Summary
Background:
The proton magnetic resonance spectroscopy (HMRS) is a non-invasive diagnostic method that allows for an assessment of the metabolite concentration in tissues. The sources of the strongest resonance signals within the brain are N-acetylaspartate (NAA), creatine (Cr), choline (Cho), myoinositol (mI) and water. The aim of our study was to analyse the ratios of metabolite signals within the brain in HMRS in the healthy population, to define the differences between the grey and white matter spectra.
Material/Methods:
We studied prospectively 90 subjects aged from 8 to 80 years (mean 43.3 years, SD=17.9), without neurological symptoms or abnormalities in magnetic resonance imaging. In all patients, brain HMRS with Signa HDx 1.5 T MR unit (GE Healthcare) was performed with PRESS sequence, using a single voxel method, at TE of 35 ms and TR of 1500 ms. Spectroscopic evaluation involved voxels placed in the white matter of parietal lobe (PWM) and the grey matter of posterior cingulate gyrus (PGM). On the basis of the intensity of NAA, Cr, Cho, mI and water signals, the proportions of these signals were calculated, as well as the ratio of the analyzed metabolite signal to the sum of signals of NAA, Cho, Cr and mI (%Met) in the PGM and PWM voxels. We compared the proportions in the same patients in PGM and PWM voxels.
Results:
There has been a statistically significant difference between the proportions of a majority of the metabolite ratios evaluated in PGM and PWM, indicating the higher concentration of NAA, Cr and mI in grey matter, and higher concentration of Cho in white matter.
Conclusions:
HMRS spectra of the brain grey and white matter differ significantly. The concentrations of NAA, Cr and mI are higher in grey matter, while of choline – in the white matter.
Keywords: MR spectroscopy, brain, white matter, grey matter
Background
The proton magnetic resonance spectroscopy (HMRS) is a non-invasive diagnostic method that allows for a quantitative assessment of the metabolite concentration in tissues, both in vitro and in vivo.
Resonance frequency of particular chemical components (metabolites) depends on so called screening effect – i.e. the influence of the electron cloud and chemical bonds surrounding the atom. This allows for fission of the spectroscopic spectrum of the examined sample and identification of particular metabolites. A constant phenomenon is so called chemical shift, σ, i.e. a proportional dependence of the resonance frequency of the substances on the value of the external magnetic field B0, under in vivo conditions. It is expressed with a formula: σ=(νA–νW)/ν0 [1]
with νA [Hz] – resonance frequency of a metabolite nucleus
νW [Hz] – resonance frequency of the nuclei of a model
ν0 [Hz] – resonance frequency of a spectrometer
The chemical shift is most frequently expressed in parts per million (ppm), which makes the graph of the spectrum independent from the applied intensity of the magnetic field. The location of the ‘peaks’ – i.e. signals of specific metabolites – is marked on axis X with a ppm value, while the peak amplitude is roughly proportional to the number of the nuclei generating magnetic resonance signals – i.e. concentration of a given substance in the examined sample. To obtain a HMRS spectrum in vivo, it is necessary to apply so called localisation techniques which consist in data acquisition from one, spatially selected volume of interest (VOI) (Figures 1, 2).
Figure 1.
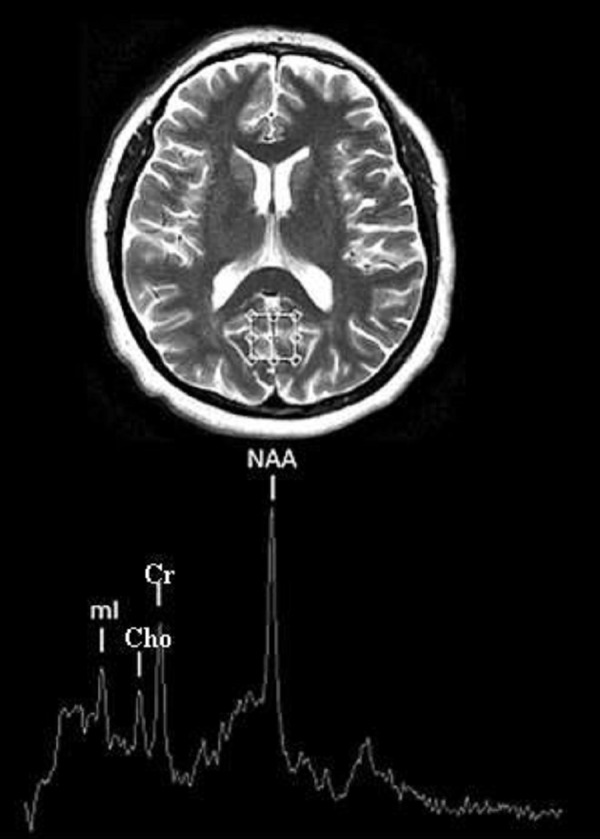
Localization of the posterior cingulate gyrus gray matter (PGM) voxel and example of spectroscopic spectrum.
