Summary
Moyamoya disease is a progressive vasculopathy leading to stenosis of the main intracranial arteries. The incidence of moyamoya disease is high in Asian countries; in Europe and North America, the prevalence of the disease is considerably lower. Clinically, the disease may be of ischaemic, haemorrhagic and epileptic type. Cognitive dysfunction and behavioral disturbance are atypical symptoms of moyamoya disease.
Characteristic angiographic features of the disease include stenosis or occlusion of the arteries of the circle of Willis, as well as the development of collateral vasculature. Currently, magnetic resonance angiography and CT angiography with multi-row systems are the main imaging methods of diagnostics of the entire range of vascular changes in moyamoya disease.
The most common surgical treatment combines the direct arterial anastomosis between the superficial temporal artery and middle cerebral, and the indirect synangiosis involving placement of vascularised tissue in the brain cortex, in order to promote neoangiogenesis. Due to progressive changes, correct and early diagnosis is of basic significance in selecting patients for surgery, which is the only effective treatment of the disease. An appropriate qualification to surgery should be based on a comprehensive angiographic and imaging evaluation of brain structures.
Despite the rare occurrence of moyamoya disease in European population, it should be considered as one of causes of ischaemic or haemorrhagic strokes, especially in young patients.
Keywords: moyamoya disease, ischaemic stroke, angiography
Background
Moyamoya disease was first described in 1957 as a “bilateral hypoplasia of internal of internal carotid arteries” [1]. The name of the disease comes from Japanese and means ‘puff of smoke’.
Commonly applied in the clinical practice, modern methods of brain vessel imaging, such as CT angiography, MR angiography, or 3D DSA (rotational digital subtraction angiography) lead to a more frequent diagnosis of asymptomatic cases of this disease. Therefore, the knowledge of radiological symptoms has a significant influence on the right diagnosis and management of patients with moyamoya disease.
Epidemiology, Aetiology and Clinical Symptoms of Moyamoya Disease
High incidence of the disease is noted mainly in Asia (in Korea and Japan predominantly), with 3 cases per 100,000 of paediatric population [2]. In non-Asian countries, the incidence is much lower - in Europe, it is ten times lower than in Japan [3,4]. In Poland, there were only single reports, and this topic has rarely been discussed in the Polish medical literature [5,6].
The aetiology of moyamoya disease is poorly understood. Genetic background is frequently mentioned because in 15% of cases, the disease is found in other family members [3]. These are most probably the cases of multifactorial autosomal inheritance. Studies by Mineharu et al. pointed to locus 17q25 as having causal relationship with the disease [7]. Genetic theory is also supported by the relationship with other genetic diseases; the disease is much more frequent in children with Down syndrome, who also reveal other vascular abnormalities, such as thrombosis of the venous sinuses [8].
Vascular lesions typical for moyamoya disease may also develop in HCV-infected individuals, patients with cryoglobulinemia, sickle-cell disease, and individuals after radiotherapy of tumours of the optic chiasm [9–11]. Cases in which the aetiological factor was determined, are termed ‘moyamoya syndrome’. However, in most of the patients, the lesions are not caused by any known risk factors, i.e. develop in healthy individuals, and are then classified as moyamoya disease [12].
The disease is most common in children and young adolescents. There are observed two peaks of incidence – in the first and 3–4 decade of life [3]. The disease is more frequent in women, and the ratio of women to men with the disease amounts to 1.8:1 [13], or even 4.25:1, according to German authors [14].
The most common clinical symptoms of the disease include: sudden onset of hemiplegia, with sensation disturbances and aphasia [15]. There may also appear headaches, vertigo, seizures and involuntary movements [16]. There were reported cases of cognitive dysfunction or psychoorganic syndrome [17].
According to the Classification of the Japanese Health Ministry, there are 4 clinical forms of the moyamoya disease: ischaemic, haemorrhagic, epileptic, and ‘other’ [16]. The ischaemic form is most common in children, while the haemorrhagic form is more popular in adults. In children, the moyamoya disease has a form of transient ischaemic attacks (TIA) or lacunar strokes, leading to mental retardation [18]. In adults, there may appear intracranial haemorrhages, including subarachnoid haemorrhages [16,19]. There were also reports on strokes of ‘the last meadow’ type in adults [19]. Rarely reported clinical symptoms include also alien limb syndrome [20]. In the European population, the disease symptoms appear mostly at a later age than in Asian countries, and the haemorrhagic lesions are less frequent [14,21].
The clinical course is variable. The disease may progress slowly, with rare stroke incidents, while in some of the cases, there is a fast progression with a sudden deterioration of the neurological status. Ischaemic incidents may repeat, especially in the first years of the disease. The risk of repeated ischaemic strokes is from 65% to 82% (for bilateral lesions) [21]. In patients from Asian countries, on the other hand, repeated haemorrhagic strokes are quite common – the risk of repeated haemorrhage amounts to 30–65% [3].
Morphological Lesions and Imaging Diagnostics in the Moyamoya Disease
Changes appearing in the course of the disease include mainly the terminal parts of internal carotid arteries and/ or proximal parts of middle and anterior cerebral arteries [13]. In the affected cerebral vessels, pathological examinations do not show atherosclerotic or inflammatory lesions and the cause of stenosis is the overgrowth of the smooth muscle layer, with thrombotic changes [22].
