Summary
Background:
Squamous cell cancer (SCC) of the head and neck, like other malignancies, should be reported with regard to TNM classification and treated accordingly. Sole anatomic imaging has its drawbacks, as early lesion detection often remains challenging, non-neoplastic processes can mimic malignancies and there are doubts concerning the extent of tumour.
The purpose of this study was to perform assessment of head and neck squamous cell cancer and surrounding tissue, in order to examine the relationship between perfusion measurements derived from CT perfusion imaging (CTP) and histologic evaluation of resected tissue.
Material/Methods:
We prospectively evaluated 21 primary SCC of the oral cavity and oropharynx, using contrast enhanced CT of the head and neck followed by CTP examination at the level of tumour. Blood flow (BF), blood volume (BV), mean transit time (MTT), and permeability (PS) values were calculated with use of manually drawn regions of interest (ROIs) over the lesions and on the contralateral side. Results were compared with histologic analysis of resected tissue.
Results:
CTP was possible in all twenty one patients, but one did not undergo surgery. Of the remaining twenty, four had retromolar trigone cancer, nine had tongue cancer and seven had tonsil cancer. We found significant differences between infiltrated and healthy tissue. Differentiation was most reliable by using blood flow (BF), permeability surface (PS) and blood volume (BV).
Conclusions:
CTP shows promise in distinguishing benign and malignant processes, primarily by means of BF, BV and PS.
Keywords: squamous cell cancer, oropharyngeal cancer, CT perfusion, functional imaging
Background
Computed tomography and magnetic resonance imaging are routinely used to diagnose and stage cancer of the head and neck. Evaluation of sole anatomic imaging has drawbacks, as early lesion detection remains difficult and benign processes can mimic malignancies. Also in many cases inflammatory response cannot be differentiated from tumour itself, what leads to over-interpretation and upstaging of the disease [1].
Morphological imaging with computed tomography (CT) and magnetic resonance (MRI) allows precise detection of pathology, but neither method can accurately differentiate benign from malignant process. Tissue biopsy for pathologic confirmation is invasive, associated with a risk of haemorrhage and infection, and may be limited by sampling error. Metabolic imaging with single photon emission CT (SPECT) and photon emission tomography (PET) can help this differentiation, but they are expensive, less available and have low spatial resolution. Ultrasound (US) and US-guided fine-needle aspiration cytology (FNAC) have been extensively used in clinical practice, but this technique is invasive and operator-dependent with high incidence of false negative results [2].
To overcome these limitations several techniques of functional imaging have been introduced. One of them is CT perfusion imaging (CTP). This technique is based on visualisation of areas of hyper-perfusion which are the result of neoangiogenesis in tumour and this feature accompanies most malignancies.
Neoangiogenesis is characteristic for every neoplasm. When visualised on functional imaging, it might be used to discriminate benign structures from aggressive lesions, enabling early detection of malignancy, prediction of tumour behaviour and the assessment of response to therapy.
The purpose of this study was to assess the value of CTP in oropharyngeal and oral cavity cancer in order to evaluate possible infiltration of surrounding structures. In addition, CTP values for different head and neck structures were compared.
Material and Methods
We prospectively examined 21 consecutive patients (17 male patients, 4 female patients, mean age 54 years, age range 34–82 years) with oral cavity or oropharyngeal squamous cell cancer, proven on biopsy. Patients were previously untreated and did not undergo any treatment or therapy except for biopsy. Of the 21 tumours (one in every patient), 11 were located in oral cavity (retromolar trigone, anterior tongue, floor of the mouth) and 10 were located in oropharynx (tonsils and tongue base). All patients were scheduled for surgery. Since one patient refused surgical treatment, twenty were left for further analysis. The detailed characteristics of patients is presented in Table 1.
Table 1.
