclipse 1,5T equipment. Each imaging was started from a single pilot image and three localisation images.
Then, proper T1-weighetd images were taken, in coronal and sagittal planes, using the FAST sequence (Fourier acquired steady-state sequence). Technical parameters of those images are presented in the Table 4.
Table 4.
Technical parameters of MRI images in the pituitary gland examination.
| Sequence | FAST T1-weighted | FAST T1-weighted |
|---|---|---|
| Plane | Coronal | Sagittal |
| Repetition time – TR (ms) | 229 | 229 |
| Echo time – TE (ms) | 4.5 | 4.5 |
| Slice thickness – THICK (mm) | 2.5/0.5 | 2.5/0.5 |
| Number of acquisitions – NSA | 2 | 2 |
| Matrix | 256×256 | 256×256 |
| Field of view – FOV (cm) | 17.0 | 18.0 |
| Acquisition time (s) | 01:57 | 02:18 |
Paramagnetic contrast medium was administered intravenously to each patient, at dose of 0.1 ml/kg BW, and further T1-weighted images were taken in coronal and sagittal planes.
Pituitary gland function was evaluated in all patients. Anterior pituitary function was evaluated by determination of trophic hormones levels – somatotrophin (GH), folliculostimmuline (FSH), luteotrophin (LH), prolactin (PRL), thyreotrophin (TSH), adrenocorticotrophin (ACTH), as well as daily profile of cortisol and daily elimination of free cortisol with urine, and free tyroxine (fT4), estradiol (E2), testosteron (T) and dehydroepiandrosteron sulphate (DHEA-S) levels in blood serum. In cases doubtful for evaluation of the adrenocorticotrophic axis function, a post-insuline hypoglycaemia test was performed, with determination of glucose, ACTH and cortisol levels. Posterior pituitary function was evaluated performing diagnostics for diabetes insipdus. Pituitary function was determined completely normal if concentrations of tested hormones were regular, and no symptoms of diabetes insipidus were nooted. Partial insufficiency of the anterior pituitary was diagnosed if one or more trophic axes were disorderd. Disorder of all trophic axes was determined as complete failure of the anterior pituitary – hypopituitarism. Complete failure of anterior and posterior pituitary – panhypopituitarism – was diagnosed if hypopituitarism was accompanied by diabetes insipidus.
McNemar’s test γ2 of a relationship between two related features was used for determination of there is a correlation between post-surical presentation of the pituitary obtained from MRI and the gland’s hormonal function.
Results
Among 124 patients post neurosurgical procedure of a tumour located in a hypothalamic-pituitary area examined with MRI, in 110 the whole gland or its part was visible. In the remaining 14 MRI did not showed presence of the pituitary gland. Among 110 patients with spared pituitary tissue, in 11 size of the gland was regular. In that group, proper function of the gland was found in 8 cases, and partial insufficiency was found in other 3 cases, in which an incomplete hormonal substitution was necessary.
Existence of a fragment of normal pituitary was shown in 99 patients. That was associated to pituitary function impairment in 53 cases. In 46 patients in whom MRI showed existence of a partial gland, its function was completely normal.
Among 14 patients in whom no pituitary gland was found, in 6 cases hormonal tests indicated a complete failure of the anterior and posterior lobe (panhypopituitarism), and in 8 cases only anterior pituitary insufficiency was found.
Results of MRI of the pituitary gland and of its hormonal function analysis is presented in Table 5. Results of statistical analysis of a relationship between MRI presentation of the pituitary gland and its hormonal function in patients post neuro-surgical procedures is presented in the Table 6. A significant statistical difference was found between post-surgical MRI presentation of the gland and its hormonal function (p=0.00000000197).
Table 5.