The disease leads not only to a different degree of stenosis and occlusions of large arteries of the anterior part of the Willis circle, but also to the development of the collateral vasculature that produces a typical angiographic image, called ‘clouds of smoke’ or ‘puff of cigarette smoke’ [12]. The vessels of the collateral circulation are formed as a result of widening of the existing vessels or development of new perforating arteries [23]. These arteries are small or medium-sized muscular arteries, branching from intracranial parts of internal carotid arteries, posterior cerebral arteries or anterior choroidal arteries. The vessels of the collateral circulation combine with distal branches of the middle cerebral arteries. There are three main pathways of collateral circulation – parenchymal, meningeal, and transdural.
Collateral parenchymal vessels (described as vessels of ‘moyamoya’ type) are small, twisting, wide vessels penetrating towards the base of the brain, along the course of thalamo-striatal arteries and lenticulo-striatal arteries [13]. Transscleral anastomoses develop between the superficial temporal artery and the middle meningeal artery or the optic artery and the anterior or middle cerebral artery, perforating the dura mater [24].
In the vessels of the collateral circulation, there may appear thrombotic changes, which are the cause of ischaemic symptoms. An increased blood flow through thin collateral walls during stress, as well as the presence of microaneurysms, is the probable cause of intracranial haemorrhages [12].
Angiographic criteria of the diagnosis of moyamoya disease were established in 1998 [25]. They include stenosis or occlusion of the distal parts of intracranial internal carotid arteries and proximal parts of anterior and middle arteries, as well as the presence of collateral vasculature in the regions of the brain base, without causal disease. In case of bilateral changes, the diagnosis is considered as sure. Unilateral changes are qualified as probable.
Naturally, CT examination is sufficient to diagnose ischaemic or haemorrhagic stroke in the course of the disease [26] (Figure 1). Ischaemic foci may be present in basal ganglia and in the white matter – periventricularly and subcortically [12]. In case of patients with TIA symptoms, the results of the studies are negative.
Figure 1.
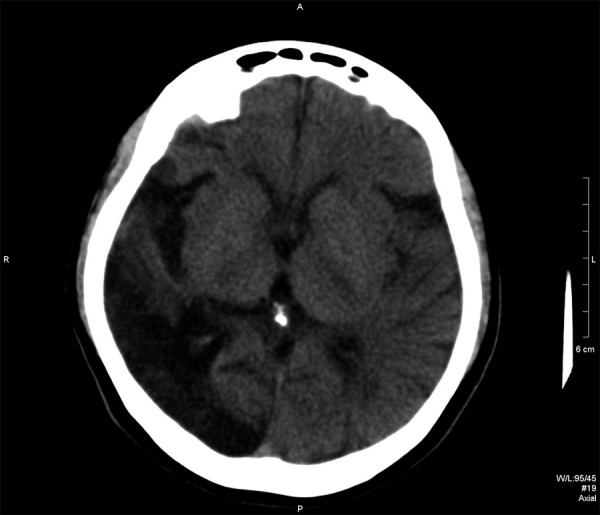
A 39-year-old women, 3 years after stroke, preceded by TIAs (Case 1). CT examination – hypodense area attributable to malacia after stroke in the right temporal and occipital lobe.
The presence of occlusions or stenoses of large intracranial vessels and the presence of collateral vasculature in a routine contrast-enhanced CT should suggest a suspicion of the disease, especially in young individuals [26]. In ambiguous cases, it is indicated to carry out MRI, which not only helps in establishing the diagnosis, but also allows for a better evaluation of the range and time phase of ischaemic lesions [27] (Figure 2). The use of diffusion techniques substantially increases the diagnostic value of the MRI studies in these cases [28]. FLAIR sequence is also very useful, as it helps to diagnose the ‘ivy sign’, i.e. an increase in signal intensity along the fissures and gyri of cerebral hemispheres, resulting most probably from the reduction of the cortical flow [29] (Figure 3A, B). The MRI study is also very useful in visualising late sequelae of the previous strokes, i.e. brain atrophy and widening of the ventricular system and of the pericerebral fluid spaces [29,30].
Figure 2.
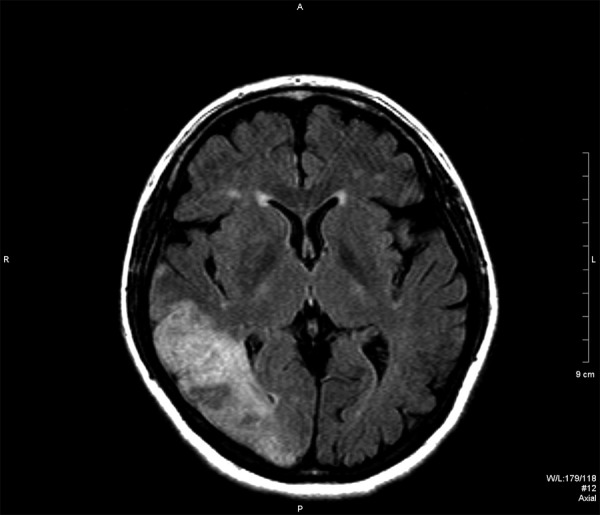
Case 1. MR study, FLAIR image in axial plane. Hyperintense lesion in the right temporal and occipital lobe.