Information about patients with SCC of oral cavity and oropharynx, scheduled for surgery and examined in CTP.
| Pt. | Tumor (space) | TNM | Surgery |
|---|---|---|---|
| 1 | retromolar trigone | T2N1M0 | middle mandibulotomy; RMND |
| 2 | retromolar trigone | T3N2aM0 | middle mandibulotomy; RMND |
| 3 | retromolar trigone | T4aN2cM0 | lateral mandibulectomy; BMRND |
| 4 | retromolar trigone | T4aN2cM0 | lateral mandibulectomy; BMRND |
| 5 | tonsil (pharyngeal mucosal space) | T2N2aM0 | intra-oral approach; BMRND |
| 6 | tonsil (pharyngeal mucosal space) | T2N2aM0 | intra-oral approach; BMRND |
| 7 | tonsil (pharyngeal mucosal space) | T2N2aM0 | intra-oral approach; BMRND |
| 8 | tonsil (pharyngeal mucosal space) | T2N2aM0 | intra-oral approach; BMRND |
| 9 | tonsil (pharyngeal mucosal space) | T2N2cM0 | intra-oral approach; RMND |
| 10 | tonsil (pharyngeal mucosal space) | T4aN2cM0 | middle mandibulotomy; RMND |
| 11 | tonsil (pharyngeal mucosal space) | T4aN2cM0 | middle mandibulotomy; RMND |
| 12 | floor of the mouth | T2N2bM0 | middle mandibulotomy; BMRND |
| 13 | floor of the mouth | T2N2cM0 | visor flap; BMRND |
| 14 | tongue (anterior 1/3) | T2N2cM0 | intra-oral approach; BMRND |
| 15 | tongue (anterior 1/3) | T4aN2cM0 | middle mandibulotomy; BMRND |
| 16 | tongue (anterior 1/3) | T4aN2cM0 | middle mandibulotomy; BMRND |
| 17 | tongue (anterior 1/3) | T4aN2cM0 | middle mandibulotomy; BMRND |
| 18 | tongue base | T2N2cM0 | middle mandibulotomy; BMRND |
| 19 | tongue base | T4aN2cM0 | middle mandibulotomy; BMRND |
| 20 | tongue base | T4aN2cM0 | middle mandibulotomy; BMRND |
RMND – radical modified neck dissection; BMRND – bilateral modified radical neck dissection.
Tumour was planned to be removed with at least 5-mm margins of healthy tissue. In addition, structures in the vicinity of tumour, suspected for malignant infiltration, were resected and separately sent for histopathological evaluation. For structures, which were suspected for infiltration on imaging studies and clinically unchanged, the intra-operative surgical evaluation was deciding.
CT imaging technique and post-processing
Standard contrast-enhanced head and neck study followed by CTP exam was obtained by using a multi-detector scanner (VCT; GE Medical Systems, Milwaukee, WI). First, 80 mL of a non-ionic iodinated contrast agent (iodixanol, 320 mg/mL; Visipaque, GE Healthcare) was injected at a rate of 1 mL/second and images were acquired with 100s delay, from the skull base to the thoracic inlet with 1.25-mm contiguous sections. CTP was performed 5–10 minutes later. For perfusion study patients received an injection of 40 ml of the same contrast agent at 4.5 mL/s and were scanned to acquire 16 contiguous 5-mm-thick slices (8 cm coverage) for 50 seconds at the predetermined levels of interest. The level of interest represented the tumour and in all cases it was possible to include the whole tumour. In patients with no defined lesion on the regular enhanced CT scans, perfusion images were obtained through the areas that were clinically suspicious.
The data was post-processed by using a commercial software package based on a deconvolution-based technique (Perfusion 3, Advantage Windows 4.2 workstation; GE Medical Systems). ROIs were placed in carotid artery and in the internal jugular vein manually, to generate contrast-enhanced curves. The data was then processed into maps that represented blood flow (BF, in mL per 100 g per minute), mean transit time (MTT, in seconds), blood volume (BV, in mL per 100 g) and capillary permeability surface product (PS, in mL per 100 g per minute).
