Summary
Background:
Great variability in the vasculature of the abdominal organs makes the pre-operative evaluation of arterial anatomical conditions extremely important and helpful. The aim of our study was to establish the prevalence of anatomical variations of the arteries branching from the abdominal aorta and to compare the results with the ones presented in the literature.
Material/Methods:
The material included computed tomography angiographies (CTA) of 201 patients (91 women and 110 men) performed between September 2007 and December 2008. The CTA examinations were conducted with a 64-detector CT scanner at the Department of Radiology of University Hospital in Wrocław. Images were obtained during the arterial phase and were analyzed for the presence of potential anomalies of the branches of the abdominal aorta.
Results:
In 88 patients (43.8%), there were anatomical variations of the arteries branching from the abdominal aorta. Variations of the renal arteries were observed in 83 (41.3%) patients, anomalies of the celiac trunk in 9 patients (4.5%), including variations of the superior mesenteric artery in 4 (2%) patients. No anatomical anomalies of the inferior mesenteric artery were shown in this study.
The most frequent anomaly of the renal vasculature was the presence of at least one additional renal artery, observed in 65 (32.3%) patients. This concerned the inferior renal polar artery mainly – in 30 (14.9%) patients. Presence of bilateral additional renal arteries was visualized in 10% (20/201) of the cases. The most frequent anomalies of the celiac trunk were the celiacmesenteric trunk (in 3 patients – 1.5%) and the hepatosplenic trunk (in 3 patients – 1.5%). The celiac-mesenteric trunk was also the most frequent variation of the superior mesenteric artery in our material.
Conclusions:
A large part of population – 43.8% of our patients – demonstrated variations of arteries branching from the abdominal aorta. The anomalies were significantly more often found within the renal arteries than within the celiac trunk or the superior mesenteric artery. Sixty-four detector CTA reveals a high sensitivity in the detection of anomalies of the arteries branching from the abdominal aorta.
Keywords: computed tomography angiography, abdominal aorta, anatomic variations
Background
Evaluation of arteries branching from the abdominal aorta (the level of their divergence, presence of atypical variants of a common origin of arteries or presence of additional arteries) plays an important role in the diagnostics of many abdominal disorders. Nowadays, it is possible owing to a minimally invasive examination – the multidetector computed tomography angiography (CTA). Apart from the standard evaluation of transverse sections of the vessels, a very important element of the CTA examination is the three-dimensional reconstruction performed with submillimeter resolution. This imaging technique is particularly important during patient’s qualification for endovascular or angiosurgical treatment, as well as during follow-up after the procedure. Thanks to the short time of the examination, the Multidetector Computed Tomography (MDCT) proves to be useful in emergency cases as well as in a quick a assessment of the vascular axes and for the purposes of immediate surgeries or endovascular interventions.
Laparoscopic surgery allows for performance of minimally invasive procedures within the abdominal cavity. However, because of a limited visualization within the operation, a very precise surgery planning is required. CT examination is perfect for that. It allows for avoidance of some serious intraoperative problems, resulting from the lacking knowledge on anatomical relations of the arteries, which is especially important in case of rarely observed variants of vasculature of the internal organs.
The aim of our study was to establish, on the basis of the 64-detector CTA examination, the prevalence of variations of the main arteries branching from the abdominal aorta and to compare the results with the ones presented in the literature.
Material and Methods
The analyzed material included 201 computed tomography angiographies (CTA) performed with a 64-detector scanner after contrast medium administration in 91 women (45%) and 110 men (55%) in the age of 2–86 years. Their mean age was 58.4. In all cases, the examination site included the whole abdominal aorta. The tests were performed between September 2007 and December 2008, at the Department of General Radiology, Interventional Radiology and Neuroradiology of Medical University in Wrocław. CT examinations were performed in emergency and elective cases, for various medical indications.
The examinations were carried out with the 64-detector CT scanner, LightSpeed VCT (GE). The examined area stretched from the diaphragm domes to the L4 vertebral body or lower. In all cases, the slice thickness was 0.67 mm, the pitch amounted to 1.3, and the average gantry rotation time to 0.5 sec. Contrast agent bolus was administrated using an automatic syringe. Vascular access was obtained with a 18G or a 20G needle inserted into the ulnar vein. The volume of the highly-iodinated contrast agent ranged from 80 to 130 ml, depending on the patient’s body mass. The rate of the contrast agent administration was 3.0–4.5 ml/s. Contrast medium administration was followed by an injection of 40 ml of a physiological salt solution, so called wash-out bolus. CT examinations were performed according to two protocols: a regular CTA (one-phase examination) or an abdominal CT (multi-phase examination), depending on indications. The multi-phase examinations evaluated the early arterial phase only (after 30 seconds following contrast agent administration). The obtained scans were analyzed using Vessel Analysis (GE) software and Advantage Workstation 4.4. Image postprocessing techniques involved two- and three-dimensional reconstructions (Maximum Intensity Projection – MIP; Volume Rendering – VR). Only those images that were free from artifacts – i.e. where the arterial phase was appropriately visualized and an adequate and comprehensive evaluation of the aortic branches was possible – were used for the analysis.