Figure 3.
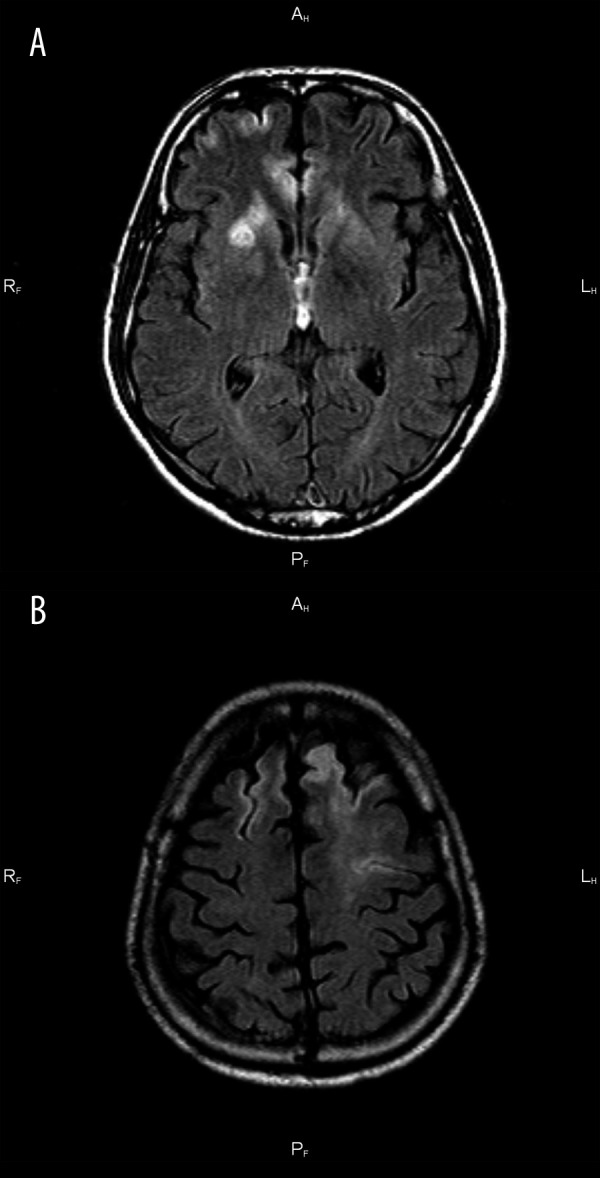
Case 2. A 35-year-old man with facial and arm paresis. MR study, FLAIR image in axial plane. Hyperintense lesion in the right basal ganglia (A). Cortical stroke in the left frontal lobe and ivy sign in the right frontal lobe (B).
Before modern angiographic examinations (such as CTA or MRA) were introduced to a wide clinical practice, the final diagnosis of the vascular changes was based on conventional angiography or digital subtraction angiography (DSA) [31]. At present, the diagnosis of the whole range of vascular changes in the course of the disease is based mainly on MRA and CTA, using multi-row systems [32–34] (Figure 4A, B). There were also single reports on the use of transcranial Doppler ultrasonography. However, this method does not have any significance in disease diagnostics [35,36].
Figure 4.
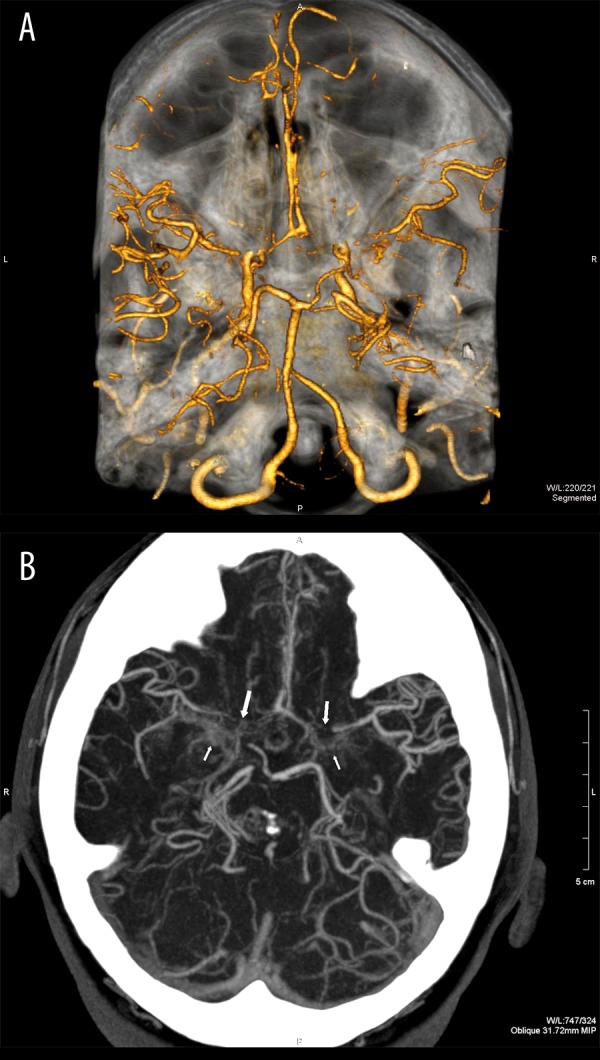
Case 1, Suzuki grades III/IV. CT angiography, 3D reconstruction. Narrowing of the distal segments of both ICAs. Narrow or locally not visible segments A1 of the anterior cerebral arteries and M1 of the middle cerebral arteries (A). Moyamoya collaterals visible only in MIP reconstruction (B).