Image analysis
For the evaluation of the tumour, a freehand ROI through the lesion or suspected lesion was drawn, using base image; other ROIs were placed on surrounding structures like muscles, salivary glands, fat, on the side of the tumour and on contralateral side. The mean values of BF, BV, MTT and PS were obtained separately for tumour and contralateral unaffected structures – like salivary glands, paraspinous muscles, muscles of mastication, muscles of the floor of the mouth, sternocleidomastoid muscle, base of the tongue, subcutaneous fat, parapharyngeal space.
Surgery and histology
Tumours were staged using the TNM classification (International Union Against Cancer, 2002) and excised based on the consensus between clinical findings (endoscopic evaluation) and imaging findings.
Resected tumour underwent standard histopathological analysis.
Statistical analysis
Differences between malignant lesions and normal structures were assessed.
We calculated the difference between CTP parameters (BV, BF, MTT, PS) within structure suspected for malignant infiltration and contralateral unaffected structure.
The tumour ROIs were hand drawn, for all anatomic sections where tumour was visible. Representative parameters values were then averaged across the sections.
Perfusion parameters from contralateral structures were similarly obtained.
Statistical analysis was performed by using the Student t-test for comparison of two data sets and P values were calculated for each comparison. Statistical significance was set at P<.05.
Results
The perfusion parameters of 20 patients with squamous cell cancer of the oral cavity and oropharynx were compared.
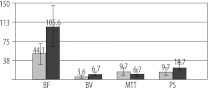
There was a significant difference in blood flow, blood volume and permeability surface (p<0.05) between tumour and contralateral unaffected tissue (Figures 1, 2), with very high values of BF, BV and PS in the tumour (Tables 2, 3, Figure 3). No significant difference was observed with regard to mean transit time.
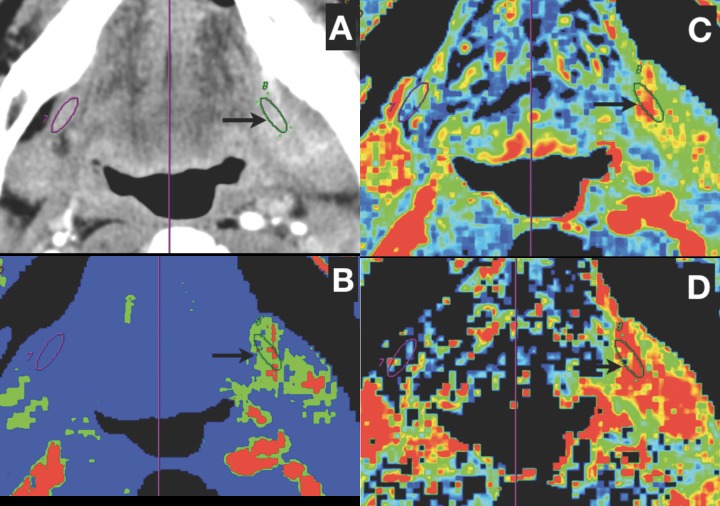
Figure 1.
A case of retromolar trigone cancer with floor of the mouth infiltration. (A) One ROI (black arrow) is manually placed over infiltrated mylohyoid muscle on the left side and another ROI is automatically created through symmetry axis (purple line) on the contralateral mylohyoid muscle. (B–D) Values of perfusion are significantly higher in tumour (BF=135.4 ml/100 g/min, BV=7.6 ml/100 g, PS=23.4 ml/100 g/min) than in contralateral unaffected muscle (BF=26.9 ml/100 g/min, BV=2.7 ml/100 g, PS=7.8 ml/100 g/min).
