Summary
Background:
Hyperdensity of the middle cerebral artery (MCA) on unenhanced CT is a recognized sign associated with brain’s early ischemia. The number of studies which showed a hyperdense posterior cerebral artery (HPCA) sign in posterior circulation infarct is relatively small. We investigated the prevalence of the HPCA sign, correlations with ischemic lesion volume, and stroke risk factors. We also determined the association with prothrombotic and inflammatory markers which have not been studied before.
Material/Methods:
In the group of 376 patients with a first acute stroke consecutively admitted to Emergency Department, early signs of brain infarction were visible in 221 (58%) cases. Fifty five (25%) subjects had ischemic lesions in the brain supplied by the posterior circulation. We analyzed the unenhanced CT scans, calculated the density of the posterior cerebral arteries, infarct volume, and assessed the relation of the HPCA sign to other factors.
Results:
The HPCA sign appeared on CT scans of 12 (22%) patients with evidence of the posterior circulation infarct. The density (in Hounsfield units) of the affected PCA was 46.5 comparing to 20.2 of an intact vessel (p<0.0001). The stroke volume was larger when the HPCA sign was observed (medians: 17.6 vs. 4.3 cm3, p=0.02); in multivariate analysis this association was still significant (OR=1.07; 95% CI, 0.99–1.13). The C-reactive protein and fibrinogen levels were significantly higher (p=0.02 for both factors) in patients with the HPCA sign in the univariate analysis.
Conclusions:
The HPCA may be considered as an additional marker of early brain infarct, especially with large lesion volume.
Keywords: stroke, imaging, computed tomography, hyperdense artery sign
Background
Visualization of acute signs of ischemic stroke is still a challenging aspect of neuroimaging. Every year new sophisticated imaging methods are introduced and their results are published showing brain ischemic lesions much more accurately. Most of these trials employ MRI techniques [1,2]. Practically, in routine emergency neurological practice head CT is still and will probably remain for many years the first step of stroke imaging. The last update of guidelines of the European Stroke Organization states that non-enhanced cranial CT is generally sufficient to conduct thrombolysis, the only one approved specific therapy for acute stroke [3]. This imaging technique identifies subtle, early signs associated with brain ischemia as: loss of gray-white matter differentiation in cortical gyri, basal ganglia or insula; focal hypoattenuation of brain structures; cortical sulcal effacement and hyperattenuation of vessel [4]. Most of them are related to the territory supplied by the middle cerebral artery (MCA). However, infarction of the region in the posterior cerebral artery (PCA) supply is not rare – according to various sources the prevalence is from 10% to 20% of all types of stroke [5]. From the above mentioned early CT signs of brain ischemia, the hyperdense MCA seems to be more reliable than parenchyma abnormalities [4]. The same phenomenon could occur in other intracranial vessels including the PCA. However, the number of studies which showed a hyperdense posterior cerebral artery (HPCA) sign in posterior circulation infarction is relatively small [6,7].
In the present study we focused on the occurrence of the HPCA sign on CT in a large group of stroke patients, and on the association of this sign with neurological status. Additionally we investigated the association between the HPCA sign and important cerebrovascular risk factors including pro-thrombotic and inflammatory markers which have not been studied before. The aim of this study was to determine the prevalence of the HPCA sign in association with PCA infarction noticeable on CT scans, density of affected vessels and the correlations with the ischemic lesion volume and fibrinogen and C-reactive protein levels.
Material and Methods
The prospective trial included 376 patients with an acute (<24 hours of symptoms onset) ischemic stroke consecutively admitted to ED in two regional hospitals between December 2006 and November 2007. Written informed consent was collected from all study participants. The protocol was approved by the Local Ethics Committee, Poznań University of Medical Sciences. Demographic data, cerebrovascular risk factors, drugs, neurological deficits, type of stroke were collected. Every patient was assigned to one of the syndromes according to the Oxfordshire Community Stroke Project (OCSP) classification. The NIH Stroke Scale (NIHSS) was used to assess the clinical status of patients at admission. At the time of collecting data hospitalized patients had not been treated by thrombolysis, so only patients with general stroke treatment are the subject of this study.