Results
Renal arteries
The total number of the analyzed renal vessels amounted to 402 in 201 patients. In 118/201 patients (58.7%) and in 292/402 kidneys (72.6%), a typical renal vasculature was found, meaning: each kidney was supplied by a single renal artery (72.6%) (Table 1).
Table 1.
The prevalence of anatomical variants of the renal arteries, N=402.
| Variant | n | Right renal artery | Left renal artery |
|---|---|---|---|
| Typical single renal artery | 292 (72.6%) | 153 | 139 |
| Early branching | 27 (6.7%) | 15 | 12 |
| Additional renal arteries: | |||
| Additional hilar artery | 28 (7.0%) | 13 | 15 |
| Superior renal polar artery | 19 (4.7%) | 6 | 13 |
| Inferior renal polar artery | 35 (8.7%) | 13 | 22 |
| Inferior and superior renal polar arteries | 1 (0.3%) | 1 | 0 |
Renal vasculature anomalies were observed in 83/201 patients (41.3%), and within 110/402 kidneys (27.4%). The revealed anomalies were divided into 2 groups: early branching of the renal artery (within 2 cm from the orifice of the renal artery at the aorta; normally observed at the level of the renal hilum) and the presence of additional arteries. Among the additional arteries, 2 groups were identified: renal polar arteries (superior and inferior) and hilar arteries.
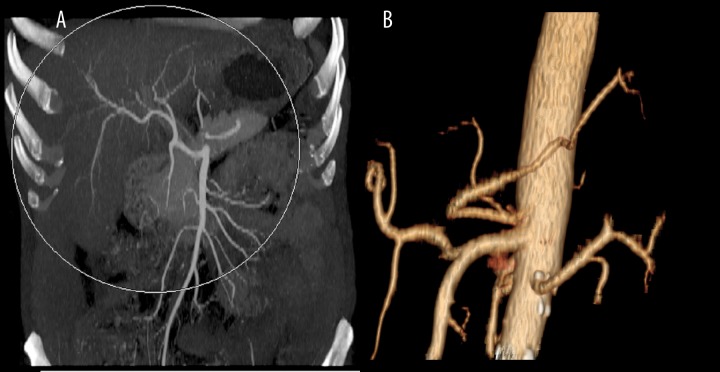
Early branching of the renal artery was observed in 24 (11.9%) patients, in 27 kidneys (6.7%) (Figure 1).
Figure 1.
Early branching of left renal artery, VR reconstruction.
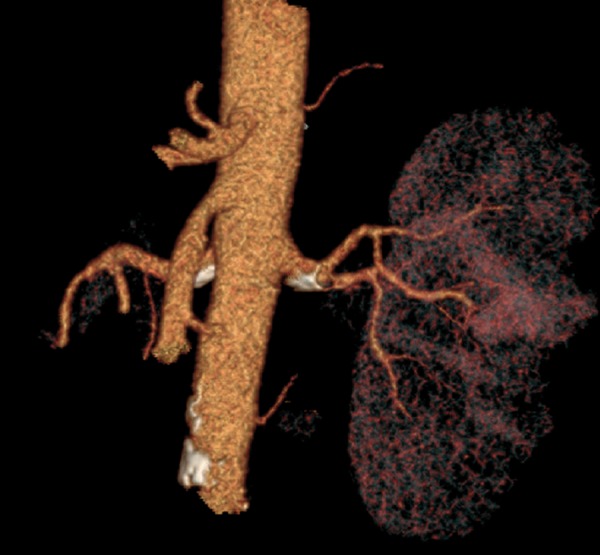
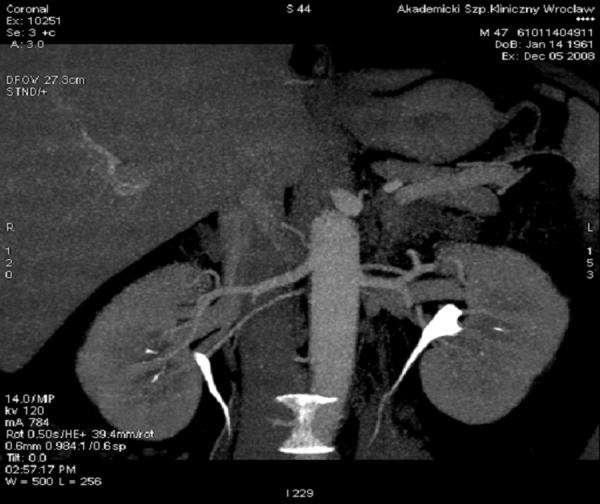
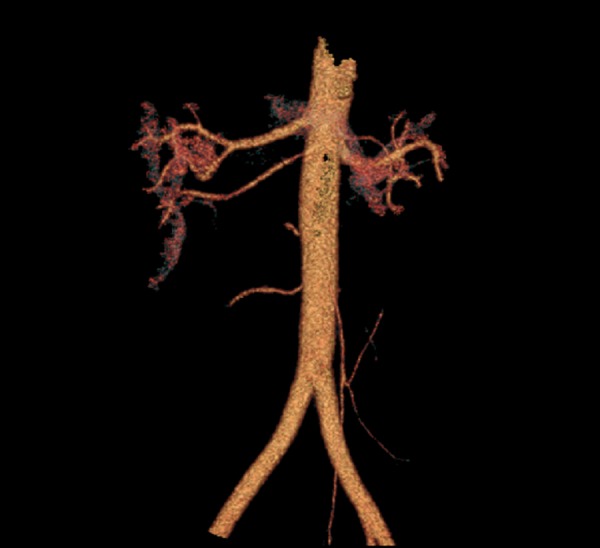
The presence of at least one additional renal artery was observed in 65 (32.3%) patients and in 83 (20.6%) kidneys. The most frequently observed anomaly in our study, was the additional inferior renal polar artery – in 35 kidneys (8.7%) (Figures 2, 3). Second the most frequent anomaly was the presence of two hilar arteries – found in 28 kidneys (7%). In 19 kidneys (4.7%), there was an additional superior renal polar artery, and in one kidney (0.3%) there was both – the superior and the inferior renal polar artery present simultaneously (Figure 4).
