Summary
Background:
In MR spectroscopy, we evaluated cerebral metabolic changes in patients 2–4 years after clipping or endovascular therapy of intracranial aneurysms.
Material/Metodhs:
A prospective study was conducted in 36 patients after SAH, treated surgically (n=23) or by endovascular embolisation (n=13). Control group consisted of 20 healthy volunteers.
The clinical evaluation was based on the Glasgow Coma Scale, Hunt and Hess grade, and Glasgow Outcome Scale. MR spectroscopy was performed with 1.5T system with PRESS sequence, at echo time of 35 ms, in frontal lobes unchanged in MR examination. Ratios of N-acetylaspartate (NAA), choline (Cho), myo-inositol (mI) and glutamine/glutamate complex (Glx) to creatine were assessed.
Results:
Only a slight, statistically insignificant reduction of NAA/Cr and an insignificant increase of mI/Cr were noted; other metabolite ratios were close to the ones in the control group. Similar results were obtained in patients after surgical clipping and after endovascular therapy. Only in patients with aneurysms of anterior communicating artery complex (AcoA), the NAA/Cr ratio showed a significant reduction as compared to that of non-AcoA patients and of the control group. No significant changes of metabolite ratios were found in patients with internal carotid artery (ICA) and middle cerebral artery (MCA) aneurysms, with regard to aneurysm lateralisation.
Conclusions:
Surgical clipping and endovascular embolisation of ICA, MCA and posterior circulatory aneurysms do not induce changes in metabolite concentration in frontal lobes assessed in MR spectroscopy. In patients with AcoA aneurysms, 2–4 years after obliteration, there were found persistent metabolic changes in unchanged brain tissue of the frontal lobes, corresponding to neuronal damage (dysfunction).
Keywords: MR spectroscopy, intracranial aneurysms, clipping, embolisation
Background
The increasingly modern techniques of cerebral vessel imaging: CT angiography, MR angiography, or rotational digital subtraction angiography (3D DSA) have raised the number of incidentally diagnosed aneurysms in the recent years. The number of aneurysm clipping procedures increases as well, together with an increasingly common application of endovascular embolisation, connected with high effectiveness and a lower incidence of complications [1,2].
Although both the neurosurgical and endovascular procedures are relatively safe, some patients develop intraand postoperative complications. The most frequent ones include intraopertive haemorrhages, vasoconstriction, or necessity of closing the main maternal vessel. Related local ischaemia is found in the imaging studies in approx. 26% of cases. In 17% of cases, there appear symptoms of neurological brain ischaemia [3].
In the recent years, it has been stressed that some patients operated on due to aneurysms experienced significant neuropsychological disturbances, despite neurological improvement after the procedure [4,5]. Moreover, some of the patients developed mood disorders and symptoms of anxiety and depression [6].
All the disorders are caused by overlapping consequences of intracranial haemorrhage, thromboembolic episodes, and results of the neurosurgical and endovascular procedure itself. These multifactorial causes lead not only to a focal damage of the CNS structures, but also to multiple metabolic disturbances. So far, there have been only single reports evaluating metabolic changes in patients after a previous clipping procedure or aneurysm embolisation.
Aim of the work
The aim of the work was to evaluate metabolic changes of the brain in patients after clipping or endovascular embolisation of intracranial aneurysms, after subarachnoid haemorrhage, long after aneurysm obliteration.
Material and Methods
Material
The study was conducted in a group of 36 patients, 13 men and 23 women (mean age of 52.5±8.3 years) with intracranial aneurysms, with a history of subarachnoid haemorrhage, who underwent a procedure of aneurysm clipping (n=23) or endovascular embolisation with detachable coils (n=13). Imaging examinations and MRI spectroscopy were carried out within 28–44 months from the procedure (35.5 months on average). Table 1 showed characteristics of the study group, including aneurysm location and type of treatment method introduced. The patients were divided into 3 groups, depending on aneurysm location: group I – patients with aneurysm of the anterior communicating artery complex (anterior artery and anterior communicating artery), group II – patients with aneurysms of the internal carotid artery and the middle cerebral artery, and group III – patients with posterior circulatory aneurysms (basilar artery and posterior arteries). The control group was composed of 20 healthy volunteers, 8 men and 12 women, in a similar age range as the patients from the study group (mean age of 50.4±5.6 years; P>0.05).