Comparison of the post-surgical pituitary gland MRI presentation and its hormonal function.
| No. | MRI presentation of the pituitary gland | Number of cases | Pituitary hormonal function | Number of cases |
|---|---|---|---|---|
| 1. | Normal size | 11 | Normal Partial anterior pituitary insufficiency |
8 3 |
| 2. | Small, at the bottom of the sella | 37 | Normal Partial anterior pituitary insufficiency Hypopituitaryzm Panhypopituitarism |
20 8 6 3 |
| 3. | A fragment on the left side of the sella | 33 | Normal Partial anterior pituitary insufficiency Hypopituitaryzm Panhypopituitaryzm |
16 9 6 2 |
| 4. | A fragment on the right side of the sella | 23 | Normal Partial anterior pituitary insufficiency Hypopituitarism |
8 8 7 |
| 5. | A fragment on the upper part of the sella | 5 | Normal Partial anterior pituitary insufficiency Hypopituitarism |
1 2 2 |
| 6. | A fragment on the posterior part of the sella | 1 | Normal | 1 |
| 7. | Invisible | 14 | Hypopituitarism Panhypopituitarism |
8 6 |
Table 6.
Results of analysis of relationship between post-surgical MRI presentation of the pituitary gland and its hormonal function.
| MRI presentation of the pituitary gland | Pituitary hormonal function | Number of cases |
|---|---|---|
| Regular size of the gland (N=11) | Normal function | 8 |
| Impaired function | 3 | |
| Visible fragment (N=99) | Normal function | 46 |
| Impaired function | 53 | |
| Invisible pituitary (N=14) | Normal function | 0 |
| Impaired function | 14 | |
| 124 | ||
| P=0.00000000197 |
Four representative cases and corresponding imaging results are presented in order to illustrate data presented above.
Case 1
A 35-y.o. female was admitted to the Department of Endocrinology, Diabetology, and Isotope Treatment at the Wroclaw Medical University. A women reported menstruation disorders with galactorrhoea for the last 5 years. Laboratory tests indicated significant hyperprolactinaemia, and MRI showed existence of pituitary adenoma sized approx. 1.5×1.3 cm. The tumour was localised intra-sellary, shifted towards the right side, compressing and relocating the pituitary gland to the left (Figure 1A). Hypothalamic infundibulum was also shifted to the left. Pharmacotherapy was attempted, but despite application of various possible regimens the patient could not use them because of numerous side effects. The patient was operated on, via the sphenoid sinus access. Control MRI performed 12 months after the tumour resection showed no residues of the adenoma or signs of its recurrence. Spared part of the pituitary gland was located on the left side of the sella, with diameter of up to 7 mm. A post-surgical defect was visible on the right side within the sella. The hypothalamic infundibulum was positioned axially. Presentation of the optic chiasm was normal. Laboratory tests of pituitary hormones level, cortisol profile and free tyroxine level were normal. No diabetes insipidus was found. The patient did not require any substitutive therapy. Another MRI performed approximately 7 years after the surgery (Figure 1B) did not show any significant changes compared to previous imaging results.
Figure 1A.
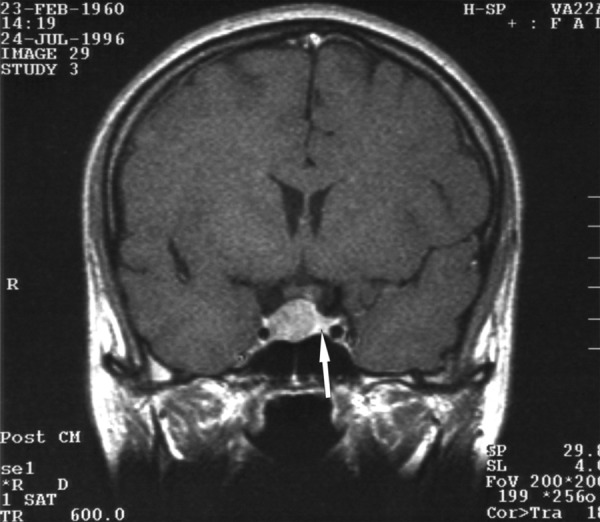
A T1-weighted image after constrast medium administration, coronal plane: the adenoma is visible, compressing and shifting the pituitary gland to the left side. The arrow indicates the compressed pituitary.
Figure 1B.