Figure 2.
Additional renal arteries: inferior right and superior left crossing with the proper renal artery and reaching the inferior pole of the left kidney, MIP reconstruction.
Figure 3.
Additional inferior renal polar artery, VR reconstruction.
Figure 4.
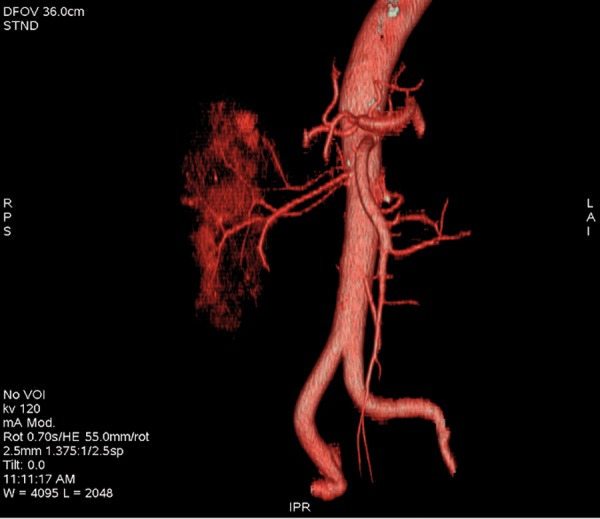
Additional renal polar arteries, inferior and superior, status post left kidney removal, VR reconstruction.
Right kidney vasculature anomalies were found in 23.9% (48/201) of patients, while the left-side anomalies in 30.8% (62/201) of patients. This was a statistically significant difference, assuming a significance level of p<0.1 (p= 0.0824 in McNemar’s test).
Bilateral coexistence of additional arteries was observed in 20/201 (10%) cases.
Variants of renal vasculature (additional arteries and early branching) were observed in 32 women and 51 men, i.e. 35.2% of women (32/91) and 46.4% of men (51/110). However, the difference was not statistically significant (p=0.14, Chi2 test).
Celiac trunk and superior mesenteric artery
Among 201 patients, a typical variant of the celiac trunk anomaly was found in 192 (95.5%) patients (Table 2). The typical variant was defined as: the vascular trunk located approx. 1 cm above the superior mesenteric artery and splitting into 3 branches: left gastric artery, common hepatic artery and splenic artery (Figure 5).
Table 2.
Prevalence of anatomical variants of the celiac trunk, N=201.
| Variant | Number | Rate |
|---|---|---|
| Typical celiac trunk (Figure 5) | 192 | 95.5% |
| Celiac-mesenteric trunk (Figure 6) (common origin of the mesenteric artery and the celiac trunk) | 3 | 1.5% |
| Splenohepatic trunk (Figure 7) | 3 | 1.5% |
| Posterosuperior pancreaticoduodenal artery (celiac trunk with 4 branches, 3 typical ones and an additional posterosuperior pancreaticoduodenal artery) | 2 | 1.0% |
| Gastrosplenic trunk (Figure 8) | 1 | 0.5% |
Figure 5.
