Summary
Background:
Both brain atrophy and decrease of perfusion are observed in dementive diseases.
The aim of the study was to correlate the results of brain perfusion CT (pCT) and CT volumetry in patients with Alzheimer’s disease (AD).
Material/Methods:
Forty-eight patients with AD (mean age of 71.3 years) underwent brain pCT and CT volumetry. The pCT was performed at the level of basal ganglia after the injection of contrast medium (50 ml, 4 ml/sec.) with serial scanning (delay 7 sec, 50 scans, 1 scan/sec). Volumetric measurements were carried out on the basis of source images, with the use of a dedicated CT software combined with manual outlining of the regions of interest in extracerebral and intraventricular CSF spaces.
Perfusion parameters of the cerebral blood flow (CBF) and cerebral blood volume (CBV) from the grey matter of frontal and temporal as well as basal ganglia were compared statistically with the volumetric measurements of frontal and temporal cortical atrophy as well as subcortical atrophy.
Results:
A statistically significant positive correlation was found between the values of CBF and CBV in the basal ganglia and the volumes of the lateral and third ventricles. The comparison of CBF and CBV results with the volumetric measurements in the areas of the frontal and temporal lobes showed mostly negative correlations, but none of them was of statistical significance.
Conclusions:
In patients with AD, the degree of cortical atrophy is not correlated with the decrease of perfusion in the grey matter and subcortical atrophy is not correlated with the decrease of perfusion in the basal ganglia region. It suggests that functional and structural changes in AD are not related to each other.
Keywords: Alzheimer’s disease, CT perfusion, CT volumetry
Background
The global tendency of increasing life expectancy and population aging in the developed countries result in a growing prevalence of dementive diseases, which becomes an increasing social, medical and economic problem. Dementive diseases constitute a very difficult challenge for physicians of different specialties and for many researchers who aim to develop new diagnostic method enebling to differentiate those diseases in their preclinical phase, as well as to invent effective medicines which would inhibit or delay the symptoms of dementia. In the recent years, much attention and time have been devoted to establish the moment of conversion from a mild cognitive impairment (MCI) to a fully symptomatic Alzheimer’s disease (AD) and to stop this conversion.
With a dynamic development of imaging diagnostics, its role in revealing the dementive diseases and monitoring their course and reaction to the applied treatment is of increasing importance. For many years now, basic structural CT and MRI examinations of the head have been a constant element of the diagnostic scheme in patients with dementia. Their main aim is to exclude the organic causes of dementia and to assess the degree and location of brain atrophy [1,2].
In recent years there have been an enormous progress not only in morphological examinations, but also in methods of functional imaging, which allow for evaluation of metabolism, biochemical composition and function of the cerebral cortex, as well as perfusion and diffusion rates in different brain regions, providing many important data on pathomechanisms of different dementive diseases. These methods include MR spectroscopy (MRS), functional MR (fMR), diffusion-weighted MR imaging (DWI) and diffusion tensor imaging (DTI), as well as perfusion techniques, such as perfusion MR (pMR) and CT (pCT).
In the course of dementive diseases both brain atrophy (cortical and subcortical) and decreased cortical perfusion can be found [3,4].
The aim of this study was to compare the volumetric measurements of brain atrophy and values of CT perfusion parameters in patients with Alzheimer’s disease and to examine whether there is any correlation of time and location between these parameters.
Material and Methods
The study group included 48 patients (30 women and 18 men) in the age ranging from 45 to 88 years (mean age of 71.3 years) who, on the basis of the valid clinical classifications, were diagnosed with AD.
To diagnose AD, ICD-10 and DSM-IV classifications were used, supplemented by NINCDS-ADRDA criteria. Most of the patients from the study group had primary or secondary education. In the Mini-Mental State Examination (MMSE) test, they received from 0 to 23 points (mean of 16.6 points), while the clock drawing test resulted in I–IV points, or II points on average.
All the patients underwent a CT, together with pCT and CT volumetry.