Table 1.
Location of aneurysms in the study group and applied treatment methods.
| Number of patients (men/women) | Mean age Mean ±SD (years) | Time from the procedure Mean ±SD (months) | Type of procedure | ||
|---|---|---|---|---|---|
| Clipping (n) | Embolisation (n) | ||||
| Group I | 11 (4/7) | 54.3±6.4 | 35.4±3.9 | 7 | 4 |
| Anterior communicating artery | 9 (3/6) | 53.6±5.0 | 35.6±4.8 | 6 | 3 |
| Anterior cerebral artery | 2 (1/1) | 57.0±12.7 | 35.0±1.4 | 1 | 1 |
| Group II | 21 (7/14) | 51.6±8.7 | 36.6±5.3 | 15 | 6 |
| Internal carotid artery | 6 (2/4) | 46.4±10.5 | 40.0±3.6 | 3 | 3 |
| Middle cerebral artery | 15 (5/10) | 54.6±6.3 | 34.0±5.2 | 12 | 3 |
| Group III | 4 (3/2) | 53.2±9.7 | 34.6±4.2 | 1 | 3 |
| Posterior cerebral artery | 1 (1/0) | 65 | 34 | 0 | 1 |
| Basilar artery | 3 (2/1) | 50.3±8.2 | 34.7±4.9 | 1 | 2 |
Methods
Physical examinations
All patients underwent a physical examination before the procedure. It was based on the Glasgow Coma Scale (GCS) [7] and Hunt-Hess scale (H-H) [8]. The evaluation of surgery results was carried out on the basis of the Glasgow Outcome Scale (GOS) [9].
MR spectroscopy examinations
MRI examinations were carried out with a Picker Eclipse 1.5T system (Picker International Inc., Highland Heights, OH) with the use of a coil designed for head examinations. Imaging examinations performed in order to locate the measurement voxel included T1-weighted images in 3D anatomical planes. The examination was carried out in the FAST sequence (Fourier-acquired steady state), with the following sequence parameters: TE (time echo) of 4.5 ms, TR (repetition time) of 300 ms, FA (flip angle) of 80°, slice thickness of 5 mm. Moreover, T2-weighted images in FSE sequence (fast spin echo) in the transverse plane were obtained, with TE amounting to 127.6 ms, TR to 5000 ms, FA to 80°, and slice thickness to 5 mm. For FLAIR, it was: TE of 120 ms, TR of 6000 ms and slice thickness of 5 mm.
MR spectroscopy was performed using PRESS sequence (point resolved spatially localised spectroscopy), with the use of the following sequence parameters: TE of 35 ms, TR of 15000 ms, number of repetitions (nex) amounting to 192. Voxels measuring 8 cm3 (2×2×2 cm) were located symmetrically in both frontal areas, above the frontal horns of the lateral ventricles, with the superior and medial frontal gyrus included in the measurement area. Voxels included brain structures that remained unchanged on MRI, i.e. white matter mainly, and cerebral cortex to a lesser degree. In every case, the measurement areas were reset, so that they did not include adjacent bones and intracranial fluid spaces [10]. Measurement results from both frontal lobes were averaged for every patient.
Spectrum recording was preceded by unification of field homogeneity in the whole head, and then in the examined area. In the next stage, the water signal was suppressed with the MOIST sequence (multiply optimised insensitive suppression train) [11,12].