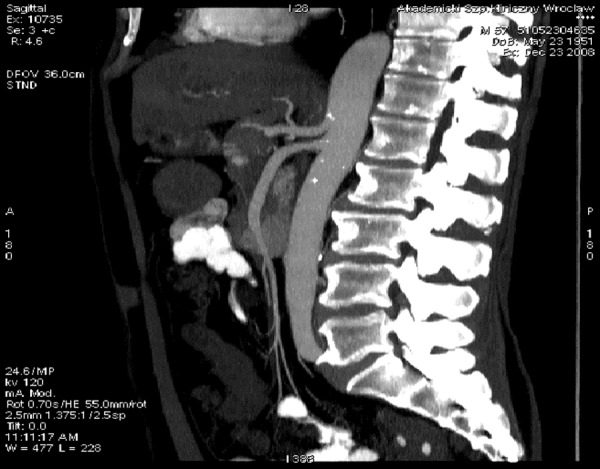
Celiac trunk and superior mesenteric artery, typical variant, MIP reconstruction.
Common origin of the celiac trunk and of the superior mesenteric artery – the celiac-mesenteric trunk – was observed in 3 patients (1.5%) (Figure 6). An independent orifice of the left gastric artery (splenohepatic trunk) was also observed in 3 patients (1.5%) (Figure 7). A trunk with an additional fourth ramification (posterosuperior pancreaticoduodenal artery) was found in 2 patients (1%). Simultaneous presence of the gastrosplenic trunk and the hepatomesenteric trunk was found in 1 patient (0.5%). Variants of the celiac trunk were observed in 5/91 (5.5%) women and in 4/110 men (3.6%), i.e. in 9/201 patients in total (4.5%).
Figure 6.
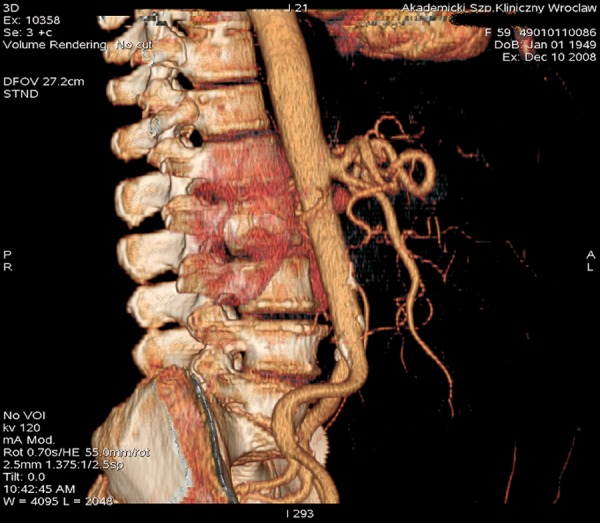
Celiac-mesenteric trunk – common origin of the superior mesenteric artery and the celiac trunk. VR reconstruction.
Figure 7.
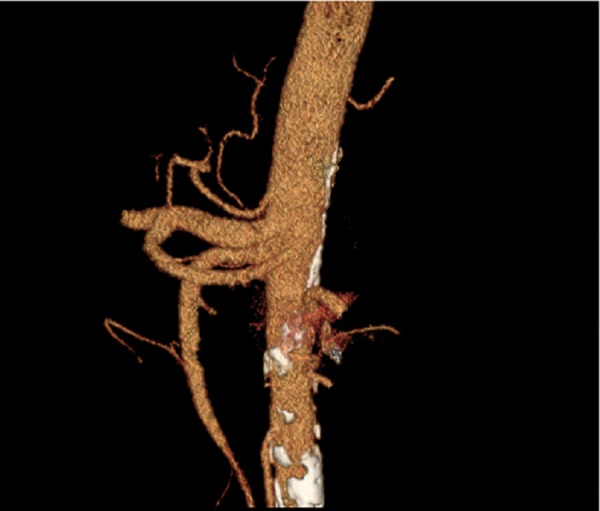
Splenohepatic trunk with an independent origin of the left gastric artery. VR reconstruction.
Superior mesenteric artery branches typically from the anterior aortal wall, approx. 1 cm below the origin of the celiac trunk. Variants of this artery orifice were found in 4 patients (2%) (Table 3). In 3 patients (1.5%), the above mentioned celiac-mesenteric trunk was observed, meaning a common origin of the superior mesenteric artery and of the celiac trunk (Figure 6). The hepatomesenteric trunk was observed in 1 patient (0.5%) (Figure 8).
Table 3.
Prevalence of anatomical variants of the superior mesenteric artery, N=201.
| Variant | Number | Percent |
|---|---|---|
| Common origin of superior mesenteric artery and celiac trunk | 3 | 1.5% |
| Common origin of superior mesenteric artery and hepatic artery | 1 | 0.5% |
Figure 8.
Simultaneous anomaly of the celiac trunk and of the superior mesenteric artery. (A) Common hepatic artery as a ramification of the superior mesenteric artery, MIP reconstruction. (B) Gastrosplenic trunk, VR reconstruction.