Angiographic examinations distinguish 6 degrees of the severity of vascular changes [37]. The first degree includes only stenosis of the carotid artery. In the 2 and 3 degree, the collateral vasculature of moyamoya type develops and increases its range (Figures 5, 6). In the fourth and fifth degree, these vessels start disappearing, and in the sixth degree they become invisible (Figure 7) – the collateral vessels branch only from external carotid arteries.
Figure 5.
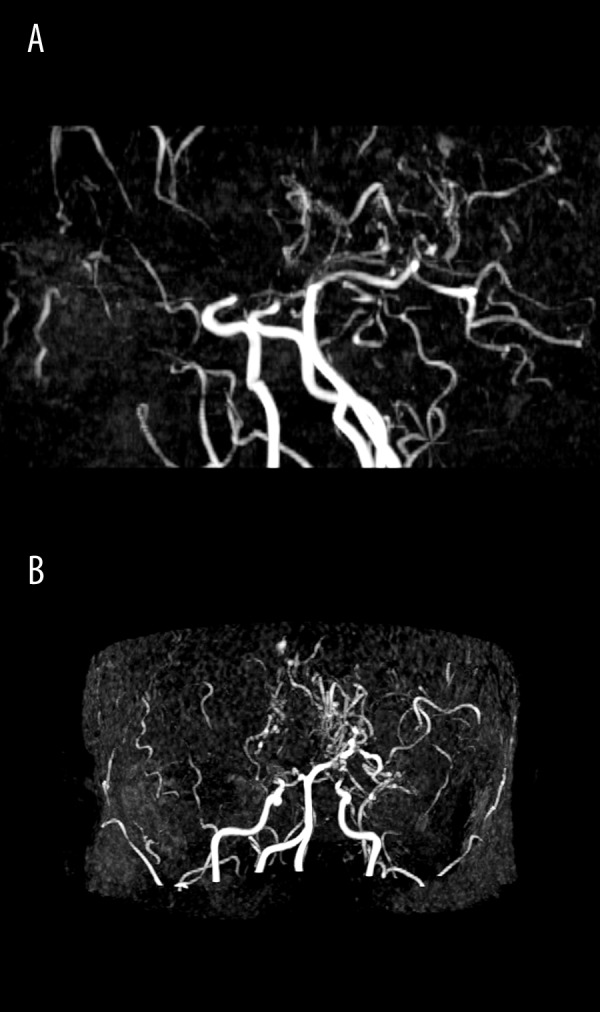
Case 1, Suzuki grades III/IV. MR angiography, ToF (time-of-flight) technique, MIP reconstructions in sagittal (A) and axial plane (B). No signal from either of the anterior cerebral and middle cerebral arteries. Collateral vessels of “moyamoya” type, visible at the base of the brain.
Figure 6.
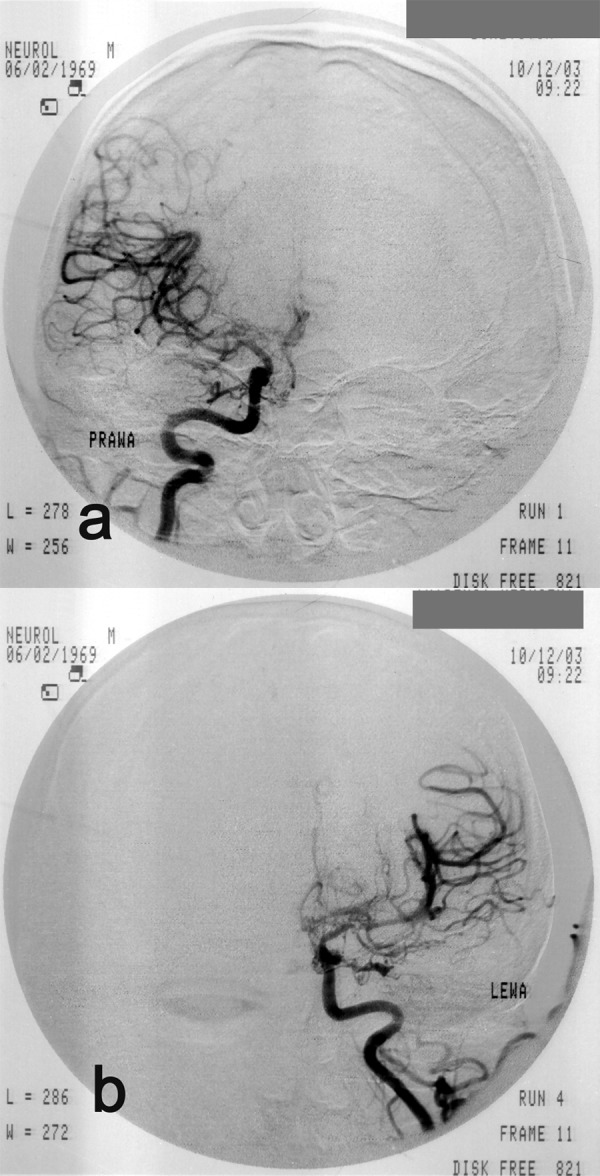
Case 3, Suzuki grade III. A 33-year-old man with seizures, aphasia and right-sided hemiparesis. Angiogram of the right (A) and of the left (B) internal carotid artery. Stenosis of the distal parts of the internal carotid arteries; proximal stenosis of anterior and middle cerebral arteries; single “moyamoya” collateral circulation vessels
Figure 7.