The same patient approx. 7 years after the adenoma resection – a T1-weighted image after contrast medium administration, coronal plane: a spared fragment of the pituitary gland visible on the left side of the sella. The hypothalamic infundibulum positioned axially. The optic chiasm free. The sphenoid sinus normally aerated.
Case 2
A 44-y.o. female was admitted to the Department of Ophthalmology in order to have temporal loss of filed of vision diagnosed. Laboratory tests indicated increased GH level, and MRI showed a large, sellar-suprasellar tumour sized approx. 3.1×3.0 cm. The tumour compressed and dislocated the optical chiasm and compressed the suprasellar cisterns and the III ventricle. No signs of the cavernous sinus infiltration were found. Acromegaly in couse of pituitary adenoma was diagnosed based on the typical clinical presentation and results of laboratory tests. The patient was qualified for surgery. In April 1997 the tumour was removed via the nasal access and the sphenoid sinus. Control tests peroformed after the surgery indicated normal GH level. Also clinical presentation suggested effectiveness of the surgery. Moreover, besides the pre-surgical primary thyroid insufficiency, function of other trophic axes seemed normal. The patient did not require any substitutive therapy, except for a previously used l-tyroxine. MRI performed in June 1998 (14 months after the surgery) revealed a hypointensive structure on the left side of the sella. A residual tumour was suspected. Spared fragment of the pituitary gland was visualised, in form of a narrow band of tissue connected to the the hypothalamic infundibulum and intensively enhanced after contrast medium administration. Subsequent MRI performed in June 1999, June 2000, May 2002 (Figure 2) and June 2004 did not show any significant changes compared to the first post-surgical examination in June 1998. Therefore, the MRI presentation had not changed for over 7 years. Repeated clinical control had not indicated recurrence of active acromegaly. For that reason, the hypointensive structure present on the left side of the sella was recognised as post-surgical fibrous changes, and the case was qualified as a false-positive for MRI result.
Figure 2.
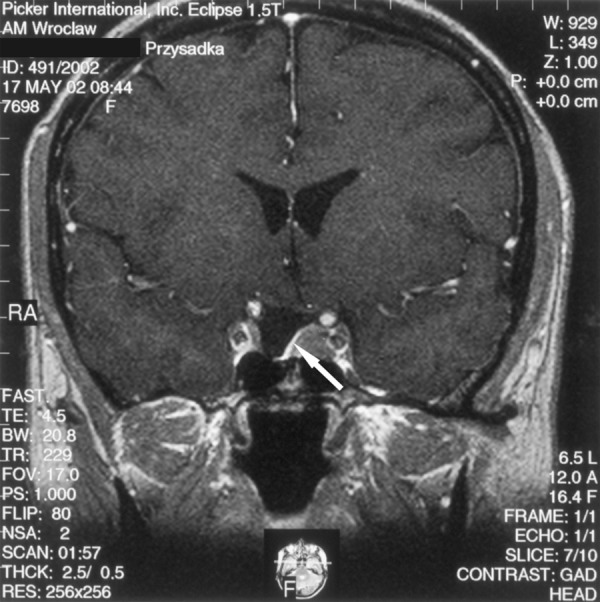
Condition post pituitary adenoma surgery performed 5 years before. A T1-weighted image after contrast medium administration, coronal plane: a narrow band of the gland connected to the hypothalamic infundibulum is visible. The hypothalamic infundibulum is slightly shifted to the right. On the left side of the sella there is a hypointensive structure visible, suggesting a residual tumour. The arrow points at a spared, small fragment of the pituitary gland. However, lack of progression in 7-year-long MRI follow-up allowed diagnosis of post-surgical fibrosis.