Inferior mesenteric artery
The artery branches off at the level of the III lumbar vertebral body. In our study, no anomalies of the orifice of the inferior mesenteric artery were found. In all patients, it was a single vessel branching off independently in its typical location.
Discussion
Multi-detector computed tomography (MDCT) and especially the fast 64-row tomography with contrast medium administration (CTA), allows for the performance of examinations with isotropic resolution. Thanks to that, the multi-plane and multi-volume imaging with a resolution of less than 1 mm, covering large anatomical areas, can be performed within a few seconds [1]. As far as the evaluation of the aorta with its branches is concerned, the possibility of a quick visualization of large sites with CT, has substantially improved the vascular diagnostics and the patient’s comfort, as well as reduced the number of examination failures resulting from, for example, motion artifacts [2]. Other advantages of the CTA examination include imaging of the arterial phase of contrast enhancement along with visualization of the vascular wall, lumen and adjacent tissues, and the possibility of presenting the anatomy of the vessels from every angle, using data from a single acquisition only.
Despite many advantages, the CTA examination has also its downsides. The most significant is the exposition to X-rays. Allergic reaction to iodine or other ingredients of the contrast medium is also possible (however, there are alternative – gadolinium-based – contrast agents, which can be administered in patients with iodine allergy [3]). Nephrotoxic properties of contrast agents should be remembered as well.
The application of examination results obtained with the 64-detector CT enabled us to conduct a profound analysis of anatomical relations of the arteries branching off from the abdominal aorta, and to distinguish a few types of such anomalies, and finally, to determine their prevalence in the study population, which was the main purpose of this study.
When evaluating vessels, we took into consideration the arterial phase of contrast enhancement or the first phase of a multi-phase examination, which corresponded to the arterial phase. The analyzed CT examinations were performed for different medical indications, e.g. in case of suspected or monitored aortic aneurysms or aortic dissection, to assess the morphology of the anatomical structures before qualifying the patient for a stent-graft or prosthesis implantation, to qualify the patient for by-pass surgery, or in case of suspected vascular pathologies and other abdominal and pelvic disorders.
The selected group of 201 patients seems to be representative and large enough to meet statistical purposes. Owing to the large number of the analyzed examinations, it was possible to find many types of anomalies of the abdominal aorta ramifications, including very rare anatomical variants.
It should be noticed that in our study, only the main arteries originating from the abdominal aorta were subjected to the analysis – celiac trunk and its three main branches: common hepatic, left gastric and splenic artery, as well as the superior mesenteric artery, renal arteries and inferior mesenteric artery. The much smaller aortic ramifications, such as: inferior phrenic, adrenal, lumbar and median sacral arteries, were not analyzed. They were often visualized in the analyzed examinations; however, their reliable evaluation was not possible in the whole study population. Moreover, the inter-variability of these small arteries results in a large number of anatomical variants [4], and thus should be, in our opinion, assessed for every patient individually, when such a clinical need arises.
The main observation of our study was that developmental abnormalities of arteries originating from the abdominal aorta are frequent, and amounting to 43.8% of the cases.
Renal arteries
Among all abdominal aorta ramifications, renal arteries show the highest anatomical variability. In our study group, different variants of the renal arteries were significantly more frequent (41.3%) than the variants of the celiac trunk (4.5% of patients) or of the superior mesenteric artery (2%) (p<0.001,McNemar’s B/C test).
There are a few theories about the embryonic origin of the renal vasculature [5,6]. Vasculature development is strictly dependent on the cephalic migration of kidneys during embryogenesis. If their final location is atypical, renal vasculature may also be atypical, which can be explained by arterial vasculature adjustments to the location of the kidneys [7]. In our study group, the atypical kidney location was not observed, and thus the additional renal arteries were probably the remains of the mesonephric blood vessels, giving rise to the renal artery [4]. During embryogenesis, there exists a genitourinary arterial system composed of a few mesonephric arteries supplying kidneys, adrenal glands and gonads. In the course of the embryonic development, the number of the kidney-supplying mesonephric arteries dwindles down to one, with the rest undergoing atrophy. Any abnormalities of this process may lead to a higher number of renal arteries [8].
The number of additional renal arteries ranges from 2 to 4 [9–11]. Their names may vary in the English literature [7,12]. In the Polish nomenclature, they are called ‘dodatkowe’ and, depending on the supplied renal region: biegunowe górne, biegunowe dolne and wnękowe [7,13].
Basing on the data from Table 4, it may be concluded that the results of our study are not significantly different from those presented in the literature. In our opinion, there are three factors underlying the differences: number of the examined patients, study method and the race or the region of the world in which the study was conducted. In our opinion, a great variability and frequency of different anatomical variants of renal vasculature demands studies in much larger populations (minimally 1000 people, on average), to obtain repeatable and similar results. The study groups in the quoted papers were rarely larger than our study population [3,7,10]. The above mentioned studies were conducted in Asia, Africa or South America, and thus the race factor can be the underlying cause of the observed differences as well. The Mongoid and the Negroid race has a different genotype than the Caucasian race, analyzed in our study. This can cause differences in renal vasculature. The influence of the race on renal vasculature was well described in the paper by Satyapal et al., showing a significantly higher rate of additional renal arteries in Africans (37.1%) and Caucasians (35.3%) than in Indians (17.4%) or Mestizos (18.5%) [7].