The obtained spectra were subjected to automatic processing with the use of an automatic procedure of the producer (Picker, Via 2.0). In this procedure, a residual water signal was removed with the use of filtration in time domain. Next, apodisation in time domain was performed (transformation of the exponential function into a Gaussian one) in order to obtain a higher resolution, together with the Fourier transformation of a signal in time domain into a signal in frequency domain. Next stages in the applied procedure included phase correction and basic line correction with the use of the Legedre function. Then, an automatic adjustment of curves was performed with the use of an iterative, nonlinear procedure of the least Levenberg-Marquardt squares. Shapes of the simulated signals were established in 85% of the Gaussian function and in 15% of the Lorenz function.
In the assessment of the spectra, we included signals of N-acetylaspartate, creatine, choline, myo-inositol, and glutamate/glutamine complex. The ratios of these chemical components were analysed with respect to the creatine level (NAA/Cr, NAA/Cho, Cho/Cr, mI/Cr, Glx/Cr).
Statistical analysis
The obtained results were subjected to a statistical analysis with the use of a SPSS 8.0 PL program. The analysis involved Mann-Whitney U test and Pearson’s chi-square test, with significance level amounting to P<0.05.
Results
A mean GCS score in the study group amounted to 14.44±1.18 points (median of 15 points). Similar values were found in the group of patients referred for aneurysm clipping (14.35±1.37), as well as patients referred for embolisation (14.62±0.77; P>0.05). The study group included mostly patients with disease of grade I and II, according to the Hunt-Hess scale (grade I – 21 patients – 58.3%; grade II – 14 patients – 38.9%), and one person with grade III (2.8%). In the group of patients with surgically clipped aneurysms, there were 14 individuals with grade I (60.9%), 8 patients with grade II (34.8%), and 1 patient with grade III (4.3%). In the group qualified for embolisation, there were 7 individuals with grade I (53.8%), and 6 patients with grade II (46.2%).
A mean GOS score in the whole study group amounted to 4.75±0.5 (median of 5 points) and was similar in both groups: after clipping (4.74±0.45; P>0.05) and after embolisation (4.77±0.6; P>0.05).
Table 2 showed the results of physical examinations including aneurysm location and the applied method of embolisation. Scores were similar for all the groups, both before the procedure and postoperatively. The difference was not statistically significant.
Table 2.
Clinical evaluation of the study group of patients, including aneurysm location and type of procedure.
| Clipping (n=23) | Endovascular embolisation (n=13) | |||||
|---|---|---|---|---|---|---|
| GCS Mean±SD (points) | H-H Grade I/II/III (n) | GOS Mean ±SD (points) | GCS Mean±SD) (points) | H-H Grade I/II/III (n) | GOS Mean±SD (points) | |
| Group I (n=11) | 14.6±0.8 | 5/2/0 | 4.7±0.5 | 14.3±0.9 | 2/2/0 | 4.3±0.9 |
| Group II (n=21) | 14.2±1.6 | 8/6/1 | 4.7±0.4 | 15.0±0.0 | 4/2/0 | 5.0±0.0 |
| Group III (n=4) | 15.0±0.0 | 1/0/0 | 5.0±0.0 | 14.3±0.9 | 1/2/0 | 4.5±1.0 |
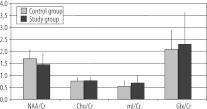
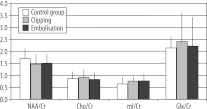
Figure 1A showed mean ratios of the examined metabolites in the study group and in the control group. In the study group, there was only a slight, statistically insignificant decrease of the NAA/Cr ratio, and a slight increase in the mI/Cr ratio. Cho/Cr and Glx/cr ratios were close to the results of the control group. Similar results were obtained in the comparison of the results of MRS examination in patients with clipped and in patients with embolised aneurysms (Figure 1B).
Figure 1A.
Metabolite ratios in patients after aneurysm obliteration and in the control group.
Figure 1B.
Metabolite ratios in patients after surgical clipping and after endovascular embolisation, in comparison to the control group.