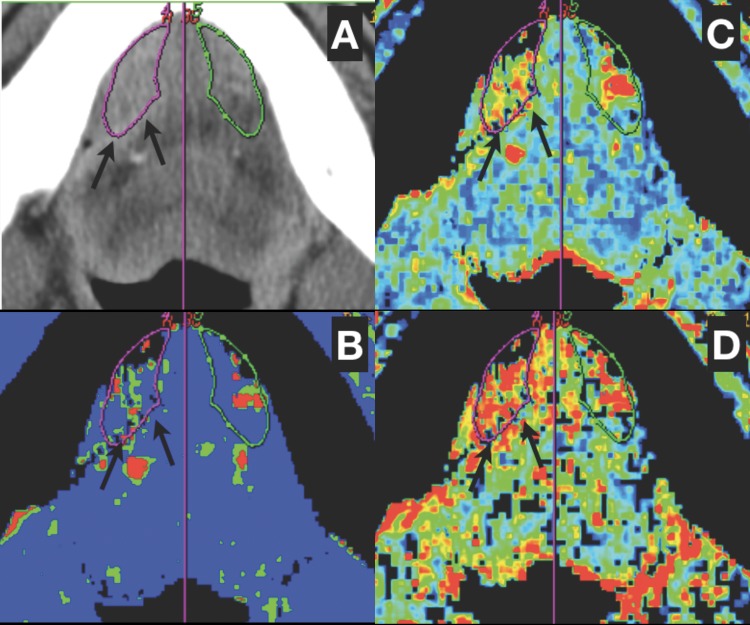
Figure 2.
A case of right-sided floor of the mouth cancer with infiltration of sublingual space. (A) One ROI is placed over the tumour (arrows) and another ROI is created through axis of symmetry (purple line) in contralateral sublingual space (outlined in green). (B–D)There is significant hyperperfusion in the right sublingual space (BF=107.4 ml/100 g/min, BV=6.9 ml/100 g, PS=20.1 ml/100 g/min) in comparison with the left side (BF=53.1 ml/100 g/min, BV=5.1 ml/100 g, PS=13.1 ml/100 g/min).
Table 2.
CT perfusion results in 20 patients with SCC of the oral cavity and oropharynx – tumour measurements.
| Parameter | Mean ± standard deviation | Range |
|---|---|---|
| BF (ml/100 g/min) | 105.6±41.5 | 40.3–193 |
| BV (ml/100 g) | 6.7±2.5 | 3.2–14.1 |
| MTT (sec) | 6.7±4.6 | 1.5–22.3 |
| PS (ml/100 g/min) | 18.7±5.5 | 6.6–32.3 |
Table 3.
CT perfusion results in 20 patients with SCC of the oral cavity and oropharynx – measurements in unaffected tissues, contra-laterally to tumour.
| Parameter | Mean ± standard deviation | Range |
|---|---|---|
| BF (ml/100 g/min) | 41.4±25.1 | 14.5–106 |
| BV (ml/100 g) | 3.6±1.5 | 1.8–6.7 |
| MTT (sec) | 9.7±5.1 | 1.5–21.6 |
| PS (ml/100 g/min) | 9.7±4.8 | 2.7–22.7 |
Figure 3.
Comparison of perfusion parameters between squamous cell cancer and contra-lateral unaffected tissue (perfusion values from healthy tissues are marked in grey and values from SCC- infiltrated tissue are marked in black).
Discussion
The morphologic information obtained on imaging studies like CT or MRI concerning squamous cell cancer is often insufficient and difficult to interpret, because of highly infiltrative character of tumour, concomitant oedema and accompanying inflammation.
Squamous cell cancer is characterised by increased angiogenic activity and neovascularity, therefore perfusion CT will have a potential role in visualising the extent of tumour and in monitoring its activity, often before it proceeds in a gross anatomical distortion [3,4].
A variety of approaches have been used for the radiologic assessment of tumour perfusion. These include contrast-enhanced dynamic CT, spin-labelling technique, blood oxygen level – dependent imaging with MR and dynamic contrast-enhanced MR imaging [5]. A CT-based method is advantageous, because CT is the most widely used diagnostic radiographic approach for assessing head and neck malignancies [6]. Deconvolution-based CTP is also a fast and robust imaging technique that is increasingly being used in the evaluation of intracranial vascular disorders [7].
The physiologic basis of contrast enhancement closely matches tumour angiogenesis.