Figure 2.
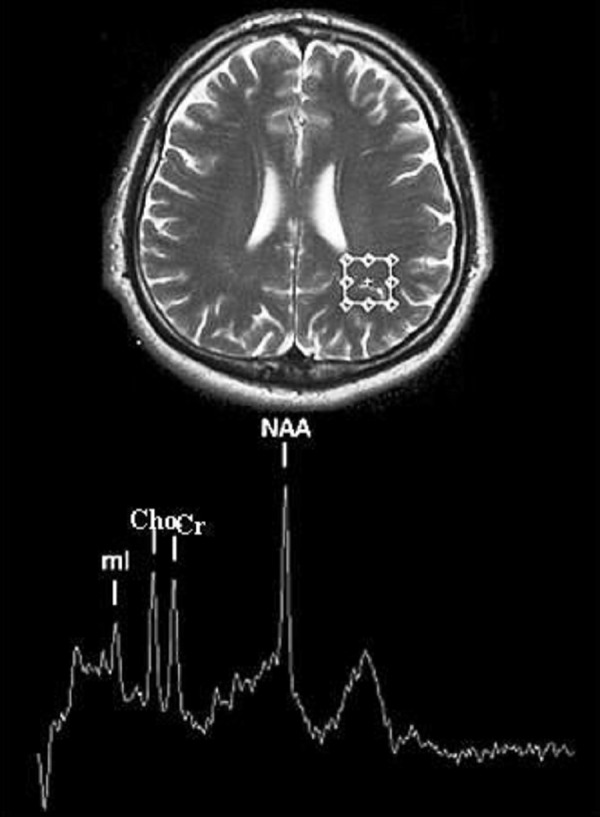
Localization of the parietal white matter (PWM) voxel and example of spectroscopic spectrum.
The sources of the strongest resonance signals within the brain are: N-acetylaspartate (NAA), creatine (Cr), choline (Cho), myoinositol (mI) and, first of all, water, the signal of which is several hundred times stronger than the signal of the remaining metabolites.
In the review of the literature on brain MRS, there are multiple articles concerning the pathological processes. However, the topic of spectrum analysis in healthy individuals (i.e. after the completion of myelinisation), with respect to the differences between the grey and white matter, is rarely introduced.
The aim of our study was to analyse the potential changes and differences in the image of the spectroscopic spectrum of the brain tissue, within the white and grey matter, and to create a model of HMRS results, including the differences in the HMRS spectrum of the white and the grey matter.
Material and Methods
In the group of 194 patients who underwent brain HMRS, 90 individuals in the age ranging from 8 to 80 years were included in the study. The study was conducted prospectively: it included logically responsive patients, not revealing any cognitive disturbances, without diseases or injuries of the central nervous system or history of systemic diseases; without neurological symptoms or pathological lesions found on brain MRI. The mean age of the whole group was 43.3 years (SD=17.9). In that group, there were 61 women (from 8 to 80 years old, mean age of 40.4; SD=17.7). All patients underwent MRI examinations (in T1- and T2-weighted images and FLAIR) and brain HMRS with a Signa HDx 1.5 T GE Healthcare unit, with PRESS sequence, using a single voxel method (voxel volume of 8 cm3, i.e. a cube with an edge of 2 cm), at TE of 35 ms and TR of 1500 ms.