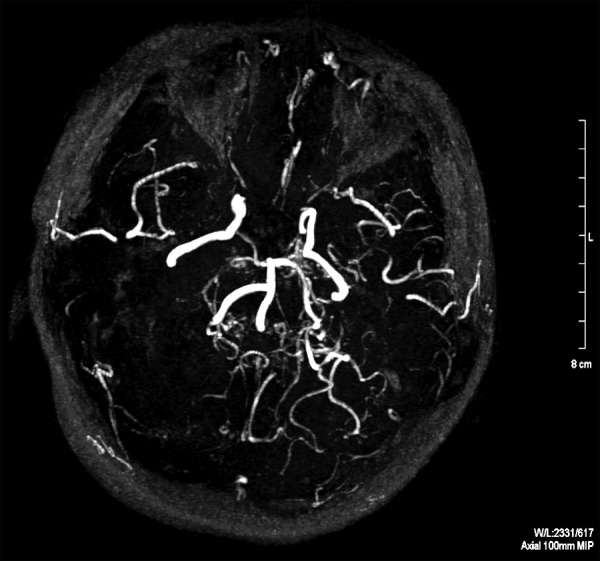
Case 2, Suzuki grades V/VI. MR angiography, MIP reconstruction in axial plane. No signal from either of the anterior or middle cerebral arteries; collateral circulation vessels not visible.
An important issue reported on in the literature is also the quantitative evaluation of haemodynamic disturbances of the cerebral circulation in the course of the moyamoya disease, evaluated on the basis of PET, SPECT, and perfusion CT and MRI [38–41].
Comparative studies, including vascular and perfusion examinations, have shown significant correlations between the angiographic image and the regional perfusion. As the disease normally affects both internal carotid arteries, and not the posterior part of the arterial circle of Willis, the individuals with moyamoya disease experience a decrease in frontal blood flow (dominant in normal conditions), with normal or increased blood flow in occipital lobes [41,42].
On the basis of perfusion studies, it was also found out that in children with moyamoya disease there is a decrease in cerebral blood flow (CBF) and increase in cerebral blood volume (CBV) and in O2 extraction fraction (OEF), much more pronounced than in adult populations [43,44]. Moreover, it was reported that the reduction of CVR (cerebrovascular reserve) is higher in children, which was evaluated on the basis of tests with acetazolamide or CO2. Such differences may explain higher incidence of ischaemic lesions in paediatric populations – as opposed to intracranial haemorrhages, which are more often in adults [45].
The results of the studies on haemodynamic disturbances in adults are not homogeneous. The majority of papers reported disturbances similar to changes observed in children [42]. However, some of the reports did not confirm the presence of significant changes in perfusion parameters in adults [44]. These differences follow most probably from heterogeneity of the studied groups and depend on severity of changes and the presence of the collateral circulation. Piao et al. [42] found that patients with clinical symptoms of ischaemia or with collateral circulation, experience an increase in CBV and decrease in CVR, as opposed to the group of patients without a well-developed collateral vasculature. On the other hand, Nariai et al. [38] showed that in cases with ischaemic stroke or TIA, there followed an increase of CBV and OEF, while in patients with haemorrahges or TIA, there were no changes in perfusion parameters.
Conservative and Surgical Treatment
Causal treatment of the disease is not known. Cases of mild clinical course are normally treated conservatively. In severe cases, it is indicated to carry out surgery [13]. It is also indicated to administer antiplatelet medicines. Anticoagulative therapy is rarely used, due to the risk of bleeding [12].
Surgery includes direct anastomoses, indirect procedures, and combined therapies. Anastomoses are usually performed between the superficial temporal artery and the middle cerebral artery (STA-MCA, i.e. superficial temporal artery – middle cerebral artery anastomosis) [17,46]. Indirect procedures include synangiosis involving placement of vascularised tissue in the brain cortex, in order to promote neoangiogenesis. This type of procedure uses dura mater (EDAS – encephaloduroarteriosynangiosis), muscle tissue (EDAMS – encephaloduroarteriomyosynangiosis) or cranial periosteum [46]. Because procedures of this type do not prevent from recurrence of ischaemic incidents, combined therapies are advised [47]. In a large material of Japanese authors, including 140 patients treated surgically, successful results were obtained in 92.9% of cases, and complications were noted only in 2.9% of patients [45]. According to the data from the metaanalysis of Fung et al. [48], including 1156 cases, the risk of symptomatic disease recurrence after surgery amounted to merely 2.6% and was much lower than in patients treated conservatively, with 2/3 of them experiencing progression of the symptoms within 5 years of the disease process [12].
