Summary
We present a case of unilateral hyperdensity of the lentiform and caudate nucleus on CT with hyperintesity on T1-weighted images on MRI in a 71-year-old woman with hemichorea-hemiballism and recently diagnosed diabetes.
Keywords: hemichorea-hemiballism, brain, computed tomography (CT), magnetic resonance imaging (MRI)
Background
Hemichorea-hemiballism may appear in older patients with hyperglycaemia, mostly unilaterally, although it can be bilateral as well. In neuroimaging studies, it is often accompanied by some characteristic lesions. Their descriptions can be found in a few dozens of patients reported on in the literature [1]. Authors of this study present their own material – there were no Polish reports on this subject in Pubmed database.
Case Report
A 71-year-old woman presented to hospital with involuntary movements of the right limbs. The movements started suddenly, in the morning, on the day of admission. As the blood tests revealed diabetes (serum glucose concentration of 606 mg%), the patient was admitted to the department of internal medicine. She had a three-year history of hyper-tension treatment.
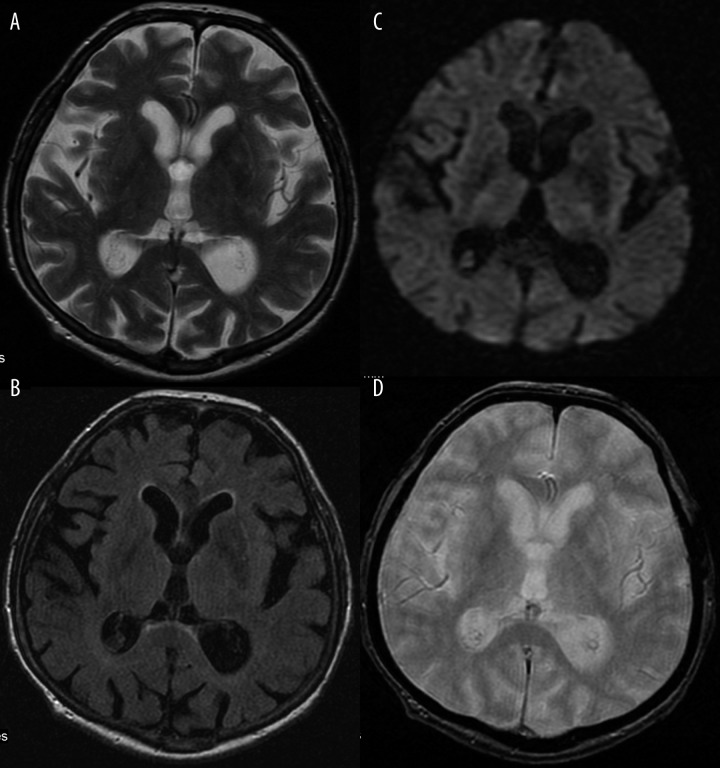
Computed tomography of the head showed hyperdensity of the left lentiform and caudate nucleus (Figure 1). The patient was referred for an MRI which revealed similar results: hyperintesity on SE/T1-weighted images (Figure 2). The lesions were slightly hypointense in other sequences: FSE/T2-, T2flair, DWI and GRE/T2*- (Figure 3A–D).
Figure 1.
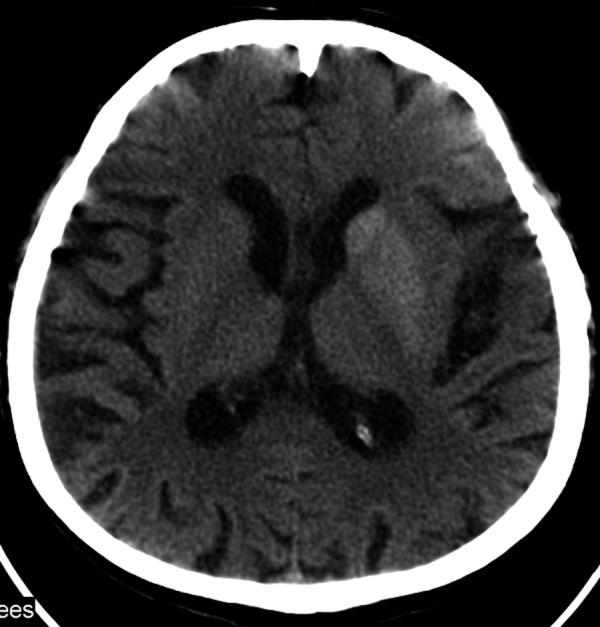
CT on the second day of symptoms. Hyperdense lentiform and caudate nucleus of the left hemisphere.
Figure 2.