The examination were performed with the use of a two-row, spiral CT unit, Dual HiSpeed (GE Medical Systems). First, a standard, unenhanced CT of the head was carried out in the sequential technique (slice thickness of 7 mm in the supratentorial space and of 4 mm within the posterior cranial fossa).
The basic head CT was used to perform volumetric measurements and to establish the level of perfusion measurements.
Methods of volumetric measurements
Source CT images were sent to the viewing station, Magic View 1000. The measurements were performed on the basis of own method, combining automatic calculations of the CSF (cerebrospinal fluid) volumes with a visual assessment of conformity between the regions marked by the program and outlines of the anatomical structures, together with manual correction of these outlines.
To perform volumetric measurements, we used axial CT scans, after excluding the highest and the lowest scans, to eliminate bias from artifacts produced by bone structures (pyramids of the temporal bones and bones of the skull vault).
The following parameters were calculated in the volumetric examination:
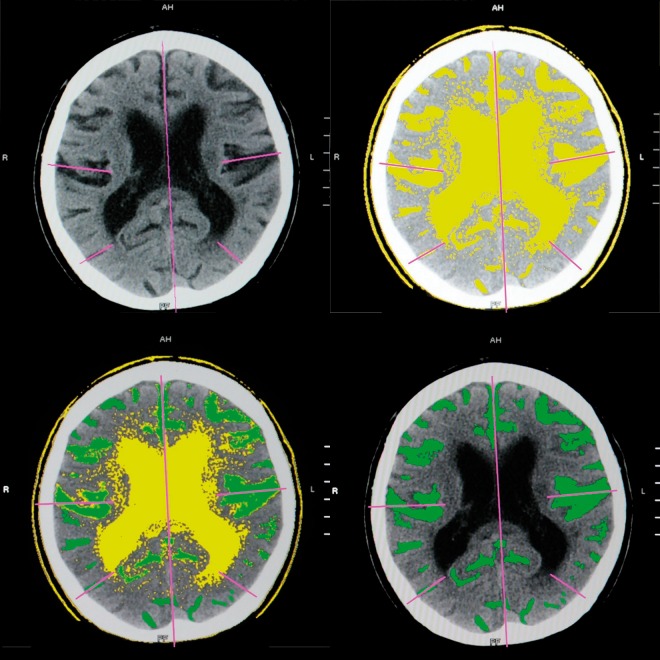
CSF volume in subarachnoid spaces of the temporal, frontal and parietal-occipital regions (for each hemisphere independently) and for the whole supratentorial region – to assess the cortical atrophy (Figure 1, Table 1),
CSF volume in the ventricular system (in the ventricular supratentorial system and in the IV ventricle, separately) – for subcortical atrophy,
Cranial volume (without the posterior fossa) – for standardisation of the obtained results,
Supratentorial volume
Figure 1.
Evaluation of cortical atrophy – CSF spaces found automatically by computer (yellow); after manual outlining (green).
Table 1.
The results of correlation of perfusion parameters (CBF and CBV) with volumetric measurements of cortical and subcortical atrophy.