Case 3
A 30-y.o. female was admitted to the Department of Neurosurgery at the Wroclaw Medical University to be surgically treated for pituitary adenoma associated with signs of acromegaly. MRI performed in August 2001 revealed an intrasellar-suprasellar tumour of approx. 2.2 cm diameter. The tumour occupied the right cavernous sinus, compressed the optic chiasm, compressed the pituitary gland and translocated it upwards and to the left (Figure 3A). The patient was operated in November 2001 using an access via the right nostril and the sphenoid sinus. Control MRI performed in June 2002 (8 months after the surgery) showed a residual adenoma on the right side of the sella and within the right cavernous sinus. A fragment of normal pituitary gland was visualised on the left side of the sella. The tissue had become slightly “expanded” compared to the MRI performed befor the resection (Figure 3B). The hypothalamic infundibulum was slightly shifter on the left side and connected to the spared fragment of the pituitary gland. Results of hormonal tests (increased GH levels) suggested non-radical resection. Moreover, partial inufficiency of the anterior pituitary (secondary adrenal and thyroid insufficiency) was diagnosed. The patient required substitutive therapy with hydrocortisone and l-tyroxine.
Figure 3A.
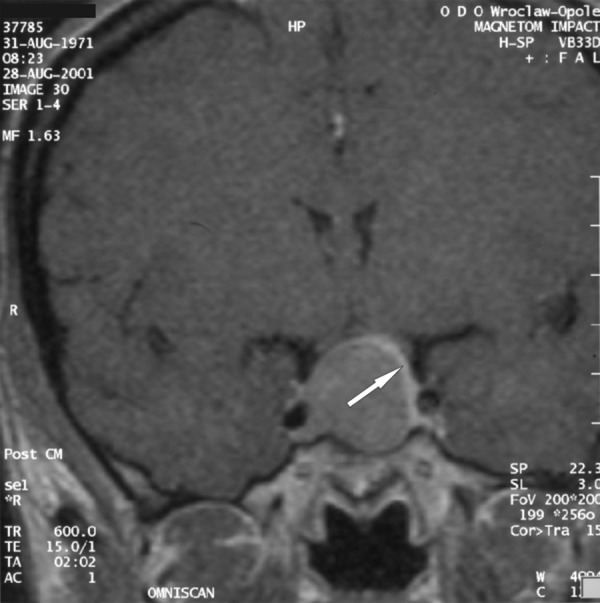
A T1-weighted image after contrast medium administration, coronal plane; the arrow points at a narrow band of the pituitary, compressed and shifted by the tumour.
Figure 3B.
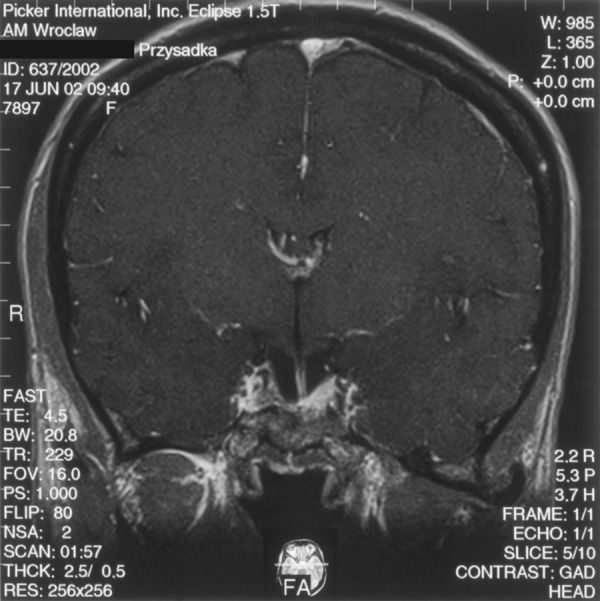
A T1-weighted image after contrast medium administration, coronal plane: on the right side of the sella there is a residual tumour visible; on the left side a spared fragment of the pituitary gland (8 months after the surgery).