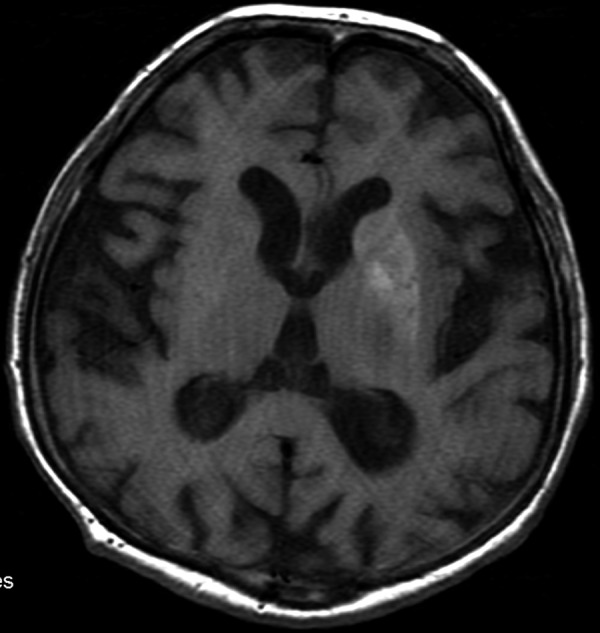
The MRI performed 4 days later. SE/T1 – Hyperintense signal from the lentiform nucleus and a less clear but also hyperintense caudate nucleus.
Figure 3.
Discretely hypointense basal ganglia on the left side, within the same layer as in Figure 2. (A) FSE/T2-. (B) T2flair. (C) DWI. (D) GRE/T2*-.
Blood glucose level was controlled with an intensive insulin treatment and then with oral antidiabetic medications. Involuntary movements were treated with clonazepam, haloperidol, depakene. Slow regression of movement disorders was observed.
Discussion
Involuntary movements are mostly present in patients with a previously diagnosed diabetes and are connected with hyperglycaemia (nonketonic one, most of the time). In our case, they preceded the diagnosis of the diabetes. The literature has known such a case already [2]. Involuntary movements are the result of metabolic disorders and tend to subside as soon as glycaemia is controlled.
There are not many causes of a shortened T1 relaxation time of the nervous tissue, expressed by a hyperintense signal on T1-weighted images. They include: iron ions in extravasated blood in the phase of free methemglobin (in subacute phase of intracerebral haemorrhage), deposits of copper (e.g. in Wilson’s disease), of manganese (in hepatic encephalopathy and during parenteral nutrition) and of calcium, fat tissue, melanin (in neurocutaneous melanosis and in melanoma metastases), as well as hamartomas in neurofibromatosis type 1 [3].
Extravasated blood is also hyperdense on CT, similarly to calcifications. However, they show a different attenuation rate. Fat tissue, on the other hand, reveals negative values of attenuation coefficient and is hypodense on CT.
The presence of manganese depositions in the brain results in movement disorders similar to the ones in our case. Manganese is deposited in the globus pallidus, in pars reticulate of the substantia nigra and, to a lesser degree, in corpus striatum, not only in the course of hepatic encephalopathy and parenteral nutrition but also in professionally-exposed individuals, e.g. miners. The lesions are bilateral, symmetrical. Movement distortions in this case are of extrapyramidal origin. They include hypokinesis, stiffness and tremor [4].
At the beginning, the increased density of lesions within basal ganglia found on CT in patients with hemichoreahemiballism was ascribed to calcifications [5,6] or hemorrhage to those structures [7]. However, due to the fact that subsequent follow-ups revealed decreased hyperdensity of the involved structures, up to its total resolution, it was concluded that the cause of lesions must be other than calcifications. Haemorrhage to basal ganglia was decided to be the cause of clinical symptoms [7]. After observing discrepancies in the evolution of lesions on CT and MRI, this theory was questioned. Shan pointed to the fact that lesions hyperintense in T1-weighted images were present for years and did not follow a standard evolution of extravasated blood. Brain biopsy in such a patient revealed gliosis with multiple gemistocytes, which, according to the author, is enough to explain the shortened T1 relaxation time [8]. There was also a theory that this is not the bleeding but a short-lasting ischaemia that leads to such an MRI image [8,9]. Finally, it was proved that ischaemia which does not lead to a full stroke results in the deposition of manganese ions in reactive astrocytes and causes a paramagnetic effect [10]. Some authors claim that this explains the diffusion restriction but not the hyperdensity on CT [11].
SPECT and PET examinations showed a decreased perfusion/metabolism within the lesions [12,13].
Nath et al. published an extensive report on autopsy findings in one of their patients from which it followed that in case of a classic triad: hyperglycaemia, hemichoreahemiballism with a sudden onset and hyperintense lesions in the basal ganglia on T1-weighted images, one may find nearly all of the following pathologies in the centralateral hemisphere: a relatively recent infarct, local calcifications, microcalcifications, gemistocytic gliosis [14].