Recently, there has also been reported a successful implantation of a stent to the internal carotid artery in a young woman with moyamoya syndrome and recurrent TIA symptoms [49].
Before a planned procedure, it is indicated to evaluate the cerebral circulation in detail. Non-enhanced MRA has a high spatial resolution but does not allow for a satisfactory time-dependent evaluation of cerebral circulation. Moreover, this technique overestimates the degree of stenosis of the Willis circle arteries and underestimates the extension of the collateral vasculature [50]. Thus, it is indicated to perform CAS including all intracerebral and external carotid arteries before surgery. Such a comprehensive imaging aims at visualising all collateral vessels, in order not to injure any of them during surgery. An alternative method is the contrast-enhanced MRA with subtraction technique, which is characterised by a high time resolution, comparable with CAS [51], or examination with the use of 3T high-field-strength MR [52]. In order to evaluate the cerebrovascular reserve before the procedure, it is indicated to perform also SPECT examination with acetazolamide test, which is the main criterion of qualification for surgery [17,53]. Helpful may be also other methods of cerebral blood flow evaluation – CT perfusion, MRI or PET [40,41,43,45]. Perfusion examinations are also used to evaluate the effectiveness of the procedure and to improve haemodynamic parameters: increase of cerebrovascular reserve and decrease of CBV and OEF [43,54].
Conclusions
Despite its low incidence in the European population, the moyamoya disease should be taken into account as a possible cause of ischaemic strokes or spontaneous intracranial haemorrhages in young individuals.
Surgery is the only successful method of treatment, preventing from disease recurrence. A proper qualification for surgery should base on a comprehensive angiographic and imaging evaluation of brain structures.
References:
- 1.Takeuchi K, Shimizu K. Hypoplasia of the bilateral internal carotid arteries. Brain Nerve. 1957;9:37–43. [Google Scholar]
- 2.Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008;79(8):900–4. doi: 10.1136/jnnp.2007.130666. [DOI] [PubMed] [Google Scholar]
- 3.Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7(11):1056–66. doi: 10.1016/S1474-4422(08)70240-0. [DOI] [PubMed] [Google Scholar]
- 4.Yonekawa Y, Ogata N, Kaku Y, et al. Moyamoya disease in Europe, past and present status. Clin Neurol Neurosurg. 1997;99(Suppl.2):S58–60. doi: 10.1016/s0303-8467(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 5.Kułakowska A, Kapica-Topczewska K, Borowik H, et al. Moyamoya disease as a rare cause of ischaemic stroke-case report. Pol Merkur Lekarski. 2009;27(160):334–37. [PubMed] [Google Scholar]
- 6.Grądzki J, Wróblewski T. Zespół “moyamoya”. Pol Przegl Radiol Med Nukl. 1980;44(4):273–77. [in Polish] [PubMed] [Google Scholar]
- 7.Mineharu Y, Liu W, Inoue K, et al. Autosomal dominant moyamoya disease maps to chromosome 17q25.3. Neurology. 2008;70(24):2357–63. doi: 10.1212/01.wnl.0000291012.49986.f9. [DOI] [PubMed] [Google Scholar]
- 8.Chaanine A, Hugonenq C, Lena G, et al. Neurological complications in Down syndrome. Arch Pediatr. 2008;15(4):388–96. doi: 10.1016/j.arcped.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Somarajan A, Ashalatha R, Syam K. Moya Moya disease: an unusual clinical presentation. J Assoc Physicans India. 2005;53:49–51. [PubMed] [Google Scholar]
- 10.Hankinson TC, Bohman LE, Heyer G, et al. Surgical treatment of moyamoya syndrome in patients with sickle cell anemia: outcome following encephaloduroarteriosynangiosis. J Neurosurg Pediatr. 2008;1(3):211–16. doi: 10.3171/PED/2008/1/3/211. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich NJ, Robertson R, Kinnamon DD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68(12):932–8. doi: 10.1212/01.wnl.0000257095.33125.48. [DOI] [PubMed] [Google Scholar]
- 12.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360(12):1226–37. doi: 10.1056/NEJMra0804622. [DOI] [PubMed] [Google Scholar]
- 13.Burke GM, Burke AM, Sherma AK, et al. Moyamoya disease: a summary. Neurosurg Focus. 2009;26(4):E11. doi: 10.3171/2009.1.FOCUS08310. [DOI] [PubMed] [Google Scholar]
- 14.Kraemer M, Heienbrok W, Berlit P. Moyamoya disease in Europeans. Stroke. 2008;39(12):3193–200. doi: 10.1161/STROKEAHA.107.513408. [DOI] [PubMed] [Google Scholar]