Table 3 showed mean metabolite ratios depending on aneurysm location and with regard to the control group. No significant differences of metabolite ratios were found between the evaluated groups and in comparison to the control group. Only in group I, i.e. in patients with aneurysms of the anterior communicating artery complex, there was a statistically significant decrease in the NAA/Cr ratio (P<0.05) with regard to other study groups and the control group. No significant differences were found in the evaluated metabolite ratios for specific study groups (formed on the basis of aneurysm location and the applied method of obliteration). There were no significant correlations between the clinical status and metabolite ratios, neither in the whole study group, nor in any of the subgroups. When comparing the results of MR spectroscopy in the II group with respect to aneurysm lateralisation, no significant differences were found in the evaluated metabolite ratios in the frontal lobe on the aneurysm side, and in the contralateral hemisphere. The ratio were as follows for the hemisphere with aneurysm: NAA/Cr 1.68±0.22, Cho/ Cr 1.0±0.14, mI/Cr 0.86±0.10, Glx/Cr 2.32±0.56 and for the contralateral frontal lobe: NAA/Cr 1.62±0.25, Cho/Cr 1.04±0.16, mI/Cr 0.78±0.21, Glx/Cr 2.35±0.51.
Table 3.
Mean ratios of the evaluated metabolites, depending on aneurysm location.
| NAA/Cr | Cho/Cr | mI/Cr | Glx/Cr | |
|---|---|---|---|---|
| Control group (n=20) | 1.82±0.23 | 0.96±0.10 | 0.65±0.18 | 2.22±0.54 |
| Group I (n=11) | 1.45±0.34* | 0.94±0.13 | 0.68±0.29 | 2.38±0.49 |
| Group II (n=21) | 1.74±0.24 | 1.0±0.15 | 0.84±0.23 | 2.81±1.64 |
| Group III (n=4) | 1.71±0.33 | 0.92±0.18 | 0.99±0.33 | 1.59±0.72 |
P<0.05.
Discussion
The incidence of intracranial artery aneurysms in the general population is reported to amount to 4.6–6.0%. This constitutes approx. 75% of all cerebrovascular anomalies [13]. Approximately 20% of aneurysms rupture, leading to spontaneous intracranial haemorrhages causing death or permanent neurological deficits. The incidence of subarachnoid haemorrhage is estimated for 6–8/100000. Mortality in the cases of bleeding is very high and amounts up to 36% in the early period and to as much as 47% after one year of follow-up [14].
A significant risk in patients with intracranial haemorrhages is posed by ischaemic lesions, occurring both during neurosurgical and endovascular procedures. The number of clinically silent and symptomatic ischaemic incidents during diagnostic and surgical endovascular procedures is estimated for 10–70% and 3.8–5.6%, respectively [3,15]. The incidence of ischaemic lesions occurring during neurosurgical clipping of aneurysms is not fully known. On the basis of pre- and postoperative DWI examinations, Krayenbuhl et al. [16] estimated the rate of asymptomatic ischaemic lesions for 9.8% and of symptomatic strokes for 2%.
In the recent years, a lot of interest has been paid to neuropsychological disturbances appearing after surgery in some of the patients [4,5]. They include disturbances of cognitive and executive functions, disturbances of attention, memory and intelligent quota [17,18]. Disturbances of cognitive functions after aneurysmal obliteration are especially common in older individuals, aged over 65, with general diseases, aneurysms of the anterior communicating artery, operated on from an intrahemispheric approach [4].
In patients after aneurysm obliteration, there are also depressive disorders [6]. In the assessment by Solheim et al. [19], the incidence of anxiety and depression symptoms is similar in the groups of patients after neurosurgical procedures and in patients with aneurysms embolised endovascularly.