We retrospectively analyzed unenhanced brain CT scans (Picker PQ 5000 and Toshiba Aquilion TSX 101A) performed at the admission to hospital. All scans were obtained based on a standard protocol for CT examination in acute stroke suspected patient: 130-kvP tube voltage, 512×512 pixel matrix, and axial 5 mm section thickness for the entire brain (from occipital foramen to the vertex).
The reviewers (clinical neurologist (WA) and neuroanatomy expert (WLN) with experience in stroke neuroimaging interpretation) were aware that the results of CT scan come from patients with acute stroke suspicion but they were blinded to the clinical information. The HPCA sign was assessed as present (according to previously defined criteria [7]) when: 1. it was unilateral, 2. PCA was denser than the surrounding brain and counterpart on the contralateral site, 3. the other early ischemic changes (brain tissue hypoattenuation and loss of precise delineation of the gray-white interface) were present. In the case of disagreement a second review was conducted to achieve consensus. The average density of the affected vessels and their intact counterparts was calculated in Hounsfield units (HU). The area of a visible acute ischemia lesion in the PCA territory on every slice was contoured manually and based on the number of slices, the slice thickness and the marked area, the volume of lesion was automatically calculated by the StrokeCAD developed in house [8,9].
Statistics
The interobserver agreement for the HPCA sign was assessed between the two readers using kappa statistics. Statistical analysis of categorical data was performed with Fisher’s exact test or a χ2 test when appropriate. For continuous data, we used the t test and Shapiro Wilk test for normal distribution of data. The non-parametric Wilcoxon rank-sum (or Kruskal-Wallis) test was also used to confirm the significant differences between subgroups. Finally, to assess the independent contribution of the parameters with significant differences across the classes in a univariate analysis to the risk of the presence of the HPCA sign we used multivariate logistic regression analysis. The results are expressed as adjusted odds ratio and corresponding 95% confidence intervals. A p value of less than 0.05 was considered significant. All tests were 2-sided and computed with the SPSS software, ver.15.
Results
Based on clinical symptoms 376 patients were assigned to OCSP classification: 71 (19%) subjects had a total anterior circulation syndrome, 176 (47%) partial anterior circulation syndrome, 72 (19%) lacunar syndrome and 57 (15%) posterior circulation syndrome. However, the 221 (58%) patients had visible early signs of brain ischemia in CT examination. Within this group 55 (24%) patients had noticeable acute infarcts lesions in brain tissue supplied by the posterior cerebral arteries. In turn in this subgroup we identified 12 (22%) patients with the HPCA sign (Figure 1) in the CT exam. The reviewers had disagreement regarding two cases. The kappa value which expresses the inter-observer agreement for the HPCA sign was 0.81 (a very good strength of agreement). The baseline characteristics (demographic, clinical and biochemical data) according to appearance or absence of the HPCA sign are presented in Table 1. The average density of the affected PCA was 46.5 (±8.8) comparing to 20.2 HU (±6.7) of the intact vessel (p<0.0001) in a univariate non-parametric analysis.
Figure 1.