| CBF | gm F+T | gm Fr | gm Fl | gm Tr | gm Tl | gm F r+l | gm T r+l | B.G. | wm F+T |
|---|---|---|---|---|---|---|---|---|---|
| CA/SV | −0.04 | 0.01 | 0.02 | 0.03 | −0.18 | 0.02 | −0.09 | 0.43 | 0.24 |
| Fr/SV | −0.11 | −0.09 | −0.04 | −0.05 | −0.21 | −0.07 | −0.14 | 0.33 | 0.20 |
| Fl/SV | −0.04 | 0.06 | 0.02 | 0.06 | −0.23 | 0.04 | −0.10 | 0.46 | 0.24 |
| Tr/SV | −0.14 | −0.14 | −0.10 | −0.15 | −0.12 | −0.12 | −0.15 | 0.29 | 0.02 |
| Tl/SV | −0.14 | −0.07 | −0.11 | −0.09 | −0.20 | −0.09 | −0.16 | 0.47 | 0.05 |
| F/SV | −0.08 | −0.01 | −0.01 | 0.01 | −0.22 | −0.01 | −0.12 | 0.40 | 0.23 |
| T/SV | −0.14 | −0.11 | −0.11 | −0.13 | −0.16 | −0.11 | −0.16 | 0.39 | 0.03 |
| v.sys./SV | −0.13 | −0.05 | −0.11 | −0.12 | −0.16 | −0.09 | −0.15 | 0.41 | 0.02 |
| CBV | gm F+T | gm Fr | gm Fl | gm Tr | gm Tl | gm F r+l | gm T r+l | B.G. | wm F+T |
|---|---|---|---|---|---|---|---|---|---|
| CA/SV | −0.01 | 0.05 | 0.01 | 0.06 | −0.12 | 0.03 | −0.04 | 0.42 | 0.13 |
| Fr/SV | −0.09 | −0.05 | −0.05 | −0.04 | −0.19 | −0.05 | −0.13 | 0.32 | 0.07 |
| Fl/SV | 0.02 | 0.08 | 0.04 | 0.09 | −0.13 | 0.06 | −0.02 | 0.42 | 0.15 |
| Tr/SV | −0.13 | −0.09 | −0.13 | −0.11 | −0.13 | −0.12 | −0.13 | 0.28 | −0.06 |
| Tl/SV | −0.08 | −0.04 | −0.10 | −0.03 | −0.12 | −0.07 | −0.08 | 0.41 | −0.01 |
| F/SV | −0.04 | 0.02 | −0.01 | 0.03 | −0.16 | 0.01 | −0.08 | 0.38 | 0.11 |
| T/SV | −0.11 | −0.07 | −0.12 | −0.07 | −0.13 | −0.10 | −0.11 | 0.35 | −0.04 |
| v.sys./SV | −0.03 | 0.01 | −0.09 | 0.02 | −0.03 | −0.05 | −0.01 | 0.42 | 0.05 |
Statistically significant values (in bold) – p<0.05. CBF – cerebral blood flow; CBV – cerebral blood volume; gm – grey matter; F – frontal area; T – temporal area; R – right; L – left; B.G. – basal ganglia; wm – white matter; ca – cortical atrophy; v.sys. – ventricular system; SV – skull volume.
Methods of the perfusion measurements
CT perfusion measurements were carried out at the level of the basal ganglia. After identifying the proper slice, contrast agent was administered with the use of an automated syringe (50 ml, rate of 4 ml/sec). After approx. 7 seconds of delay, a serial scanning was introduced at a rate of 1 scan per second and lasted for about 1 minute. As a result, we obtained 50 CT scans during the firts pass of the contrast agent through brain parenchyma. The thickness of the examined slice was 1 cm.
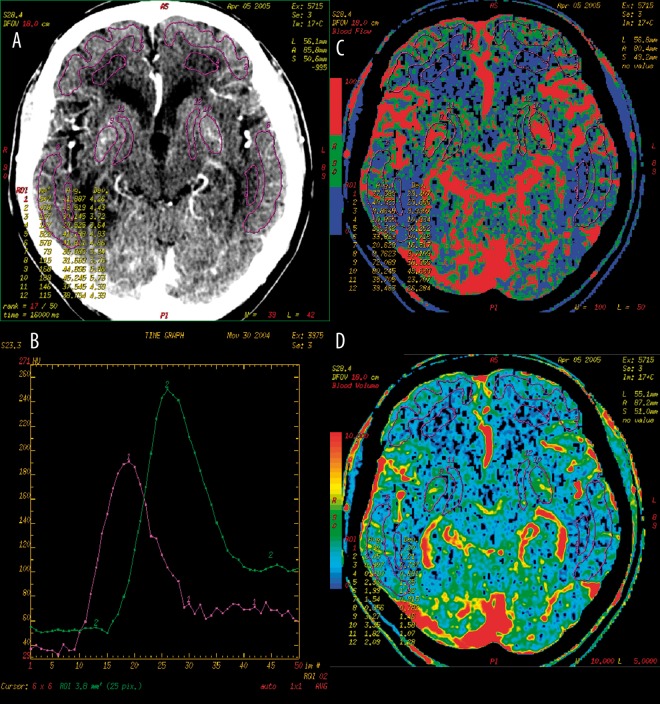
Row data obtained from the perfusion examination were then processed on AW workstations with the use of Perfusion 2 software dedicated for the diagnostics of ischaemic lesions (brain stroke). Further stages of postprocessing included the elimination of movement artifacts, establishment of an optimal density range (0–120 HU, normally) and identification of the reference vessels. Due to the analysis of perfusion data with the use of the deconvolution method, it was necessary to identify two reference vessels: arterial (pericallosal artery) and venous (superior or transverse sagittal sinus). After generating colour perfusion maps for CBF (cerebral blood flow) and CBV (cerebral blood volume) parameters, we marked 12 regions of interest (ROI) on them: within the grey and white matter of the frontal and temporal lobes and within the lentiform nuclei and internal capsules (Figure 2). Regions of interests were manually outlined (freehand drawing option) by a precise contouring of different anatomical structures, excluding the cerebral grooves or large vessels. For each of the identified ROIs, values of CBF and CBV parameters were obtained. Moreover, by adding the results from a few ROIs and by calculating a mean value, values of perfusion parameters were obtained for larger brain areas (Table 1).
Figure 2.