There is an established relationship between malignant infiltration, or tumour growth, and contrast enhancement on CT scans, because the basis of contrast enhancement results from the physiologic effects of tumour angiogenesis [8]. Angiogenesis is associated with increased perfusion, what is seen as an elevation of the value of the following parameters: BV, BF, PS. It seems that CT perfusion of the head and neck would become a valuable tool in differentiating normal tissue from malignant process. Gandhi et al. [9] demonstrated increased values of PS, BF, and BV in head and neck SCC, compared with values for normal adjacent structures. Similarly, Rumboldt et al [10] were able to differentiate malignant from non-malignant lesions of the head and neck with CT perfusion parameters.
Our results show, that based on perfusion parameters, such as blood flow, blood volume and capillary permeability it is possible to differentiate between tumour (squamous cell cancer) and healthy tissue in oropharynx and oral cavity. We observed a significantly higher value of CT perfusion parameters, such as BF, BV and PS, measured in squamous cell cancer of the oral cavity and oropharynx, when compared to contralateral unaffected tissue. Although further prospective studies with larger patient populations are needed, in our opinion the potential for CT perfusion to detect malignant lesion and to evaluate its extent is promising and warrants further investigations.
Compared with the reported values in the literature, our measurements are little bit higher that parameters reported by Bisdas et al. [11–13] and this may be attributed to inclusion of necrotic tumoral areas in their evaluation, while in our patients we did not evaluate large lesions with visible necrotic foci.
Perfusion CT, except for helping in the delineation of tumour, may have a very powerful role in predicting outcome in head and neck cancer after radiotherapy. Results of some recent studies prove, that microvessel density in the tumour and high median tumour perfusion value are independent predictors of local failure [14].
However, Hermans [15] also suggests, that anatomic tumour extent, which is to some extent reflected in the tumour stage, and especially tumour bulk (as evaluated in CT) may be more important predictors of cause-specific survival than perfusion rate in the tumour.
Information about the tumour, obtained during perfusion examination, are being widely used to assess tumour response to treatment with antiangiogenic and vascular disrupting agents [16,17], since angiogenesis is a classical target of radiotherapy and chemotherapy.
Of course, perfusion studies have their shortcomings. In many cases tumour perfusion assessment is limited to one or two levels, what provides with average and unsatisfactory evaluation of the whole mass of tumour. This technique is also connected with ionising radiation and administration of iodine contrast medium. In cases of suspected lymph node metastases, the range of examination is not wide enough to include all groups of lymph nodes which should be evaluated [18].
Perfusion can be also evaluated based on MRI studies, but when compared with perfusion-weighted MR, perfusion CT is indeed more widely available and accessible, also less time-consuming. In contrast to MR perfusion, CTP has the major advantage of being able to assess all perfusion parameters in a robust quantitative way and allows a direct insight into vascular auto-regulation and neo-angiogenesis [19].
Summarising, it can be stated, that a variety of clinical situations may benefit from quantitative assessment of tumour perfusion in the future. Potential applications include differentiation between infiltrated and healthy tissue, non-invasive in vivo assessment of tissue hypoxia during radiation therapy, measurement of response to therapy by using angiogenesis inhibitors and differentiation of recurrent/residual tumour from post-treatment changes.
Conclusions
The obtained perfusion parameters were sufficient for differentiating neoplastic from normal tissue, enabling more detailed delineation of tumour and better evaluation of its extent. Results of our study are in close agreement with those published using the same tracer kinetic model. Thus, deconvolution analysis of perfusion images remains a robust technique and despite some limitations is reliable for measuring perfusion parameters both for research and clinical use.