In every patient, two voxels were subjected to analysis: within the grey matter of the posterior cingulated gyrus (PGM) and in the parietal white matter (PWM) (Figures 1, 2). The location of every voxel was supposed to omit bone structures and lateral ventricles. Signal amplitudes were determined automatically on the AW 4.4 workstation with SVS software, for analysis. After establishing signal intensity of N-acetylaspartate (NAA), creatine (Cr), choline (Cho), myoinositol (mI) and water, the proportions of these signals were calculated. To evaluate the differences in the spectroscopic image of the grey and the white matter, we introduced (apart from proportions using creatine or water as an internal standard) some additional proportions of NAA, Cr, Cho and mI signals in PGM to signals in PWM (e.g. NAAPGM/NAAPWM). In case of NAA, Cr, Cho and mI, we calculated also the ratio of the analysed metabolite signal (%Met) to the sum of signals of all the remaining metabolites in a given voxel – e.g.% NAAPGM=NAAPGM/(NAAPGM+CrPGM+ChoPGM+mIPGM). The obtained values were compared for the grey and for the white matter.
To calculate mean values, we applied a student’s t-test and Mann-Whitney U test. To assess the correlation between the changes in the proportions and the age, we applied the Pearson and Spearman coefficient.
Results
We found statistically significantly higher values of the NAA/Cr, Cho/Cr, mI/Cr, Cho/NAA, and Cho/H2O proportions within the white matter than within the grey matter. In the grey matter, the following proportions were significantly higher than in the white matter: mI/Cho, NAA/H2O, Cr/H2O and mI/H2O. The mI/NAA proportion revealed a higher mean value in PWM, which, however, was not statistically significant (Table 1). When comparing the proportions of individual metabolites in the voxels, the following values were found: NAAPGM/NAAPWM=1.055; CrPGM/CrPWM=1.19; ChoPGM/ChoPWM=0.67; MiPGM/mIPWM=1.04 (Table 2). No correlation was found between the NAAPGM/NAAPWM, CrPGM/CrPWM, ChoPGM/ChoPWM, and mIPGM/mIPWM proportions and the age of the examined patients.
Table 1.
The comparison of the grey and of the white matter – Student’s t-test, mean values of metabolite proportions, N=90.
| PGM | PWM | |
|---|---|---|
| NAA/Cr* | 1.637 | 1.847 |
| Cho/Cr* | 0.562 | 0.997 |
| mI/Cr* | 0.570 | 0.658 |
| Cho/NAA* | 0.345 | 0.543 |
| mI/Cho* | 1.024 | 0.666 |
| mI/NAA | 0.350 | 0.358 |
| H2O/Cr* | 1610.368 | 2159.436 |
| NAA/H2O* | 0.0010224 | 0.0008726 |
| Cr/H2O* | 0.0006251 | 0.0004746 |
| Cho/H2O* | 0.0003509 | 0.0004702 |
| mI/H2O* | 0.000356 | 0.000311 |
Statistically significant differences, p<0.01.
Table 2.
The comparison of the intensity of metabolite signals in PGM and PWM voxels, N=90.
| Mean | Standard deviation | |
|---|---|---|
| NAAPGM/PWM | 1.055495 | 0.131290 |
| CrPGM/PWM | 1.189104 | 0.144824 |
| ChoPGM/PWM | 0.674067 | 0.101038 |
| mIPGM/PWM | 1.039426 | 0.168765 |
When evaluating %Met values within PGM and PWM, we found statistically significant differences between the white and the grey matter: for %NAA, %Cr and %mI, a higher value was found in PGM, while for %Cho, a higher percentage was noted within PWM (Table 3).
Table 3.
Comparison of %Met values between the grey and the white matter. All differences are statistically significant, Student’s t-test, p<0.01, N=90.
| Metabolite | %MetPGM | %MetPWM |
|---|---|---|
| NAA | 0.434011 | 0.410118 |
| Cr | 0.265717 | 0.222786 |
| Cho | 0.149096 | 0.221055 |
| mI | 0.151176 | 0.146040 |
Discussion
The study was conducted prospectively. From among 194 patients who underwent spectroscopic brain examination, we excluded individuals suspected for dementive, vascular, demyelinisation, proliferative and inflammatory lesions, as well as patients with lesions found on basic MR imaging (100 persons in total). Other exclusion criteria involved artifacts in HMRS images (4 cases). Finally, 90 individuals (aged 8–80 years) were included in the study group.