CT perfusion of the brain. (A) – twelve regions of interest (ROIs) in the grey and white matter of frontal and temporal lobes, both caudate nuclei and internal capsules. (B) – normal perfusion curves in the reference vessels (violet – artery, green – vein). CBF (C) and CBV (D) perfusion maps with ROIs.
Values of CBF and CBV parameters from 9 selected ROIs were mathematically correlated with the results of volumetric measurements. Perfusion parameters of the grey matter were compared with cortical atrophy of a given region, while the perfusion in the area of basal ganglia, with subcortical atrophy. The Pearson’s correlation coefficient with p<0.05 was used for the statistical analysis.
Results
We found a statistically significant positive correlation between CBF and CBV values within the basal ganglia and the CSF volume in the supratentorial ventricular system (subcortical atrophy). i.e. increasing perfusion with increasing ventricular volume.
No correlation was found between the perfusion measurements within the whole white matter, and the subcortical atrophy.
The degree of cortical atrophy in the frontal and temporal areas did not correlate significantly with CBF and CBV values obtained for those areas (Table 1).
Discussion
Brain atrophy which goes beyond the physiological aging process is one of the basic pathological processes in patients with dementia. The characteristic feature of atrophy found in imaging examinations is the decreasing volume of brain structures, which results in a secondary dilation of the extracerebral fluid spaces (cortical atrophy) and of the intraventricular system (subcortical atrophy) [5].
On the basis of neuropathological examinations, 3 main stages of the development of brain atrophy in AD were identified. In the first stage, mostly asymptomatic, morphological lesions are found selectively within the parahippocampal areas (hippocampal gyrus and entorhinal cortex). In the next stage, the degenerative process involves additional structures of the medial part of the temporal lobe and limbic system, and especially the hippocampal system. In the last stage, the pathological lesions spread to the remaining parts of the cortex, including most of the temporal, parietal and frontal lobes [6–9].
The results of the previously reported volumetric CT and MRI examinations correlate with the presented neuropathological lesions. The examinations performed in patients in a preclinical stage of AD with MCI showed a decreased volume of the parahippocampal gyrus, which speaks for early morphological lesions of ‘dementive atrophy’ type [10].
Results of the volumetric MRI examinations in patients with AD confirmed the loss of volume of the medial structures of the temporal lobe – ERC cortex (enthorhinal cortex) and hippocampus mainly. The loss of the volume increases with the development of the disease [11–17].
Also the previous volumetric CT measurements conducted by the authors of this article among patients with AD showed a significantly higher volume of the cerebrospinal fluid in the occipital-parietal regions and in the supratentorial ventricular system, than in the control group, which remains in accordance with the results of the studies by other authors [18–23]. However, no differences were shown in the measurements of the CSF volume in the temporal regions between the patients with AD and the control group, which resulted most probably from the type of measuring method accepted by the authors. The evaluation of the volumes of extracerebral CSF spaces in the temporal region included sections from above the apexes of the pyramids of the temporal bones, which was supposed to avoid bias induced by artifacts from bone structures within the skull base.