Intracranial haemorrhage, ischaemic incidents, as well as traumatising influence of the surgery lead to permanent or transient neurological deficits and to the reported functional disorders of the CNS. It seems that such overlapping brain damages must also influence cerebral metabolic processes. However, so far, there have appeared only scarce reports evaluating the influence of aneurysmal obliteration on brain metabolism with the use of MR spectroscopy. MR allows for detection of early lesions, even before their appearance on morphological images [20,21]. Rowe et al. [22] found decreased NAA levels and the presence of lactate bands in the area of oedema/ischaemia in T2-weighted images in the early post-clipping and post-embolisation period. Metabolic changes were significantly correlated with the extent of oedema and with the clinical state. In patients in a good clinical condition (according to Hunt-Hess scale), the spectra were normal. Regression of metabolic disturbances was found in follow-up studies in patients with clinical improvement. Similar studies, using phosphorus spectroscopy in patients after subarachnoid bleeding, showed decreased values of high-energy phosphates and acidosis. These changes regressed with clinical improvement as well [23].
Kobayashi et al. [24] on the other hand found no significant changes of the NAA/Cr ratios in the group of 21 patients after surgical clipping of unruptured aneurysms. A significant decrease in the NAA/Cr ratio in the white matter of the frontal lobes was noted only among individuals after clipping of the aneurysms of the anterior communicating artery. Decrease in the NAA/Cr ratio in the frontal and parietal lobes was also observed in the group of patients aged over 70.
These results are in accordance with own observations. In the study group of patients, there was only a slight decrease in the NAA/Cr ratio and a slight increase in the mI/Cr ratio, with no changes of the ratios of other metabolites. Only in patients with aneurysm of the anterior communicating artery complex, there was a statistically significant reduction of the NAA/Cr ratio as compared to patients with aneurysms in a different location and to the control group. Such metabolic changes, proving a damage or dysfunction of neurocytes are, without any doubt, the cause of functional disorders, especially severe in patients with aneurysms of the anterior communicating artery complex. An indirect confirmation is the Japanese research which found a reduction in the total or verbal IQ (evaluated with WAIS-R tests), being a result of recent memory disturbance in a group of patients after surgical clipping of unruptured aneurysms of the anterior communicating artery. No such disturbances were found in patients after clipping of aneurysms in different location [24].
However, no significant differences were found in the study group within the metabolite ratios with regard to the applied method of obliteration. The lack of such differences is also consistent with the results of neuropsychological studies, according to which the type of surgery does not have any significant influence on formation of functional disorders. Preiss et al. [17] did not find any significant differences in the results of neuropsychological tests, cognitive functioning and degree of mood disorders between the study group subjected to surgery and the group treated with endovascular methods.
Own studies did not show any asymmetry in metabolite concentrations between two brain hemispheres depending on aneurysm lateralisation (neither in the whole study group, nor in patients with aneurysms of the interior carotid artery and of the middle cerebral artery). The results presented by Ohue et al. [4] we different. They found rCBF (regional cerebral blood flow) asymmetry on SPECT, in the frontal lobes, significantly correlating with disturbed cognitive function.
Own studies did not show any significant correlation between the clinical status and the level of the assessed metabolites. However, the lack of such correlations may be a result of small diversity of the study group as far as the clinical state is concerned (the group included patients with the Glasgow score of 13–15 points, median of 15 points).
Surely the most reliable verification of the evaluated metabolic changes would be showing their connection with blood flow disturbances. However, so far, the literature has not presented any reports comparing the results of perfusion and spectroscopic tests. Moreover, the results of long-term follow-ups of perfusion are not homogeneous. Among multiple reports confirming the presence of these disturbances in patients with subarachnoid bleeding and vasoconstriction, there are also reports that do not reveal any changes in regional blood flow rate; these include the report by Otawara et al. [25] who did not show any rCBF changes in patients after surgical clipping of unruptured aneurysms.