Case 4
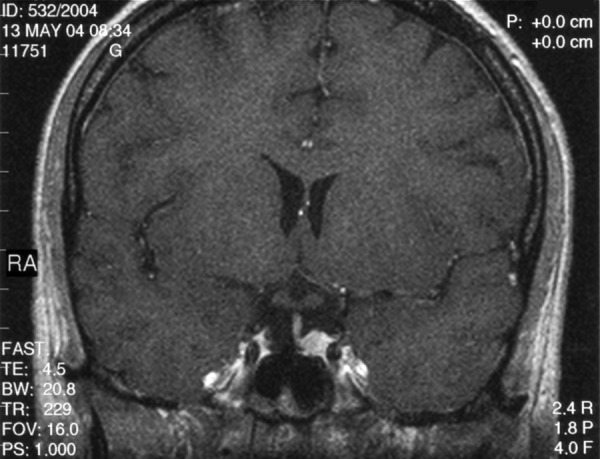
A 59-y.o. female with clinical features of acromegaly was admitted to the Department of Endocrinology, Diabetology, and Isotope Treatment at the Wroclaw Medical University. Hormonal tests showed increased GH level not inhibited in glucose test, as well as associated hyperprolactinaemia. No disorders in other pituitary trophic axes were found. MRI performed in June 2002 showed a tumour located intrasellary, on the right side, sized approx. 1.3×1.1×1.7 cm, with weak contrast enhancement. The tumour adhered closely to the right cavernous sinus and caused compression and displacement of the pituitary gland with the hypothalamic infundibulum to the left (Figure 4A). The optic chiasm and perisellar structures were normal. The patient was operated in September 2002 via the trans-sphenoid access. Control MRI in January 2003 (4 months after the surgery), and next in May 2004 (20 months after the recection) did not show any residual tumour. Normal pituitary gland was visualised, which “expanded” after the tumour removal and regained its normal size and location (Figure 4B). After intravenous contrast medium administration the gland had become uniformly enhanced. The hypothalamic infundibulum was positioned axially. Hormonal test results confirmed effectiveness of the surgery, and GH level normalised. Despite a normal presentation of the pituitary gland in the MRI, insufficiency of the anterior pituitary was shown within the adrenal axis. The patient required substitutive therapy in that scope. The patient was examined again in April 2005 (31 months after the surgery). MRI image of the pituitary gland was not changed compared to previous examinations.
Figure 4A.
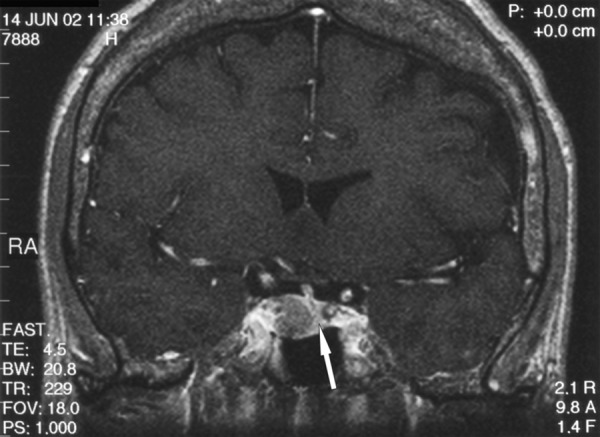
A T1-weighted image after contrast medium administration, coronal plane: on the left side of the sella there is the compressed pituitary gland visible (arrow), connected to the hypothalamic infundibulum shifted to the left.
Figure 4B.
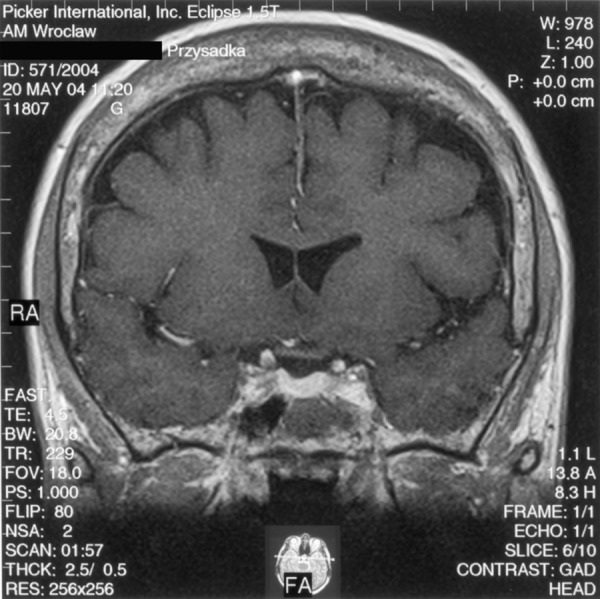
The same patient – condition approx. 1.5 years after the pituitary tumour resection. A T1-weighted image after contrast medium administration, coronal plane: normal pituitary gland visible, uniformly enhanced.