The authors did not show any significant cortical atrophy in the frontal regions in patients with AD, which is in accordance with the reports by other authors. The presence of atrophy in the frontal regions is typical for patients with frontotemporal dementia (FTD), and due to a high similarity and overlapping of clinical symptoms in the course of FTD and AD, it is used in the differential diagnostics between these forms of dementia [24,25].
In AD, apart from brain atrophy also major injury of the cerebral vessels can be seen, which according to the traditional view of AD pathomechanism, is caused by toxicity of the accumulating beta-amyloid [26]. The significant role of vascular lesions in the pathomechanism of AD is becoming more frequently mentioned. According to the vascular hypothesis of AD these lesions are believed to be not only the result of the neuronal damage but also its primary cause [27,28].
In the light of the recent histological and molecular studies, lesions found in AD within the vascular system are varied and concern both the large and the small vessels. Patients with AD are diagnosed with disturbed angiogenesis, degeneration of small vessels, improper functioning of the brain-blood barrier (disturbed transfer of beta-amyloid and its decreased elimination from the brain tissues) as well as atheromatous lesions within large vessels [29–32]. All these pathologies of the vascular system lead to a chronic brain hypoperfusion.
Nuclear medicine imaging examinations (PET, SPECT), and, with increasing frequency, also the perfusion examinations, such as PWI and pCT, allow for an in vivo, noninvasive evaluation of the cerebral microcirculation. The results of PET, SPECT, and PWI examinations confirm the significant hypoperfusion of the temporo-parietal regions, and medial aspects of the temporal lobes, frontal regions and the posterior cingulate cortex in AD, as well as in MCI – a prestage of AD [33–37]. Previous pCT examinations, carried out by the authors of this article, also showed a statistically significant decrease in CBF and CBV values in the grey and white matter of the frontal and temporal lobes, as well as in both internal capsules in patients with AD [38]. Additionally, it was found that in those patients, the values of CBF and CBV within the cortex and the white matter of the frontal and the temporal lobes correlate positively with the results of the MMSE test, i.e. the more severe the hypoperfusion of these regions, the more pronounced the disturbances of the cognitive function [39].
Until present, there have not been many articles comparing the results of volumetric and perfusion examinations in patients with AD. Most of the studies based on the evaluation of MRI, SPECT or PET examinations, and none of them concerned the CT methods.
Our assessment of the correlation between volumetric measurements of brain atrophy and perfusion parameters did not show any statistically significant relations between the degree of the cortical atrophy and the decrease in perfusion rate in the selected regions of the frontal and temporal areas, neither in the white matter, nor in the grey matter. Similar results were obtained by Bozzao et al., who compared the PWI results with the degree of cortical atrophy in patients with AD, and showed that CBV values do not depend on brain atrophy. According to the authors, the decreased values of CBV in patients with AD do not follow directly from brain atrophy, but, most probably, from a limited brain perfusion in patients with a disturbed cognitive function resulting from a lower brain demand [36].
The obtained results may be also caused by a different course and rate of changes in both processes, which was suggested by Kitayama et al. They concluded that in the development of the AD from an early to an advanced stage, the dynamics of perfusion disturbances and of hippocampal volume decrease are not the same. In the early AD stages, the hippocampal atrophy (22.2%) exceeds the perfusion decrease (12.4%), while in the advanced stages of AD, the authors of the study found a very similar degree of both those processes (perfusion decreased by 25.9%, hyppocampal volume decreased by 29.3%) [40].
Also other SPECT and PET studies perfomed in the patients with early AD changes showed significant disturbances of metabolism and brain perfusion in the posterior cingulate cortex as well as, the typical for AD, degeneration of the neurons in the enthorinal cortex. Lesions in the posterior cingulate cortex were considered to be a late functional result of the structural injury of the enthorinal cortex [33]. Such a finding points to the fact that changes in brain perfusion, secondary to the structural damage, do not have to concern the same region in the brain, but may be found in distant regions.