Table 4.
The prevalence of renal artery anomalies – review of literature.
| Author | Number of patients | Anomaly rate | Examination type | Country | Comments | |||
|---|---|---|---|---|---|---|---|---|
| Early branching | Additional renal arteries | |||||||
| Patients | Kidneys | Patients | Kidneys | |||||
| Cicekcibasi et al. [14] | 90 | X | X | 25 | X | A | Turkey | |
| Khamanarong et al. [10] | 267 | X | X | 18 | X | A | Thailand | |
| Kurcz et al. [3] | 216 | 9.3 | X | 32.9 | X | T | Poland | 10-row CT |
| Patil et al. [16] | 102 | X | 10 | X | 25.5 | T | USA | No information on scanner resolution |
| Saldarriaga et al. [5] | 196 | X | 12.95 | X | 24.9 | A | Columbia | |
| Sampaio et al. [15] | 70 | 42.8% of patients*, 25.7% of kidneys* | A | Brazil | * Total number of all anomalies | |||
| Satyapal et al. [7] | (180 kidneys) | X | X | X | 27.7 | Angiography | RPA | 130 angiograms+180 kidneys in autopsy examination |
| Tarzamni et al. [18] | 117 | 36 | 21.4 | 40.2 | 26 | T | Iran | 64-row CT |
| Bordei et al. [9] | (272 kidneys) | X | X | X | 21 | A | Romania | |
| Kawamoto et al. [19] | 74 | X | 9.5 | X | 24 | T | USA | 4-row CT |
| Our results | 201 | 12 | 6.7 | 32.3 | 20.7 | T | Poland | 64-row CT |
| Mean | 148 | 19 | 12 | 30 | 24 | – | – | – |
A – autopsy examination; T – computed tomography; X – not assessed.
Study method is also important, and in case of studies based on CTs – scanner resolution is of significance. It should be noticed that the precision of our method is not worse than the one of autopsy-based studies [10,14,15].
In our study, anomalies of the renal arteries were more frequent on the left (30.8% of left kidneys – 62/201) than on the right side (23.9% of right kidneys – 48/201). This was a statistically significant difference, with the level of significance of p<0.1 (p=0.0824, McNemar’s B/C test). The majority of authors presented similar results [5,7,16,15]. Other authors: Cicekcibasi et al. [14] and Ayuso et al.[17] described an opposite laterality of anomalies; this was also the case in the study by Tarzamni et al., who observed 32.47% of right-sided anomalies and 17.09% of left-sided anomalies, p=0.01 [18]. On the basis of our results and the data by other authors, it is now impossible to determinate a significantly dominant side; however, it is of a great clinical importance, because the left kidney is easier to collect laparoscopically (for transplantation purposes), due to the longer renal vein and to more convenient anatomical conditions [19].
In our study, bilateral anomalies were observed in 10% (20/201) of patients. In the literature, the prevalence of bilateral anomalies of the renal arteries ranged between 3.1% and 12% [5,3,15,18,20] (Table 5).
Table 5.
Prevalence of bilateral anomalies of renal arteries in the literature.
Early renal artery branching, i.e. before entering the renal hilum [4,3] was observed in our study in 6.7% (27/402) of kidneys and 12% of patients (24/201). Data of other authors were as follows: 10% of kidneys [16], 11.8% of kidneys [17], 12.95% of kidneys [5] and 32.9% of patients [3]. This kind of anomaly may cause some complications during renal transplant collection, because the first 2 centimeters of the donor renal artery are used for anastomosis with the recipient’s aorta [5,18]. Moreover, early renal artery branching or the presence of additional renal arteries constitute the exclusion criteria from laparoscopic nephrectomy [5].
In our study, one in 402 kidneys (0.25%) was supplied by three arteries simultaneously: hilar, renal polar superior and renal polar inferior artery. Such a rare anomaly was also found in other studies. Kurcz et al. found three unilateral renal arteries in 3 patients (1.4% – 3/216) and three arteries on one side and two arteries on the second side in one patient [3]. Bordei et al. revealed the presence of three arteries on one side and two arteries on the second side, in one autopsy examination [9]. In our study, there were no cases of the kidney supplied by two hilar arteries and by one polar artery, as it was the case in the study by Khamanarong et al. [10]. We did not find any cases of the kidney supplied by 4 arteries either, as it was observed by Pollak et al. in their study including 400 autopsy examinations [21]. This is most probably because of a small sample size of our population and a great variability of anomalies of the renal arteries.