Footnotes
Source of support: Financial disclosure: this study was entirely financed by the State Committee of Scientific Research grant number NN 402 436833
References:
- 1.Vokes E, Weichselbaum R, Lippman S, et al. Head and neck cancer. N Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 2.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg. 1994;120:699–702. doi: 10.1001/archotol.1994.01880310005001. [DOI] [PubMed] [Google Scholar]
- 3.Gleich LL, Biddinger PW, Pavelic ZP, et al. Tumor angiogenesis in T1 oral cavity squamous cell carcinoma: role in predicting tumor aggressiveness. Head Neck. 1996;18:343–46. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<343::AID-HED5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 4.Miles K. Tumour angiogenesis and its relation to contrast enhancement on computed tomography. Eur J Radiol. 1999;30:198–205. doi: 10.1016/s0720-048x(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 5.Dammann F, Horger M, Mueller-Berg M, et al. Rational diagnosis of squamous cell carcinoma of the head and neck region: comparative evaluation of CT, MRI, and 18FDG PET. AJR Am J Roentgenol. 2005;184(4):1326–31. doi: 10.2214/ajr.184.4.01841326. [DOI] [PubMed] [Google Scholar]
- 6.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 7.Miles KA. Functional computed tomography in oncology. Eur J Cancer. 2002;38:2079–84. doi: 10.1016/s0959-8049(02)00386-6. [DOI] [PubMed] [Google Scholar]
- 8.Ash L, Teknos T, Gandhi D, et al. Head and neck squamous cell carcinoma: CT perfusion can help noninvasivly predict intratumoral microvessel density. Radiology. 2009;251(2):422–28. doi: 10.1148/radiol.2512080743. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi D, Hoeffner EG, Carlos RC, et al. Computed tomography perfusion of squamous cell carcinoma of the upper aerodigestive tract. Initial results. J Comput Assist Tomogr. 2003;27(5):687–93. doi: 10.1097/00004728-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Rumboldt Z, Al-Okailli R, Develkis J. Perfusion CT of head and neck tumors: Pilot study. AJNR. 2005;26:1178–85. [PMC free article] [PubMed] [Google Scholar]
- 11.Bisdas S, Baghi M, Smolarz A, et al. Quantitative measurements of perfusion and permeability of oropharyngeal and oral cavity cancer, recurrent disease, and associated lymph nodes using first-pass contrast-enhanced computed tomography studies. Invest Radiol. 2007;42(3):172–79. doi: 10.1097/01.rli.0000252496.74242.0b. [DOI] [PubMed] [Google Scholar]
- 12.Bisdas S, Spicer K, Rumboldt Z. Whole-tumor perfusion CT parameters and glucose metabolism measurements in head and neck squamous cell carcinomas: a pilot study using combined positron-emission tomography/CT imaging. AJNR Am J Neuroradiol. 2008;29(7):1376–81. doi: 10.3174/ajnr.A1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisdas S, Baghi M, Wagenblast J, et al. Differentiation of benign and malignant parotid tumors using deconvolution-based perfusion CT imaging: feasibility of the method and initial results. Eur J Radiol. 2007;64(2):258–65. doi: 10.1016/j.ejrad.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Bisdas S, Nguyen SA, Anand SK, et al. Outcome prediction after surgery and chemoradiation of squamous cell carcinoma in the oral cavity, oropharynx, and hypopharynx: use of baseline perfusion CT microcirculatory parameters vs. tumor volume. Int J Radiat Oncol Biol Phys. 2009;73(5):1313–18. doi: 10.1016/j.ijrobp.2008.06.1956. [DOI] [PubMed] [Google Scholar]
- 15.Hermans R, Lambin P, Van der Groten A, et al. Tumoral perfusion as measured by dynamic computed tomography in head and neck carcinoma. Radiotherapy and Oncology. 1999;53:105–11. doi: 10.1016/s0167-8140(99)00132-2. [DOI] [PubMed] [Google Scholar]
- 16.Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet. 2008;371(9625):1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisdas S, Konstantinou GN, Lee PS, et al. Dynamic contrast-enhanced CT of head and neck tumors: perfusion measurements using a distributed-parameter tracer kinetic model. Initial results and comparison with deconvolution-based analysis. Phys Med Biol. 2007;52(20):6181–96. doi: 10.1088/0031-9155/52/20/007. [DOI] [PubMed] [Google Scholar]
- 18.Vandecaveye V, De Keyzer F, Nuyts, et al. Hermans R. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: correlation between radiologic and histopathologic findings. Int J Radiat Oncol Biol Phys. 2007;67(4):960–71. doi: 10.1016/j.ijrobp.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Hermans R, Vandecaveye V. Diffusion-weighted MRI in head and neck cancer. JBR-BTR. 2007;90(4):264–67. [PubMed] [Google Scholar]