The HMRS examination may be performed (as in the case of this study) using a single voxel method (SVS) or chemical shift imaging (CSI), i.e. multi-voxel spectroscopy (MVS) in other words. The CSI method is especially useful in case of evaluation of larger pathological lesions, as it allows for selection of a specific plane of brain section and its division into voxels, owing to which it is possible to evaluate potential changes in the spectroscopic spectrum in a specific, larger area. However, the examination lasts longer, and the applied parameters hinder the evaluation of the amplitudes of metabolites with shorter relaxation times [1]. The SVS method allows for a quick assessment of a wide range of metabolites in a specific, reproducible site of brain, in which a single voxel will be placed. In the presented study group, voxels were located both in the white, and in the grey matter, due to the presence of differences between their spectroscopic spectrum, reported by the literature [2,3]. Currently, for SVS evaluation, most of the centres apply the PRESS sequence, which, in comparison to the STEAM sequence, results in a nearly twofold higher signal to noise ratio (SNR) [3]. Thanks to the application of a double spin echo (two RF impulses 180°), this sequence is also less prone to disturbances caused by blood flow and brain motion [4].
A maximal NAA signal intensity is observed at ppm of 2.02. NAA is defined as a marker of neurons - its signal intensity decreased with age, and with neurodegenerative processes, as well as with (among others) brain tissue injuries caused by proliferative, inflammatory, and vascular processes, past trauma or, potentially also, chemotherapy or radiotherapy, and with a toxic brain injury (e.g. carbon monoxide poisoning). The increase in the NAA level in the brain is much more seldom and can be found in i.a. Canavan disease (spongiform leukodystrophy) and, physiologically, during the myelinisation processes in the early years of life [1,5].
A band of creatine and phosphocreatine (Cr) is observed at 3.03 ppm. Due to its relative stability of concentration and signal intensity in the brain, creatine is used as an ‘internal standard’ – to calculate its ratios to other metabolites [1]. In cells, it acts as a ‘quickly available energy source’ [1], which can be confirmed by a fact of its higher concentration in the grey matter (found by us). Changes in the brain levels of creatine are connected with a significant injury of the white and the grey matter (e.g. as a result of chemotherapy or radiotherapy, or a strong injury).
Choline (Cho) band is present at 3.20 ppm. In brain cells, choline is i.a. a precursor of phosphatydylocholine and a marker of cell membrane integrity, and thus its concentration is higher in the white matter of the brain. Choline is also an indicator of the myelin sheath breakdown – its level increases in demyelination diseases (e.g. multiple sclerosis) [1,5]. Its increase is typical for proliferative processes, and is accompanied by a decreased NAA level. Cho increase is also observed in posttraumatic brain injuries (e.g. in axonal injury) [5].
Myoinositol (mI) band is found at 3.56 ppm. According to the majority of the literature sources, myoinositol is present in astrocytes only – its levels increase in astrocyte proliferation [1]. Increase in mI signal intensity is also observed in dementive diseases (e.g. Alzheimer’s disease) [5].
Literature describing differences between the white and the grey matter bases on the measurements of absolute metabolite concentrations mainly. Schuff et al. [1] found a higher NAA and Cho concentration within the white matter, and a higher Cr concentration in the grey matter. Noworolski et al. [6] also revealed a higher Cr concentration in the grey matter and Cho in the white matter, with (as the authors of this study) a higher NAA level in the grey matter (data acquisition was carried out in the white matter of the fornix and the occipital grey matter). Noworolski et al examined also the proportions of metabolites: in the grey matter, higher NAA/Cho, NAA/Cr and Cr/Cho values were found than in the white matter, with the data acquisition carried out in the white matter of the fornix and in the occipital grey matter. In the study by Angelie et al. [2], a higher value of the Cho/Cr proportion was found within the white matter, similarly to our results.