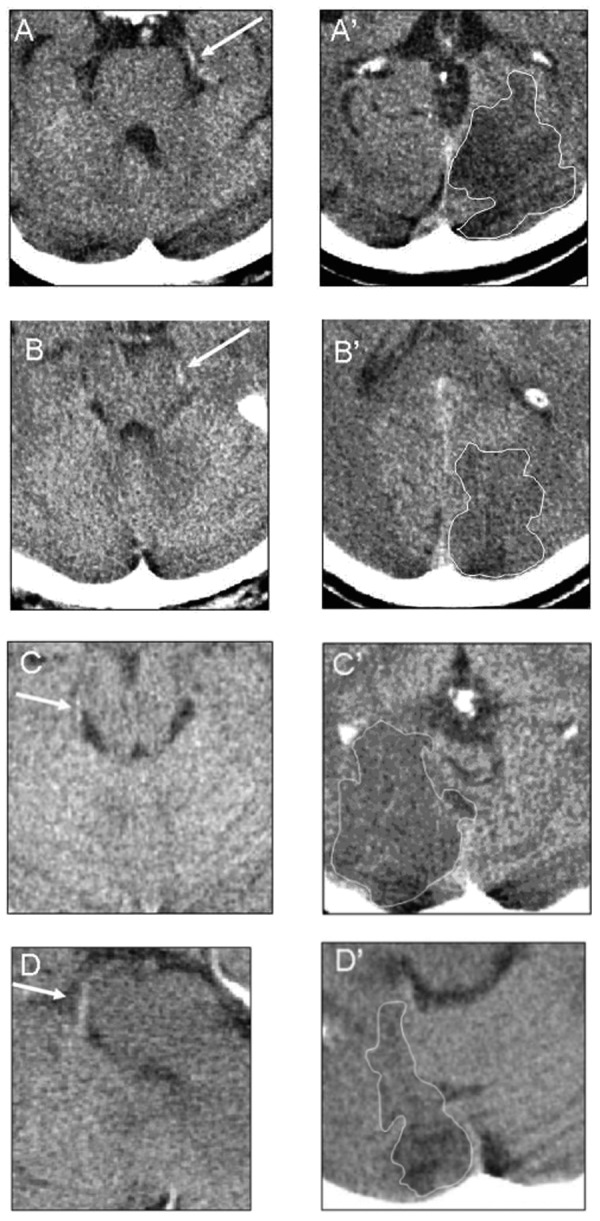
The HPCA sign on CT. Examples of the HPCA sign (A–D, marked by the white narrows) in proximal and distal parts of the P2 segment with the corresponding ischemic lesions (A’–D’ respectively). The infarct areas marked by contours in white.
Table 1.
The baseline characteristics of patients with a presence or absence of the HPCA sign.
| Parameter | HPCA Sign (n=12) | no HPCA Sign (n=43) | P-value |
|---|---|---|---|
| Age, years | 72 (62–79) | 65 (58–75) | NS |
| Female | 4 (33%) | 15 (34%) | NS |
| Baseline NIHSS | 3 (2–4.5) | 3 (2–5) | NS |
| Hematocrit at baseline (%) | 45.2 (42.5–50.5) | 42.6 (40–45.4) | 0.05 |
| WBC (K/uL) | 9.95 (7.3–12.4) | 8.1 (6.5–10.8) | NS |
| Glucose at baseline (mg/dL) | 117.5 (103.5–174.5) | 136 (104.5–172) | NS |
| C-reactive protein (mg/L) | 17.2 (8.05–28.6) | 2.6 (0.7–7.9) | 0.02 |
| Fibrinogen (g/L) | 4.8 (3.7–5.04) | 3.8 (3.0–4.4) | 0.02 |
| Time to CT scan from onset (h) | 11.5 (7.5–22) | 9.5 (5–21.5) | NS |
| Infarct's volume (cm3) | 17.6 (4.6–31.8) | 4.3 (1.5–14.1) | 0.02 |
| Secondary hemorrhage | 3 (25%) | 1 (2.3%) | 0.03 |
| Diabetes | 3 (25%) | 12 (28%) | NS |
| Coronary artery disease | 6 (50%) | 20 (45%) | NS |
| Previous stroke or TIA | 4 (33%) | 11 (25%) | NS |
| Atrial fibrillation | 1 (8%) | 8 (19%) | NS |
| Hyperlipidemia | 5 (42%) | 12 (28%) | NS |
| Current smoking | 7 (58%) | 24 (56%) | NS |
Values are median (interquartile range) or n (%). NIHSS – NIH Stroke Scale, mRS – modified Rankin Score, NS – not significant.
The HPCA sign was unilateral to the affected hemisphere in all patients.
In the univariate analysis patients with the HPCA sign had a significantly larger volume of ischemic lesion (mean 19 cm3, median 17.3 cm3) compared to those without this feature (mean 8.9 cm3, median 4.3 cm3; p=0.02). The secondary hemorrhage appeared significantly more frequent in the group with manifestation of HPCA sign compared to the patients without this symptom (p=0.03).