- 15.Pineda Sánchez J, Palomeque Rico A, Cambra Lasaosa FJ, et al. A cause of vascular occlusion in childhood. An Esp Pediatr. 1999;50(1):44–48. [PubMed] [Google Scholar]
- 16.Shamim S, Kumar J, Jamalvi SW, et al. Moya Moya disease in a child. J Coll Physicians Surg Pak. 2008;18(4):252–53. [PubMed] [Google Scholar]
- 17.Sirucek P, Vaclav P, Hrbac T, et al. Brain single photon emission tomography and hypercapnia test in testing cerebrovascular reserve capacity, in Moya moya disease. Hell J Nucl Med. 2008;11(3):179–81. [PubMed] [Google Scholar]
- 18.Smith JL. Understanding and treating moyamoya disease in children. Neurosurg Focus. 2009;26(4):E4. doi: 10.3171/2000.01.FOCUS08306. [DOI] [PubMed] [Google Scholar]
- 19.Cerrato P, Grasso M, Lentini A, et al. Atherosclerotic adult Moya-moya disease in a patient with hyperhomocysteinaemia. Neurol Sci. 2007;28(1):45–47. doi: 10.1007/s10072-007-0748-6. [DOI] [PubMed] [Google Scholar]
- 20.Rabbani O, Bowen LE, Watson RT, et al. Alien limb syndrome and Moya-moya disease. Mov Disord. 2004;19(11):1317–20. doi: 10.1002/mds.20155. [DOI] [PubMed] [Google Scholar]
- 21.Hallemeier C, Rich K, Brubb R, et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Stroke. 2006;37(6):1490–96. [Google Scholar]
- 22.Takagi Y, Kikuta K, Nozaki K, et al. Histological features of middle cerebral arteries from patients treated for Moyamoya disease. Neurol Med Chir. 2007;47(1):1–4. doi: 10.2176/nmc.47.1. [DOI] [PubMed] [Google Scholar]
- 23.Lim M, Cheshier S, Steinberg G. New vessel formation in the central nervous system during tumor growth, vascular malformations, and Moyamoya. Curr Neurovasc Res. 2006;3(3):237–45. doi: 10.2174/156720206778018730. [DOI] [PubMed] [Google Scholar]
- 24.Plasencia-Fernández E, Vázquez-López ME, Pulpeiro JR, et al. Transdural anastomosis in a juvenile form of moyamoya disease. Rev Neurol. 1997;25(148):1939–41. [PubMed] [Google Scholar]
- 25.Hasuo K, Mihara F, Matsushima T. MRI and MR angiography in moyamoya disease. J Magn Reson Imaging. 1998;8(4):762–66. doi: 10.1002/jmri.1880080403. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed R, Ahsan H. Imaging of Moya Moya disease. J Pak Med Assoc. 1997;47(7):181–85. [PubMed] [Google Scholar]
- 27.Hasuo K, Yasumori K, Yoshida K, et al. Magnetic resonance imaging compared with computed tomography and angiography in moyamoya disease. Acta Radiol. 1990;31(2):191–95. [PubMed] [Google Scholar]
- 28.Chabbert V, Ranjeva JP, Sevely A, et al. Diffusion- and magnetisation transfer-weighted MRI in childhood Moya-moya. Neuroradiology. 1998;40(4):267–71. doi: 10.1007/s002340050583. [DOI] [PubMed] [Google Scholar]
- 29.Fujiwara H, Momoshima S, Kuribayashi S. Leptomeningeal high signal intensity (ivy sign) on fluid-attenuated inversion-recovery (FLAIR) MR images in moyamoya disease. Eur J Radiol. 2005;55(2):224–30. doi: 10.1016/j.ejrad.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Fujisawa I, Asato R, Nishimura K, et al. Moyamoya disease: MR imaging. Radiology. 1987;164(1):103–5. doi: 10.1148/radiology.164.1.3588894. [DOI] [PubMed] [Google Scholar]
- 31.Hasuo K, Tamura S, Kudo S, et al. Moya moya disease: use of digital subtraction angiography in its diagnosis. Radiology. 1985;157(1):107–11. doi: 10.1148/radiology.157.1.3898215. [DOI] [PubMed] [Google Scholar]
- 32.Takanashi JI, Sugita K, Niimi H. Evaluation of magnetic resonance angiography with selective maximum intensity projection in patients with childhood moyamoya disease. Eur J Paediatr Neurol. 1998;2(2):83–89. doi: 10.1016/s1090-3798(98)80046-9. [DOI] [PubMed] [Google Scholar]
- 33.Yamada I, Matsushima Y, Suzuki S. Moyamoya disease: diagnosis with three-dimensional time-of-flight MR angiography. Radiology. 1992;184(3):773–78. doi: 10.1148/radiology.184.3.1509066. [DOI] [PubMed] [Google Scholar]
- 34.Yamada I, Suzuki S, Matsushima Y. Moyamoya disease: comparison of assessment with MR angiography and MR imaging versus conventional angiography. Radiology. 1995;196(1):211–18. doi: 10.1148/radiology.196.1.7784569. [DOI] [PubMed] [Google Scholar]
- 35.Lee YS, Jung KH, Roh JK. Diagnosis of moyamoya disease with transcranial Doppler sonography: correlation study with magnetic resonance angiography. J Neuroimaging. 2004;14(4):319–23. doi: 10.1177/1051228404264958. [DOI] [PubMed] [Google Scholar]