In our study, we based on the evaluation of metabolite proportions. As Noworolski et al. [6], we found similar proportions in Cho/Cr and Cho/NAA but higher values of NAA/Cr proportions in the white matter compared to the grey matter, than the above mentioned authors. When trying to explain contrary results on NAA values, obtained by Schuff et al. [1] and by Noworolski et al. [6], we concluded that such discrepances may follow from the difference in signal intensity of Cr rather than NAA. In both cases, our data correlate with the results of studies showing a higher NAA and Cr concentration in the grey matter. At the same time, values of the PWM/PGM proportion suggest a higher signal intensity of NAA, Cr and mI in the grey matter, and of Cho in the white matter. Similar conclusions can be drawn on the basis of values of the%Met proportion, which were significantly different for the grey and for the white matter. It should be added that when analysing data for choline concentration in the grey matter, we evaluated in our study only the grey matter of the occipital lobe, where, according to the analysis by Pouwels et al. [7], Cho concentration is two times lower than in the frontal lobes.
When carrying out a spectroscopic evaluation of a voxel located in the grey matter, we should take into consideration a potential influence of fluid spaces and signal intensity of water included in the cerebrospinal fluid (CSF), increasing with patient’s age. The age factor and its influence on the water signal increase was confirmed by Brooks et al. in a comparatively located voxel [8]. The PGM voxel was located in the grey matter of both posterior cingulate gyra, including the longitudinal fissure of the brain. The influence of water on signal intensity may be eliminated within the grey matter with the use of proportions including metabolites other than water. At the same time, we believe that the increase in CSF signal intensity does not have any influence on signal disturbance in the PWM voxel – i.e. voxel not containing fluid spaces. This opinion was confirmed with studies conducted by Brooks et al. [8], who did not reveal age-dependent changes in the rate of water included in the nervous tissue of the brain. Therefore, in our opinion, although the proportions including water should be approached with reserve in PGM, they are reliable for PWM.
Significant differences in the signals of metabolites and their proportions, showed in our study, require spectroscopic evaluation of potential pathologies in comparison to either PWM or PGM, depending on lesion location.
Conclusions
The obtained results showed a higher NAA, Cr and mI concentration in the grey matter and Cho in the white matter, which is expressed by higher values of Cho/Cr and Cho/NAA proportions in the white matter. NAA/Cr values higher in PWM than in PGM may be influenced by the level of the Cr signal rather than of the NAA signal, which correlates also with the results showing higher NAA and Cr concentrations in PGM. A higher NAA concentration in the grey matter confirms the literature data, according to which this metabolite functions as a neuronal marker. A strong creatine signal intensity in the grey matter may be explained (most probably) by an intensive metabolism of this compound, while a strong signal of choline in the white matter results from the role of this compound – being a component of myelin sheaths.
References:
- 1.Schuff N, Ezekiel F, Gamst A, et al. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45(5):899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelie E, Bonmartin A, Boudraa A, et al. Regional differences ad metabolic changes in normal aging of the human brain: proton MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2001;22(1):119–27. [PMC free article] [PubMed] [Google Scholar]
- 3.van der Graaf M. In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur Biophys J. 2010;39(4):527–40. doi: 10.1007/s00249-009-0517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pattany P, Massand M, Bowen B, et al. Quantitative Analysis of the Effects of Physiologic Brain Motion on Point-Resolved Spectroscopy. AJNR Am J Neuroradiol. 2006;27:1070–73. [PMC free article] [PubMed] [Google Scholar]
- 5.Osborn A, et al. Diagnostic Imaging Brain. Amirsys; New York: 2004. [Google Scholar]
- 6.Noworolski SM, Nelson SJ, Henry RG, et al. High spatial resolution 1H-MRSI and segmented MRI of cortical gray matter and subcortical white matter in three regions of the human brain. Magn Reson Med. 1999;41:21–29. doi: 10.1002/(sici)1522-2594(199901)41:1<21::aid-mrm5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Pouwels PJ, Frahm J. Regional metabolite concentrations inhuman brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39:53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- 8.Brooks JC, Roberts N, Kemp GJ, et al. A proton magnetic resonance spectroscopy study of age-related changes in frontal lobe metabolite concentrations. Cereb Cortex. 2001;11:598–605. doi: 10.1093/cercor/11.7.598. [DOI] [PubMed] [Google Scholar]