The prevalence of the superior renal polar artery accompanied by the hilar renal artery ranges, according to the study, from 4.3 to 7% of kidneys [5,10] or 3.3–7.5% of cases [3,14]. For the inferior renal polar artery, this is: 3–10.8% of kidneys [5,10] and about 10% of patients [14, 3]. Two hilar arteries were observed in 7–12.1% of kidneys [5, 10] and 11.1 in 19% of patients [3,14]. Such discrepancies between the results may have various causes: size of the study group, study method (autopsy examination [5,10,14], CT examination [3]) and the race of the examined patients [5]. It is worth noticing that the results of our study are similar to the results of the quoted papers, including the autopsy-based ones [3,5,7,10,14–16,18,20]. Sometimes, our study revealed even more anomalies, e.g. higher prevalence of renal artery early branching [3] or of additional renal arteries [10,14].
In case of kidneys with arterial vasculature anomalies, the abnormalities were often present in both kidneys simultaneously (29% – 20/70 of patients). In our study population, this was three times more frequent in men than in women. No relevant data were found in the studies by other authors.
Renal vasculature anomalies were observed in 35.2% (32/91) of women and 46.4% (51/110) of men. The difference was no statistically significant (p=0.14). Satyapal et al. found that the prevalence of additional renal arteries is statistically significantly higher in men than in women [7]. Other studies did not show statistically significant differences between genders with respect to the prevalence of renal artery variants [15,18]. Cicekcibasi et al. showed that vasculature variants were more frequent in men, which was similar to our results [14].
Celiac trunk and superior mesenteric artery
Celiac trunk is the most superior branch of all three single branches of the abdominal aorta (Figure 5). It splits into 3 branches: left gastric, common hepatic and splenic artery. Normally, the left gastric artery arises just before the orifice of the common hepatic and the splenic artery, but it may also have a common origin with those vessels or it may be a splenic artery ramification. In our study, the above mentioned different types of the inferior gastric artery branching were classified as a typical variant of the celiac trunk.
Typical celiac trunk division into three arteries was observed by us in 192 patients (95.5%) (Figure 5). Other authors, analyzing larger populations, reported a much lower prevalence of the typical celiac trunk division: in 378 patients (72.1%) and 875 patients (89.8%) [22,23]. However, the common origin of the celiac trunk and of the superior mesenteric artery was observed in 1.5%, of our patient, which is more frequent than in the literature – 0.4% [22] (Figure 6). In our study, the hepatosplenic trunk was present in 1.5% of the patients (Figure 7), and the gastrosplenic trunk in 0.5% (Figure 8). This was much less frequent than in other studies: 2.7–4.4% and 3.4–4.0%, respectively [22,23].
In the available literature, we did not find any variant which would correspond to the anomaly observed by us in two patients, i.e. celiac trunk with the fourth ramification – the posterosuperior pancreaticoduodenal artery. On the other hand, we did not find in our study any variants observed by Iezzi et al., such as the gastrohepatic trunk or the absence of the celiac trunk. This was probably due to the rareness of such variants and a larger study sample in report by Iezzi et al. [22].
Conclusions
Developmental anomalies of the main arteries branching from the abdominal aorta were frequent seen in our study – in 43.8% of patients. They were mostly concerning renal arteries and revealing a great variability of variants, with the most common one being the presence of an additional inferior renal polar artery. The study showed a statistically significantly higher number of vasculature anomalies of the left kidney in comparison to the right kidney. In our study group, renal vasculature anomalies were clearly more frequent in men, but the difference was not statistically significant.
Celiac trunk and superior mesenteric artery showed less anatomical variabilities and the prevalence of these arterial anomalies was low in our study group – 4.5% and 2% of the patients, respectively. No anomalies of the inferior mesenteric artery branching were observed.
Correlation between our study results and the literature data, including data from autopsy examinations, confirms the sensitivity and precision of the presented diagnostic method, i.e. of the multi-detector computed tomography. The high rate of atypical anatomical variants of the arteries branching from the abdominal aorta, observed in our study, speaks for the necessity of presurgical vascular diagnostics in this respect. Evaluation of the renal vasculature seems especially important due to the frequency of the anomalies.