The characteristics of lesions found on CT and MRI are not always the same. All the reports mention an increased signal in T1-weighted images. Some authors report that CT was normal and the lesions were hyperintense in T1-weighted images [12,15]. The lesion are also claimed to have different characteristics in images other than T1-weighted ones. Apart from the cases similar to ours (with a hypointense signal in diffusion-weighted imaging), there are also cases with diffusion restriction [11,12]. There are also publications on hyperintensity on T2-weighted images [7,13]. This may follow from a combination of different anomalies in different ratios in different patients.
An unchanging finding in all patients is the involment of the lentiform nucleus (putamen). In some of such cases, the lesions can be found in caudate nucleus as well – as it was in our case. Some of the patients present with bilateral lesions and with bilateral involuntary movements. An important feature of this syndrome is the resolution of MRI abnormalities with time and with follow-ups.
Despite multiple attempts, there is still no hypothesis that would unambiguously and coherently account for the type and origin of the lesions: the density on CT with the intensity of signal on MRI at the same time.
References:
- 1.Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with nonketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002;200(1–2):57–62. doi: 10.1016/s0022-510x(02)00133-8. [DOI] [PubMed] [Google Scholar]
- 2.Felicio AC, Chang CV, Godeiro-Junior C, et al. Hemichoreahemiballism as the first presentation of type 2 diabetes mellitus. Arq Neuropsiquiatr. 2008;66(2A):249–50. doi: 10.1590/s0004-282x2008000200022. [DOI] [PubMed] [Google Scholar]
- 3.Bekiesińska-Figatowska M, Walecki J. Zmiany hiperintensywne w mózgowiu w obrazach T1-zależnych badania rezonansu magnetycznego – przyczyny i możliwości różnicowania. Pol Przegl Radiol. 2001;66(2):15–18. [Google Scholar]
- 4.Rovira A, Alonso A, Cordoba J. MR imaging findings in hepatic encephalopathy. Am J Neuroradiol. 2008;29(9):1612–21. doi: 10.3174/ajnr.A1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanfield JA, Finkel J, Lewis S, et al. Alternating choreoathetosis associated with uncontrolled diabetes mellitus and basal ganglia calcification. Diabetes care. 1986;9:100–1. doi: 10.2337/diacare.9.1.100b. [DOI] [PubMed] [Google Scholar]
- 6.Inbody S, Jankovic J. Hyperkinetic mutism: bilateral ballism and basal ganglia calcification. Neurology. 1986;36:825–27. doi: 10.1212/wnl.36.6.825. [DOI] [PubMed] [Google Scholar]
- 7.Chang MH, Chiang HT, Lai PH, et al. Putaminal petechial haemorrhage as the cause of chorea: a neuroimaging study. J Neurol Neurosurg Psychiatry. 1997;63:300–3. doi: 10.1136/jnnp.63.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan DE. Hemichorea-hemiballism associated with hyperintense putamen on T1-weighted MT images: an update and a hypothesis. Acta Neurol Taiwan. 2004;13(4):170–77. [PubMed] [Google Scholar]
- 9.Fujioka M, Taoka T, Matsuo Y, et al. Novel brain ischemic change on MRI. Delayed ischemic hyperintensity on T1-weighted images and selective neuronal death in the caudoputamen of rats after brief focal ischemia. Stroke. 1999;30(5):1043–46. doi: 10.1161/01.str.30.5.1043. [DOI] [PubMed] [Google Scholar]
- 10.Fujioka M, Taoka T, Matsuo Y, et al. Magnetic resonance imaging shows delayed ischemic striatal neurodegeneration. Ann Neurol. 2003;54(6):732–47. doi: 10.1002/ana.10751. [DOI] [PubMed] [Google Scholar]
- 11.Wintermark M, Fischbein NJ, Mukherjee P, et al. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. Am J Neuroradiol. 2004;25:975–76. [PMC free article] [PubMed] [Google Scholar]
- 12.Lee EJ, Choi JY, Lee SH, et al. Hemochorea-hemiballism in primary diabetic patients: MR correlation. J Comput Assist Tomogr. 2002;26(6):905–11. doi: 10.1097/00004728-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Shobha N, Sinha S, Taly AB, et al. Diabetic nonketotic hyperosmolar state: interesting imaging observations in 2 patients with involuntary movements and seizures. Neurol India. 2006;54:440–42. doi: 10.4103/0028-3886.28126. [DOI] [PubMed] [Google Scholar]
- 14.Nath J, Jambhekar K, Rao C, et al. Radiological and pathological changes in hemiballism-hemichorea with striatal hyperintensity. J Magn Reson Imaging. 2006;23(4):564–68. doi: 10.1002/jmri.20548. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa K, Suzuki Y, Kamei S, et al. Choreic involuntary movement that occurred during therapy for diabetes mellitus. Nippon Ronen Igakkai Zasshi. 2008;45(2):225–30. doi: 10.3143/geriatrics.45.225. [DOI] [PubMed] [Google Scholar]