We did not find any significant differences in age, sex, conventional risk factors, baseline NIHSS score and most of biochemical parameters between patients without or with the HPCA sign, except C-reactive protein (CRP) and fibrinogen. In the univariate analysis levels of those two markers were significantly higher in patients with the presence of the HPCA sign than in those lacking this sign (p=0.02 for both factors). The association between higher hematocrit values and the hyperdensity of the PCA with borderline significance (p=0.05) was also observed. The results of multivariable analysis are shown in Table 2; only the infarct’s volume was an independent factor related to the presence of the HPCA sign. The statistical trend was observed between the presence of the HPCA sign and the occurrence of a secondary hemorrhage in CT scans (p=0.07) and hematocrit (p=0.05). The overall prediction of this model was 90.2% correct (100% for the absence of HPCA class and 54.5% for the presence of HPCA class).
Table 2.
Evaluation of independent factors associated with the HPCA sign by a multinomial logistic regression analysis.
| Variable | Odds Ratio | 95% Confidence Interval | Significance |
|---|---|---|---|
| Infarct's volume | 1.07 | 0.99–1.13 | P=0.04 |
| C-reactive protein | 1.01 | 0.98–1.03 | NS |
| Hematocrit | 1.22 | 0.99–1.5 | p=0.05 |
| Fibrinogen | 1.14 | 0.56–2.29 | NS |
| Secondary hemorrhage | 9.48 | 0.9–105.6 | p=0.07 |
NS – not significant.
Discussion
In our study the HPCA sign was observed among 22% patients who experienced PCA territory stroke and had noticeable acute infarcts lesions in CT in comparison to 35.4% given by a similar report [6], however, the discrepancy could arise from a different methodology used. We studied consecutively admitted patients with stroke and visible ischemic changes in CT, whereas the mentioned study had a retrospective character in which the authors were particularly interested in finding frequency of the HPCA sign. Additionally, in our study we reviewed a larger (more than twice the number) group of patients.
The agreement between the raters was very good, however, variations in interpretation were mostly because of the course and diameter of the vessel (in older patient in whom the basilar artery can be elongated and kinked and thus mimics the PCA) and localization within the cistern (closed to the brain stem which causes difficulty in interpretation, especially with a possible partial volume effect from nearest bone structures).
According to many studies, the incidence of the hyperdense MCA sign is variable. Most often it fluctuates up to 30% of initial CT scans in patients with acute ischemic stroke [10,11]. In our study, the HPCA sign is observed in 22% of CT scans carried out in posterior circulation infarction patients. Presumably the difference is the result of more heterogeneous etiology of posterior circulation stroke than in anterior circulation infarction. It is believed those cardiac and intra-arterial embolisms are the leading mechanisms [12]. Specific relations among the vertebrobasilar system cause that the emboli mostly arrest not in the PCA but in the distal parts of the basilar artery or vertebral artery [5]. Other studies showed that also atherosclerotic plaques localized in the PCA are relatively rare (comparing to the basilar and vertebral arteries) [13,14].
A standard CT scanning procedure has the orbitomeatal orientation (which not always properly visualizes the posterior cerebral arteries) could be the additional reason of a HPCA sign low detection [6].
Our study discovered that in the group of patients with the HPCA sign the infarct volume was larger compared to patients which did not present this feature. This association seems independent from other factors and is consistent with the results of the earlier study [6]. However, the advantage of our analysis is an accurate quantitative measurement of lesion’s absolute volume whereas Krings et al estimated the size of infarct by qualitative 3-point scale. The data about association between a well know hyperdense MCA sign and the infarct size is inconsistent as some studies have not shown any correlation [15] while in others the patients with the HMCA sign have a significantly larger infarct volume compared to the patients without this feature [16].
We found that the HPCA sign was significantly related (but only in the univariate analysis) to higher fibrinogen and CRP levels. To our best knowledge no previous report revealed an association between a hyperdense artery sign appearance and the considered acute phase markers, however, a strong association between inflammation, prothrombotic state and stroke is well established [5,17]. We also know that the increase of CRP levels correlates with the lesion volume [18]. That is why we assume that the link between the HPCA and acute phase markers levels comes from a more intense inflammatory state in stroke with a larger infarct area. Another explanation could be that pro-inflammatory and prothrombic states predispose to a hyperdense sign appearance – we know that the signal augmentation of artery in stroke is indicative of an intra-luminal thromboembolic clot [19]. Further population studies are necessary.