Despite all these differences, the correlation between vasoconstriction, regional blood flow disturbances, and long-term formation of foci of vascular origin seems indisputable [26]. However, it is still unknown how long such disturbances last and when they tend to subside. Fukunaga et al. [27] revealed perfusion disturbances after surgical clipping of unruptured aneurysms on SPECT, appearing one month after surgery. However, the disturbances regressed within 3 months in most of the cases.
The results of own observations seem to show that long after obliteration of aneurysms of the anterior communicating artery complex, and despite the lack of morphological foci of ischaemia, there are found persisting metabolic disturbances that may result in the disturbances of neuropsychological functions.
Conclusions
Surgical clipping and endovascular embolisation of aneurysms of the internal carotid artery, of the middle cerebral artery, and of the posterior cerebral arterial circle do not lead to significant changes in metabolite levels in the frontal lobes on MR spectroscopy at long term follow-up.
In patients after obliteration of aneurysms of the anterior communicating artery complex, metabolic disturbances are found in morphologically unchanged frontal lobes, after 2–4 years following surgery. These disturbances are indicative of neuronal damage (dysfunction).
Footnotes
Source of support: The report was constructed owing to the financial support of the Ministry of Science and Higher Education – own research project N403 040 32/2157 in the years 2007–2010
References:
- 1.Rabinstein AA, Pichelmann MA, Friedman JA, et al. Symptomatic vasospasm and outcomes following aneurysmal subarachnoid haemorrhage: a comparison between surgical repair and endovascular coil occlusion. J Neurosurg. 2003;98(2):319–25. doi: 10.3171/jns.2003.98.2.0319. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux A, Kerr R, Stratton I, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–74. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 3.Bendszus M, Koltzenburg M, Burger R, et al. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999;354(9190):1594–97. doi: 10.1016/S0140-6736(99)07083-X. [DOI] [PubMed] [Google Scholar]
- 4.Ohue S, Oka Y, Kumon Y, et al. Importance of neuropsychological evaluation after surgery in patients with unruptured cerebral aneurysms. Surg Neurol. 2003;59(4):269–75. doi: 10.1016/s0090-3019(03)00043-0. [DOI] [PubMed] [Google Scholar]
- 5.Fontanella M, Perozzo P, Ursone R, et al. Neuropsychological assessment after microsurgical clipping or endovascular treatment for anterior communicating artery aneurysm. Acta Neurochir. 2003;145(10):867–72. doi: 10.1007/s00701-003-0111-5. [DOI] [PubMed] [Google Scholar]
- 6.Haug T, Sorteberg A, Sorteberg W, et al. Surgical repair of unruptured and ruptured middle cerebral artery aneurysms: impact on cognitive functioning and health-related quality of life. Neurosurgery. 2009;64(3):412–20. doi: 10.1227/01.NEU.0000338952.13880.4E. [DOI] [PubMed] [Google Scholar]
- 7.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 8.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 9.Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1975;1(7905):480–84. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 10.Sobiecka B, Urbanik A, Walecki J, et al. The evaluation of physiological ageing of the brain using MR proton spectroscopy. Pol J Radiol. 2004;69(3):68–75. [Google Scholar]
- 11.Murdoch JB, Lampman DA. Beyond WET and DRY: Optimized Pulses for Water Suppression. Society of Magnetic Resonance in Medicine, Twelfth Annual Meeting; New York. 1993; p. 1191. [Google Scholar]
- 12.Kamińska K, Walecki J, Grieb P, et al. Magnetic resonance spectroscopy – state of art and future. Pol J Radiol. 2007;72(1):71–75. [Google Scholar]