On the other hand, it is difficult to explain the results of positive correlations between the increased subcortical atrophy and the increased value of perfusion parameters in the region of basal ganglia, which were found in our study. Such a result can confirm a thesis different dynamics of both degenerative and perfusion changes and may suggest an hyperperfusion of the deep cortical structures to be a protective mechanism in the condition of the increased subcortical atrophy. However, in the situation of no similar results obtained by other authors, it is necessary to carry out further studies, which would aim to define the exact nature of the changes.
Conclusions
In patients with AD, the degree of cortical atrophy does not correlate with decreased pCT parameters within the grey matter, and the degree of subcortical atrophy does not correlate with the decrease in perfusion of the basal ganglia.
The obtained results suggest that structural and functional lesions in the course of AD do not follow concurrently and are not strictly dependent from one another.
References:
- 1.Scheltens P. Early diagnosis of dementia: neuroimaging. J Neurol. 1999;246:16–20. doi: 10.1007/s004150050300. [DOI] [PubMed] [Google Scholar]
- 2.Sobów T. „Otępienia odwracalne” – historia koncepcji, krytyczny przegląd badań i praktyczne implikacje kliniczne. Postępy Psychiatrii i Neurologii. 2005;14(4):331–36. [Google Scholar]
- 3.Frisoni GB. Structural imaging in the clinical diagnosis of Alzheimer’s disease: problems and tools. J Neurol Neurosurg Psychiatry. 2001;70:711–18. doi: 10.1136/jnnp.70.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varma AR, Adams W, Lloyd JJ, et al. Diagnostic patterrns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer’s disease, frontotemporal dementia and vascular dementia. Acta Neurol Scand. 2002;105(4):261–69. doi: 10.1034/j.1600-0404.2002.1o148.x. [DOI] [PubMed] [Google Scholar]
- 5.Walecki J, Pawłowska-Detko A, Adamczyk M. Rola współczesnych metod obrazowania w rozpoznaniu i monitorowaniu otępienia. Polski Przegląd Neurologiczny. 2007;3(2):69–89. [Google Scholar]
- 6.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 9.Haroutunian V, Purohit DP, Perl DP. Neurofibrillary tangles in nondemented elder subjects and mild Alzheimer disease. Arch Neurol. 1999;56:713–18. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- 10.Thomann PA, Pantel J, Wuestenberg T, et al. Structural MRI-findings in Mild Cognitive Impairment and Alzheimer’s Disease. Psychogeriatia Polska. 2005;2(1):1–10. [Google Scholar]
- 11.Xu Y, Jack CR, O’Brien PC, et al. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2000;54:1760–67. doi: 10.1212/wnl.54.9.1760. [DOI] [PubMed] [Google Scholar]
- 12.Bobinski M, de Leon MJ, Convit A, et al. MRI of entorhinal cortex in mild Alzheimer’s disease. Lancet. 1999;3(53):38–40. doi: 10.1016/s0140-6736(05)74869-8. [DOI] [PubMed] [Google Scholar]
- 13.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22:747–54. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 14.Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;71:441–47. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du AT, Schuff N, Zhu XP, et al. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology. 2003;60:481–86. doi: 10.1212/01.wnl.0000044400.11317.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schott JM, Fox NC, Frost C, et al. Assessing the onset of structural change in familial Alzheimer’s disease. Ann Neurol. 2003;53:181–88. doi: 10.1002/ana.10424. [DOI] [PubMed] [Google Scholar]
- 17.Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–89. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du AT, Schuff N, Kramer JH, et al. Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain. 2007;130:1159–66. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley KM, Bydder GM, Budge MM. Serial brain MRI at 3–6 month intervals as a surrogate marker for Alzheimer’s disease. Br J Radiol. 2002;75:506–13. doi: 10.1259/bjr.75.894.750506. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Chalk JB, Rose SE. MR image-based measurement of rates of change in volumes of brain structures. Part II: application to a study of Alzheimer’s disease and normal aging. Magn Res Imag. 2002;20:41–48. doi: 10.1016/s0730-725x(02)00472-1. [DOI] [PubMed] [Google Scholar]