Discussion
Pituitary tumours, especially macroadenomas, cause compression, displacement and deformation of the gland. Level of the pituitary displacement and deformation depends on size, localisation and expansion of the tumour [29]. It was observed that majority (91% of cases) of adenomas showing an suprasellar growth cause the pituitary displacement towards the suprasellar space. Adenomas with diameter of over 2 cm are in 78% of cases a cause for major pituitary deformation. The gland assumes then a characteristic, sickle shape. If a tumour occupies initially a supra- or infrasellar area (sphenoid sinus) and does not penetrate the intersellar area, it happens that the pituitary gland maintains an almost regular shape and position [30]. Compression on the gland is manifested by various level of its function disorder [17,29]. Evaluation of compressed pituitary with MRI before a scheduled surgery may prevent its intrasurgical damage, and therefore reduce the risk of post-surgical insufficiency of the gland [15,29].
Commonly, the pituitary gland becomes visible better in MRI performed after the tumour resection, when released compression allows return to its physiological location. The gland becomes “reexpanded” and rarely it may even reassume a regular shape and size. That situation is observed more frequently in young patients and with relatively small tumours [28,29].
After the tumour is removed, the post-resection defect becomes filled with implanted materials. Therefore, resorption time of the filling material and its quantity are additional factors influencing the process of post-surgical repositioning and reexpansion of the pituitary gland [29].
In majority of cases, the pituitary gland does not return to its normal shape and volume after a surgery, and that condition is reflected by its function impairment. According to Gołkowski et al. [17], post-surgical pituitary insufficiency was observed in approx. 31% of patients, who had a normal endocrine function of the gland before the surgery. And in approx. 28% of patients, intensification of previously diagnosed pre-surgical insufficiency was observed. Most commonly the hormone deficiency was associated with ACTH, and the least commonly – with prolactine. Patients with macroadenomas dominated in a group of patients with postsurgically formed or intensified pituitary insufficiency. A risk of pituitary function impairment is higher in case of patients who had had an irregular pituitary function before a tumour resection, and in patients with large tumours [17].
In available literature, radiologists [29] pay attention to post-surgical presentation of the pituitary gland in MRI and endocrinologists [17] focus on evaluation of the gland’s hormonal function and appropriate therapy of possible post-surgical insufficiency. No analysis of a possible relation between the MRI presentation of the gland and its hormonal function has been attempted previously. We have made that attempt in this study.
In the analysed material of 124 cases, the pituitary gland was visible in the MRI in 110 patients after surgical removal of pituitary tumour. Almost regular size and shape was visualised in 11 patients, which constituted only 10% of all cases in which presence of the pituitary gland was visualised in MRI. Among 11 patients with normal MRI presentation of the gland, 8 showed normal pituitary function, and in 3 patients a partial anterior pituitary insufficiency within one axis was diagnosed. Those results are consistent with other literature data indicating that the pituitary gland rarely regains its normal configuration after a surgery [28,29]. A fact should be noted that not all patients presenting normal MRI presentation of the pituitary gland have normal hormonal function of the organ preserved (case 4 is an example).
In 99 patients, constituting as much as 90% of cases, MRI indicated existence of only a fragment of a normal gland. That presentation may suggest existence of other pituitary function disorders. It was shown than 46 patients of that group had completely normal pituitary function and did not require any substitutive therapy (cases 1 and 2). Only 48 patients in whome existence of fragment of a normal pituitary was found, had partial impairment of the gland endocrine function adequate to the MRI presentation of the organ. Five patients had a complete failure of the anterior and posterior pituitary (panhypopituitaryzm), despite the fact that MRI indicated existence of a spared fragment of a normal gland. In a group of 14 patients in which no pituitary gland could be visualised in MRI, none showed normal function of the organ.