- 36.Laborde G, Harders A, Klimek L, et al. Correlation between clinical, angiographic and transcranial Doppler sono-graphic findings in patients with moyamoya disease. Neurol Res. 1993;15(2):87–92. doi: 10.1080/01616412.1993.11740115. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki J, Kodama N. Moyamoya disease: a review. Stroke. 1983;14(1):104–14. doi: 10.1161/01.str.14.1.104. [DOI] [PubMed] [Google Scholar]
- 38.Nariai T, Matsushima Y, Imae S, et al. Severe haemodynamic stress in selected subtypes of patients with moyamoya disease: a positron emission tomography study. J Neurol Neurosurg Psychiatry. 2005;76(5):663–69. doi: 10.1136/jnnp.2003.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito N, Nakagawara J, Nakamura H, et al. Assessment of cerebral hemodynamics in childhood moyamoya disease using a quantitative and a semiquantitative IMP-SPECT study. Ann Nucl Med. 2004;18(4):323–31. doi: 10.1007/BF02984471. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki R, Nariai T, Matsushima Y, et al. Xe-CT in cerebrovascular disease and moyamoya disease. Acta Neurol Scand, Suppl. 1996;166:69–71. doi: 10.1111/j.1600-0404.1996.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 41.Togao O, Mihara F, Yoshiura T, et al. Cerebral hemodynamics in Moyamoya disease: correlation between perfusion-weighted MR imaging and cerebral angiography. AJNR Am J Neuroradiol. 2006;27(2):391–97. [PMC free article] [PubMed] [Google Scholar]
- 42.Piao R, Oku N, Kitagawa K, et al. Cerebral hemodynamics and metabolism in adult moyamoya disease: comparison of angiographic collateral circulation. Ann Nucl Med. 2004;18(2):115–21. doi: 10.1007/BF02985101. [DOI] [PubMed] [Google Scholar]
- 43.Ikezaki K, Matsushima T, Kuwabara Y, et al. Cerebral circulation and oxygen metabolism in childhood moyamoya disease: a perioperative positron emission tomography study. J Neurosurg. 1994;81(6):843–50. doi: 10.3171/jns.1994.81.6.0843. [DOI] [PubMed] [Google Scholar]
- 44.Taki W, Yonekawa Y, Kobayashi A, et al. Cerebral circulation and metabolism in adults’ moyamoya disease – PET study. Acta Neurochir. 1989;100(3–4):150–54. doi: 10.1007/BF01403603. [DOI] [PubMed] [Google Scholar]
- 45.Lee M, Zaharchuk G, Guzman R, et al. Quantitative hemodynamic studies in moyamoya disease: a review. Neurosurg Focus. 2009;26(4) doi: 10.3171/2009.1.FOCUS08300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byval’tsev VA, Suzuki Y. Combined treatment for Moya-moya disease, by using direct anastomosis and revascularization: experience of 225 operations. Zh Vopr Neirokhir Im N N Burdenko. 2007;3:11–16. [PubMed] [Google Scholar]
- 47.Baaj AA, Agazzi S, Sayed ZA, et al. Surgical management of moyamoya disease: a review. Neurosurg Focus. 2009;26(4):E7. doi: 10.3171/2009.01.FOCUS08293. [DOI] [PubMed] [Google Scholar]
- 48.Fung LW, Thompson D, Ganesan V. Revascularisation surgery for paediatric moyamoya: a review of the literature. Childs Nerv Syst. 2005;21(5):358–64. doi: 10.1007/s00381-004-1118-9. [DOI] [PubMed] [Google Scholar]
- 49.Kornblihtt LI, Cocorullo S, Miranda C, et al. Moyamoya syndrome in an adolescent with essential thrombocythemia: successful intracranial carotid stent placement. Stoke. 2005;36(8):E71–73. doi: 10.1161/01.STR.0000174193.89864.55. [DOI] [PubMed] [Google Scholar]
- 50.Yoon HK, Shin HJ, Lee M, et al. MR angiography of moyamoya disease before and after encephaloduroarteriosynangiosis. AJR Am J Roentgenol. 2000;174(1):195–200. doi: 10.2214/ajr.174.1.1740195. [DOI] [PubMed] [Google Scholar]
- 51.Aoki S, Yoshikawa T, Hori M, et al. Two-dimensional thick-slice MR digital subtraction angiography for assessment of cerebrovascular occlusive diseases. Eur Radiol. 2000;10(12):1858–64. doi: 10.1007/s003300000584. [DOI] [PubMed] [Google Scholar]
- 52.Fushimi Y, Miki Y, Kikuta K, et al. Comparison of 3.0- and 1.5-T three dimensional time-of-flight MR angiography in moyamoya disease: preliminary experience. Radiology. 2006;239(1):232–37. doi: 10.1148/radiol.2383042020. [DOI] [PubMed] [Google Scholar]
- 53.Marcinkevicius E, Liutkus D, Gyazdatis A. Experience of treatment of moyamoya disease at the Clinic of Neurosurgery of Kaunas University of Medicine. Medicina. 2006;42(2):130–36. [PubMed] [Google Scholar]
- 54.Touho H, Karasawa J, Ohnishi H. Preoperative and postoperative evaluation of cerebral perfusion and vasodilatory capacity with 99mTc-HMPAO SPECT and acetazolamide in childhood Moyamoya disease. Stroke. 1996;27(2):282–89. doi: 10.1161/01.str.27.2.282. [DOI] [PubMed] [Google Scholar]