- 13.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123(Pt 2):205–21. doi: 10.1093/brain/123.2.205. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson OG, Lindgren A, Brandt L, et al. Prediction of death in patients with primary intracerebral hemorrhage: a prospective study of a defined population. J. Neurosurg. 2002;97(3):531–36. doi: 10.3171/jns.2002.97.3.0531. [DOI] [PubMed] [Google Scholar]
- 15.Grunwald IQ, Papanagiotou P, Politi M, et al. Endovascular treatment of unruptured intracranial aneurysms: occurrence of thromboembolic events. Neurosurgery. 2006;58(4):612–18. doi: 10.1227/01.NEU.0000204101.00996.D9. [DOI] [PubMed] [Google Scholar]
- 16.Krayenbuhl N, Erdem E, Oinas M, et al. Symptomatic and silent ischemia associated with microsurgical clipping of intracranial aneurysms: evaluation with diffusion-weighted MRI. Stroke. 2009;40(1):129–33. doi: 10.1161/STROKEAHA.108.524777. [DOI] [PubMed] [Google Scholar]
- 17.Preiss M, Koblihova J, Netuka D, et al. Ruptured cerebral aneurysm patients treated by clipping or coiling: comparison of long-term neuropsychological and personality outcomes. Zentralbl Neurochir. 2007;68(4):169–75. doi: 10.1055/s-2007-985855. [DOI] [PubMed] [Google Scholar]
- 18.Proust F, Martinaud O, Gerardin E, et al. Quality of life and brain damage after microsurgical clip occlusion or endovascular coil embolization for ruptured anterior communicating artery aneurysms: neuropsychological assessment. J Neurosurg. 2009;110(1):19–29. doi: 10.3171/2008.3.17432. [DOI] [PubMed] [Google Scholar]
- 19.Solheim O, Elogayli H, Muller TB, et al. Quality of life after treatment for incidental, unruptured intracranial aneurysms. Acta Neurochir. 2006;148(8):821–30. doi: 10.1007/s00701-006-0804-7. [DOI] [PubMed] [Google Scholar]
- 20.Walecki J, Tarasów E, Czernicki Z, et al. The importance of 1H MRS examination in the assessment of peritumor infiltration in patients with cerebral glioma. Pol J Radiol. 2004;69(2):16–23. [Google Scholar]
- 21.Ślubowska E, Walecki J, Grieb P, et al. 1H MRS spectroscopy in brain tumors -in search of the highest efficacy. Pol J Radiol. 2009;74(1):50–58. [Google Scholar]
- 22.Rowe J, Blamire AM, Domingo Z, et al. Discrepancies between cerebral perfusion and metabolism after subarachnoid haemorrhage: a magnetic resonance approach. J Neurol Neurosurg Psychiatry. 1998;64(1):98–103. doi: 10.1136/jnnp.64.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooke N, Ouwerkerk R, Adams CBT, et al. Phosphorus-31 magnetic resonance spectra reveal prolonged intracellular acidosis in the brain following subarachnoid haemorrhage. Proc Natl Acad Sci USA. 1994;91(5):1903–7. doi: 10.1073/pnas.91.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi M, Takayama H, Suga S, et al. Changes in proton magnetic resonance spectroscopy and Wechsler adult intelligence scale revised after clipping of unruptured aneurysms. No Shinkei Geka. 2000;28(8):691–98. [PubMed] [Google Scholar]
- 25.Otawara Y, Ogasawara K, Ogawa A, et al. Cognitive function before and after surgery in patients with unruptured intracranial aneurysm. Stroke. 2005;36(1):142–43. doi: 10.1161/01.STR.0000149925.36914.4e. [DOI] [PubMed] [Google Scholar]
- 26.Sviri GE, Britz GW, Lewis DH, et al. Dynamic perfusion computed tomography in the diagnosis of cerebral vasospasm. Neurosurgery. 2006;59(2):319–25. doi: 10.1227/01.NEU.0000222819.18834.33. [DOI] [PubMed] [Google Scholar]
- 27.Fukunaga A, Uchida K, Hashimoto J, et al. Neuropsychological evaluation and cerebral blood flow study of 30 patients with unruptured cerebral aneurysms before and after surgery. Surg Neurol. 1999;51(2):132–39. doi: 10.1016/s0090-3019(98)00090-1. [DOI] [PubMed] [Google Scholar]