Statistical analysis showed that there is no strict relationship between post-surgical presentation of the pituitary gland and its hormonal function.
Conclusions
Post-surgical MRI presentation of the pituitary gland does not have a strict relationship with the gland’s hormonal function. Statistically significant difference between MRI presentation of the pituitary gland and endocrinological evaluation of its function was found in the whole group. Therefore, evaluation of surgery effectiveness, and effect on post-surgical pituitary function must be based on both imaging results and a hormonal profile. It should be noted that post-surgical visualisation of a partial pituitary in MRI does not exclude the gland’s normal function, and vice versa – determination of post-surgical presence of a properly sized gland does not necessarily mean its normal function and lack of necessity for hormonal substitution in a particular patient.
References:
- 1.Evanson J. Imaging the pituitary gland. Imaging. 2002;14:93–102. [Google Scholar]
- 2.Yoon PH, Kim DI, Jeon P, et al. Pituitary adenomas: early postoperative MR imaging after transsphenoidal resection. AJNR. 2001;22:1097–104. [PMC free article] [PubMed] [Google Scholar]
- 3.Bolanowski M. Guzy przysadki – możliwości diagnostyczne i terapeutyczne. Terapia. 2001;102:37–39. [Google Scholar]
- 4.Zhang X, Horwitz GA, Heaney AP, et al. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. 1999;84:761–67. doi: 10.1210/jcem.84.2.5432. [DOI] [PubMed] [Google Scholar]
- 5.Zgliczyński S, Brzezińska A. Rozpoznawanie i leczenie guzów przysadki. Med Prakt. 1995;3:69–71. [Google Scholar]
- 6.Bladowska J, Sokolska V, Czapiga E, et al. Postępy w diagnostyce obrazowej przysadki mózgowej i okolicy okołosiodłowej. Adv Clin Exp Med. 2004;13:709–17. [Google Scholar]
- 7.Keogh BP. Recent advances in neuroendocrine imaging. Curr Opin Endocrinol Diabetes Obes. 2008;15(4):371–75. doi: 10.1097/MED.0b013e328305085b. [DOI] [PubMed] [Google Scholar]
- 8.Portman O, Flemming S, Cox JP, et al. Magnetic resonance imaging of the normal pituitary gland using ultrashort TR (UTE) pulse sequences (REV 1.0) Neuroradiology. 2008;50(3):213–20. doi: 10.1007/s00234-007-0329-7. [DOI] [PubMed] [Google Scholar]
- 9.Kozic D, Medic-Stojanowska M, Ostojnic J, et al. Application of MR Spectroscopy and treatment approaches in a patient with extrapituitary growth hormone secreting macroadenoma. Neuro Endocrinol Lett. 2007;28(5):560–64. [PubMed] [Google Scholar]
- 10.Chernov MF, Kawamata T, Amano K, et al. Possible role of single-voxel (1)H- MRS in differential diagnosis of suprasellar tumors. J Neurooncol. 2008 doi: 10.1007/s11060-008-9698-y. (Epub adead of print) [DOI] [PubMed] [Google Scholar]
- 11.Rennert J, Doerfler A. Imaging of sellar and parasellar lesions. Clin Neurol Neurosurg. 2007;109:111–24. doi: 10.1016/j.clineuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Bonneville JF. Pituitary adenomas: value of MR imaging. J Radiol. 2000;81:939–42. [PubMed] [Google Scholar]
- 13.Rodriguez O, Mateos B, de la Pedraja R, et al. Postoperative follow-up of pituitary adenomas after transsphenoidal resection: MRI and clinical correlation. Neuroradiology. 1996;38:747–54. doi: 10.1007/s002340050341. [DOI] [PubMed] [Google Scholar]