Previous studies have indicated that the hyperdense artery was related to a severe clinical status [15,20], however, most of them have been referred to the hyperdense MCA sign and stroke in an area supplied by the MCA. The results of published large trials have shown that the mean baseline NIHSS score was lower among posterior than anterior circulation stroke patients [21]. Our results are consistent with the above mentioned study because the baseline mean clinical status was relatively good. This could explain why we did not find any association between the presence of the HPCA sign and the baseline NIHSS score. The other possible explanation is that the use of NIHSS in posterior circulation stroke appears to have some inaccuracy [22,23]. As until now there is no recognizable clinical scale particularly suited to stroke in the posterior circulation, we thus applied commonly used measures. Our study has some limitations as all clinical data (demographics, risk factors, comorbid conditions, neurological status) were assessed prospectively except the CT scans analysis which had a retrospective character. Unfortunately, we did not have any other reference method for visualization intra-arterial pathology (like MR or CT angiography), however, our study is based on a routine clinical practice with stroke patients in most ED settings. Differentiating the HPCA sign from other conditions: above mentioned partial volume effect (from bones) and calcifications of dura mater are critical. Checking the neigh-boring slices with analysis of artery course and dura location can help in resolving the problem. The calcified atherosclerosis in vessels also should be considered. The calcifications of arteries have usually a higher density, and are permanent as opposed to temporal persistence of thrombus which is asymmetrical and undergoes resolving (with the disappearance of the HPCA sign in serial CT scans).
The HPCA sign is not so frequent but still a useful unenhanced CT scan finding of posterior circulation ischemia, especially, associated with large lesions. It should direct the next steps of diagnosing (i.e. angiography) and also treatment course [24].
Conclusions
We conclude that the HPCA sign can be a helpful additional early CT marker of stroke in the PCA territory.
Acknowledgments
The authors thank A. Wencel-Warot, D. Adamczewska-Kocialkowska, M. Kmieckowiak for collecting data and S. Huang, J. Liu for contribution to software development.
Footnotes
Source of support: This study is a part of the joined Singaporean-Polish project focused on development of the computer-aided decision support system for assessment of thrombolytic therapy in acute ischemic stroke in Emergency Department funded by Agency for Science, Technology and Research, Singapore and Ministry of Science and Higher Education, Poland (project no. 072-134 0049)
Disclosure of conflict of interest
All authors report no disclosures.
References:
- 1.Rosso C, Drier A, Lacroix D, et al. Diffusion-weighted MRI in acute stroke within the first 6 hours: 1.5 or 3.0 Tesla? Neurology. 2010;74(24):1946–53. doi: 10.1212/WNL.0b013e3181e396d1. [DOI] [PubMed] [Google Scholar]
- 2.Thomalla G, Rossbach P, Rosenkranz M, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65(6):724–32. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 3.Guidelines for management of ischaemic stroke and transient ischaemic attack. Cerebrovasc Dis. 2008;25(5):457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment – systematic review. Radiology. 2005;235(2):444–53. doi: 10.1148/radiol.2352040262. [DOI] [PubMed] [Google Scholar]
- 5.Warlow C, van GIjn J, Dennis M, Wardlaw JM, et al. 3rd ed. Oxford: Blackwell Publishing; 2007. Stroke: practical management; p. 995. [Google Scholar]
- 6.Krings T, Noelchen D, Mull M, et al. The hyperdense posterior cerebral artery sign: a computed tomography marker of acute ischemia in the posterior cerebral artery territory. Stroke. 2006;37(2):399–403. doi: 10.1161/01.STR.0000199062.09010.77. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi A, Wardlaw JM, Lindley RI, et al. on behalf of the IST-3 Collaborative Group Oxfordshire Community Stroke Project Clinical Stroke Syndrome and Appearances of Tissue and Vascular Lesions on Pretreatment CT in Hyperacute Ischemic Stroke Among the First 510 Patients in the 3rd International Stroke Trial (IST-3) Stroke. 2009;40(3):743–48. doi: 10.1161/STROKEAHA.108.526772. [DOI] [PubMed] [Google Scholar]