- 21.Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology. 2003;61:487–92. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- 22.Czarnecka A, Sąsiadek M. Value of volumetric head CT in diagnostics and differentiation of selected dementive disorders. Pol J Radiol. 2009;74(2):7–13. [Google Scholar]
- 23.Czarnecka A, Sąsiadek M, Hudyma E, et al. Correlation of volumetric and fractal measurements of brain atrophy with neuropsychological tests in patients with dementive disorders. Pol J Radiol. 2008;73(4):16–20. [Google Scholar]
- 24.Bocti C, Rockel C, Roy P, et al. Topographical patterns of lobar atrophy in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21(5–6):364–72. doi: 10.1159/000091838. [DOI] [PubMed] [Google Scholar]
- 25.Chan D, Fox NC, Jenkins R, et al. Rates of global and regional cerebral atrophy in AD and fontotemporal dementia patients. Neurology. 2001;57:1156–63. doi: 10.1212/wnl.57.10.1756. [DOI] [PubMed] [Google Scholar]
- 26.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–56. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 27.Zlokovic BV. Neurovascular mechanism of Alzheimer’s neurodegeneration. Trends in Neurosci. 2005;28(4):202–8. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Bell RD, Zlokovic BV. Neurovascular mechanism and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–13. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De la Torre JC. Alzheimer’s disease is a vasculopathy: a new term to describe its nature. Neurol Res. 2004;26:517–24. doi: 10.1179/016164104225016254. [DOI] [PubMed] [Google Scholar]
- 30.Kalback W, et al. Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s disease. Neurol Res. 2004;26:525–39. doi: 10.1179/016164104225017668. [DOI] [PubMed] [Google Scholar]
- 31.Bailey TL, et al. The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol Res. 2004;26:573–78. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- 32.Zlokovic BV. Clearing amyloid through the blood-brain barrier. J Neurochem. 2004;89:807–11. doi: 10.1111/j.1471-4159.2004.02385.x. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann Nucl Med. 2001;15(2):85–92. doi: 10.1007/BF02988596. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda H. The role of neuroimaging in mild cognitive impairment. Neuropathology. 2007;27:570–77. doi: 10.1111/j.1440-1789.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 35.Harris GJ, Lewis RF, Satlin A, et al. Dynamic Susceptibility Contrast MR Imaging of Regional Cerebral Blood Volume in Alzheimer Disease: A Promising Alternative to Nuclear Medicine. AJNR. 1998;19:1727–32. [PMC free article] [PubMed] [Google Scholar]
- 36.Bozzao A, Floris R, Baviera ME, et al. Diffusion and Perfusion MR Imaging in Cases of Alzheimer’s Disease: Correlation with Cortical Atrophy and Lesion Load. AJNR. 2001;22:1030–36. [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson N, Jahng G, Weiner MW, et al. Pattern of Cerebral Hypoperfusion in Alzheimer Disease and Mild Cognitive Impairment Measured with Arterial Spin-labeling MR Imaging: Initial Experience. Radiology. 2005;234:851–59. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimny A, Sąsiadek M, Leszek J, et al. Does perfusion CT enable differentiating Alzheimer’s disease from vascular and mixed dementia? A preliminary report. J Neurol Sci. 2007;257:114–20. doi: 10.1016/j.jns.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 39.Zimny A, Leszek J, Kiejna A, et al. Analysis of correlation between the degree of cognitive impairment and the results of perfusion CT in patients with dementia. Med Sci Monit. 2007;13(Suppl.1):23–30. [PubMed] [Google Scholar]
- 40.Kitayama N, Kogure D, Ohnishi T, et al. MRI-based volumetry of hippocampal gray-matter, and SPECT measurements of hippocampal blood flow for the diagnosis of Alzheimer’s disease: comparison with Statistical Parametric Mapping. Brain Science and Mental Disorders. 1999;10:299–306. [Google Scholar]