- 14.Edal AL, Skjodt K, Nepper-Rasmussen HJ. SIPAP – a new MR classification for pituitary adenomas. Acta Radiol. 1997;38:30–36. doi: 10.1080/02841859709171238. [DOI] [PubMed] [Google Scholar]
- 15.Hald JK, Nakstad PH, Kollevold T, et al. MR imaging of pituitary macroadenomas before and after transsphenoidal surgery. Acta Radiol. 1992;33:396–99. [PubMed] [Google Scholar]
- 16.Hardy J, Czepko R. Guzy przysadki. Zarys neurochirurgii. PZWL; Warszawa: 1999. pp. 83–107. [Google Scholar]
- 17.Gołkowski F, Trofimiuk M, Huszno B, et al. Ocena czynności hormonalnej przysadki po operacji gruczolaka z dojścia przez zatokę klinową. Przeg Lek. 2001;58:825–27. [PubMed] [Google Scholar]
- 18.Bonicki W, Michalik R, Krajewski R, et al. Zastosowanie technik chirurgii podstawy czaszki w leczeniu gruczolaków przysadki. Neurol Neurochir Pol. 2002;36:1121–33. [PubMed] [Google Scholar]
- 19.Czepko R, Danilewicz B, Anilewicz M, et al. Bezpośrednie wyniki leczenia gruczolaków przysadki metodą przezczaszkową i przezklinową. Przeg Lek. 1999;56:638–43. [PubMed] [Google Scholar]
- 20.Jho H-D, Carrau RL, Ko Y, et al. Endoscopic pituitary surgery: an early experience. Surg Neurol. 1997;47:213–23. doi: 10.1016/s0090-3019(96)00452-1. [DOI] [PubMed] [Google Scholar]
- 21.Patterson RH. The role of transcranial surgery in the management of pituitary adenoma. Acta Neurochir (Suppl) 1996;65:16–17. doi: 10.1007/978-3-7091-9450-8_6. [DOI] [PubMed] [Google Scholar]
- 22.Kassam AB, Gardner PA, Snyderman CH, et al. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 2008;108(4):715–28. doi: 10.3171/JNS/2008/108/4/0715. [DOI] [PubMed] [Google Scholar]
- 23.Liebert W, Szyfter W, Tokarz F, et al. Chirurgiczne leczenie gruczolaków przysadki mózgowej z dojścia przez zatokę klinową. Otolaryng Pol. 1994;48:260–65. [PubMed] [Google Scholar]
- 24.Sheehan MT, Atkinson JLD, Kasperbauer JL, et al. Preliminary comparison of the endoscopic transnasal vs the sublabial transseptal approach for clinically nonfunctioning pituitary macroadenomas. Mayo Clinic Proc. 1999;74:661–70. doi: 10.4065/74.7.661. [DOI] [PubMed] [Google Scholar]
- 25.Bolanowski M. Treatment of pituitary tumours. W: International Intensive Course in Oncology ERASMUS 2000 “Recent advances in the knowledge of cancer ”; Wrocław. 2000. pp. 49–54. [Google Scholar]
- 26.Wilson CHB. Surgical management of pituitary tumors. J Clin Endocrinol Metab. 1997;82:2381–85. doi: 10.1210/jcem.82.8.4188. [DOI] [PubMed] [Google Scholar]
- 27.Fahlbush R, Hadani M, Perrin G, et al. Neurosurgical treatment of pituitary adenomas: operative and/or medical therapy. Acta Neurochir. 1995;133:211–38. [Google Scholar]
- 28.Steiner E, Knosp E, Herold ChJ, et al. Pituitary adenomas: Findings of postoperative MR imaging. Radiology. 1992;185:521–27. doi: 10.1148/radiology.185.2.1410366. [DOI] [PubMed] [Google Scholar]
- 29.Steiner E, Math G, Knosp E, et al. MR-appearance of the pituitary gland before and after resection of pituitary macroadenomas. Clin Radiol. 1994;49:524–30. doi: 10.1016/s0009-9260(05)82929-0. [DOI] [PubMed] [Google Scholar]
- 30.Kurowska M, Tarach JS, Zgliczyński W, et al. Acromegaly in a patient with normal pituitary gland and somatototropic adenoma located in the sphenoid sinus. Endokrynol Pol. 2008;59(4):348–51. [PubMed] [Google Scholar]