- 8.Nowinski WL, Qian G, Bhanu Prakash KN, et al. Stroke Suite: CAD systems for acute ischemic stroke, hemorrhagic stroke, and stroke in ER. 1st ed. Berlin/Heidelberg: Springer; 2008. Gupta V Medical imaging and informatics, Lecture Notes in Computer Science; pp. 377–86. [Google Scholar]
- 9.Nowinski WL, Qian G, Kirgaval Nagaraja BP, et al. Analysis of ischemic stroke MR images by means of brain atlases of anatomy and blood supply territories. Acad Radiol. 2006;13(8):1025–34. doi: 10.1016/j.acra.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Leys D, Pruvo JP, Godefroy O, et al. Prevalence and significance of hyperdense middle cerebral artery in acute stroke. Stroke. 1992;23(3):317–24. doi: 10.1161/01.str.23.3.317. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi AI, Ezzeddine MA, Nasar A, et al. Is IV tissue plasminogen activator beneficial in patients with hyperdense artery sign? Neurology. 2006;66(4):1171–74. doi: 10.1212/01.wnl.0000208407.69544.5a. [DOI] [PubMed] [Google Scholar]
- 12.Brandt T, Steinke W, Thie A, et al. Posterior cerebral artery territory infarcts: clinical features, infarct topography, causes and outcome. Multicenter results and a review of the literature. Cerebrovasc Dis. 2000;10(3):170–82. doi: 10.1159/000016053. [DOI] [PubMed] [Google Scholar]
- 13.Mazighi M, Labreuche J, Gongora-Rivera F, et al. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39(4):1142–47. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- 14.Weimar C, Goertler M, Harms L, et al. Distribution and outcome of symptomatic stenoses and occlusions in patients with acute cerebral ischemia. Arch Neurol. 2006;63(9):1287–91. doi: 10.1001/archneur.63.9.1287. [DOI] [PubMed] [Google Scholar]
- 15.Roberts HC, Dillon WP, Furlan AJ, et al. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke. 2002;33(6):1557–65. doi: 10.1161/01.str.0000018011.66817.41. [DOI] [PubMed] [Google Scholar]
- 16.Tomsick T, Brott T, Barsan W, et al. Prognostic value of the hyperdense middle cerebral artery sign and stroke scale score before ultraearly thrombolytic therapy. Am J Neuroradiol. 1996;17(1):79–85. [PMC free article] [PubMed] [Google Scholar]
- 17.Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32(11):2575–79. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 18.Audebert HJ, Rott MM, Eck T, et al. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke. 2004;35(9):2128–33. doi: 10.1161/01.STR.0000137607.61697.77. [DOI] [PubMed] [Google Scholar]
- 19.Leary MC, Kidwell CS, Villablanca JP, et al. Validation of computed tomographic middle cerebral artery “dot”sign: an angiographic correlation study. Stroke. 2003;34(11):2636–40. doi: 10.1161/01.STR.0000092123.00938.83. [DOI] [PubMed] [Google Scholar]
- 20.Manelfe C, Larrue V, von Kummer R, et al. Association of hyperdense middle cerebral artery sign with clinical outcome in patients treated with tissue plasminogen activator. Stroke. 1999;30(4):769–72. doi: 10.1161/01.str.30.4.769. [DOI] [PubMed] [Google Scholar]
- 21.Libman RB, Kwiatkowski TG, Hansen MD, et al. Differences between anterior and posterior circulation stroke in TOAST. Cerebrovasc Dis. 2001;11(4):311–16. doi: 10.1159/000047659. [DOI] [PubMed] [Google Scholar]
- 22.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–12. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- 23.Sato S, Toyoda K, Uehara T, et al. Stroke Scale Score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008;70(24):2371–77. doi: 10.1212/01.wnl.0000304346.14354.0b. [DOI] [PubMed] [Google Scholar]
- 24.De Keyser J, Gdovinová Z, Uyttenboogaart M, et al. Intravenous alteplase for stroke: beyond the guidelines and in particular clinical situations. Stroke. 2007;38(9):2612–18. doi: 10.1161/STROKEAHA.106.480566. [DOI] [PubMed] [Google Scholar]