References:
- 1.Prokop M, Galanski M. Spiralna i wielorzędowa tomografia komputerowa człowieka. Medipage; Warszawa: 2007. pp. 812–907. chapter 1: 1–25, chapter 4; [Google Scholar]
- 2.Creager MA, Zadu VJ, Loscalzo J. Choroby naczyń. Czelej; Lublin: 2008. pp. 231–38. [Google Scholar]
- 3.Kurcz J, Nienartowicz E, Słonina J, et al. The usefulness of CT-angiography in detecting anatomical variants of arteries arising from the abdominal aorta and aortic arch. Adv Clin Exp Med. 2007;16(6):751–60. [Google Scholar]
- 4.Bochenek A, Reicher M. Anatomia człowieka. Vol. 2. PZWL; Warszawa: 2006. pp. 512–15. 3: 284–89, 272–93. [Google Scholar]
- 5.Saldarriaga B, Pérez AF, Ballesteros LE. A direct anatomical study of additional renal arteries in a Colombian mestizo population. Folia Morphol. 2008;67(2):129–34. [PubMed] [Google Scholar]
- 6.Sykes D. The arterial supply of the human kidney with special reference to accessory renal arteries. Br J Surg. 1963;50:368–74. doi: 10.1002/bjs.18005022204. [DOI] [PubMed] [Google Scholar]
- 7.Satyapal KS, Haffejee AA, Singh B, et al. Additional renal arteries incidence and morphometry. Surg Radiol Anat. 23:33–38. doi: 10.1007/s00276-001-0033-y. [DOI] [PubMed] [Google Scholar]
- 8.Ozkan U, Oguzkurt L, Tercan T, et al. Renal artery origins and variations: angiographic evaluation of 855 consecutive patients. Diagn Interv Radiol. 2006;12:183–86. [PubMed] [Google Scholar]
- 9.Bordei P, Sapte E, Iliescu D. Double renal arteries originating from the aorta. Surg Radiol Anat. 2004;26(6):474–79. doi: 10.1007/s00276-004-0272-9. [DOI] [PubMed] [Google Scholar]
- 10.Khamanarong K, Prachaney P, Ultraravichien A, et al. Anatomy of renal arteria supply. Clin Anat. 2004;17:334–36. doi: 10.1002/ca.10236. [DOI] [PubMed] [Google Scholar]
- 11.Woźniak WT. Origin of the renal arteries from sides of aorta. Folia Morphol. 1999;60:337–41. [PubMed] [Google Scholar]
- 12.Satyapal K. Reply to “Anatomy of renal arterial supply”. Clin Anat. 2004;17:688. doi: 10.1002/ca.20075. [DOI] [PubMed] [Google Scholar]
- 13.Putz R, Pabst R. Sobotta – Atlas Anatomii Człowieka. Vol. 2. Elsevier Urban&Partner; Wrocław: 2006. p. 216. [Google Scholar]
- 14.Cicekcibasi AE, Ziylan T, Salbacak A, et al. An investigation of the origin, localization and variations of the renal arteries in human fetuses and their clinical relevance. Ann Anat. 2005;187:421–27. doi: 10.1016/j.aanat.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Sampaio FJB, Passos MA. Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat. 1992;14:113–17. doi: 10.1007/BF01794885. [DOI] [PubMed] [Google Scholar]
- 16.Patil UD, Ragavan A, Nadaraj, et al. Helical CT angiography in evaluation of live kidney donors. Nephrol Dial Transplant. 2001;16:1900–4. doi: 10.1093/ndt/16.9.1900. [DOI] [PubMed] [Google Scholar]
- 17.Ayuso JR, Openheimer F, Ayuso C, et al. Living donor kidney transplantation: helical CT evaluation of candidates. Actas Urol Esp. 2006;30(2):145–51. doi: 10.1016/s0210-4806(06)73416-7. [DOI] [PubMed] [Google Scholar]
- 18.Tarzamni MK, Nezami N, Rashid RJ, et al. Anatomical differences in the right and left renal arterial patterns. Folia Morphol. 2008;67(2):104–10. [PubMed] [Google Scholar]
- 19.Kawamoto S, Montgoery RA, Lawler LP, et al. Multidetector CT angiography for preoperative evaluation of living laparoscopic kidney donors. AJR Am J Roentgenol. 2003;180(6):1633–63. doi: 10.2214/ajr.180.6.1801633. [DOI] [PubMed] [Google Scholar]
- 20.Spring DB, Salvatierra O, Jr, Palubinskas AJ, et al. Results and significance of angiography in potential kidney donors. Radiology. 1979;133(1):45–47. doi: 10.1148/133.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Pollak R, Prusak BF, Mozes MF. Anatomic abnormalities of cadaver kidneys produced for purposes of transplantation. Am Surg. 1986;52:233–35. [PubMed] [Google Scholar]
- 22.Iezzi R, RaVaele Cotroneo A, Giancristofaro D, et al. Multidetectorrow CT angiographic imaging of the celiac trunk: anatomy and normal variants. Surg Radiol Anat. 2008;30:303–31. doi: 10.1007/s00276-008-0324-7. [DOI] [PubMed] [Google Scholar]
- 23.Huayue C, Ryuichiro Y, Shoichi E, Shizuko S. Anatomic variation of the celiac trunk with special reference to hepatic artery patterns Annals of Anatomy. Anatomischer Anzeiger. 2009;191:399–407. doi: 10.1016/j.aanat.2009.05.002. [DOI] [PubMed] [Google Scholar]